Abstract
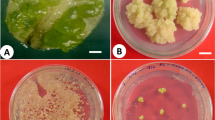
Bromus auleticus (Trin.) is a grass native to the southern cone with important agronomical potential as fodder. Different breeding programs have been initiated with this grass, but plant tissue culture techniques could not be used because B. auleticus is recalcitrant. The aim of the present study was to develop a micropropagation protocol in the genus Bromus and to investigate if the association between B. auleticus and Epichloë endophytes affected in vitro culture and growth of micropropagated plantlets. In different micropropagation stages, better results were obtained with endophyte-infected (E+) seeds compared to endophyte-free (E−) seeds. The E+ seeds presented higher percentages of in vitro germination (82 ± 5 vs. 57 ± 6%), callus induction (72 ± 6 vs. 37 ± 6%), and plant regeneration from callus (89 ± 5 vs. 13 ± 5%). We also compared the biomass of shoot complexes and regenerated plantlets. After 4 weeks of culture, shoot complexes obtained from E+ seeds reached greater weight than the ones regenerated from E− seeds (173 ± 24 vs. 74 ± 9 mg). More than the 80% of the regenerated shoot complexes were rooted ex vitro and acclimated, regardless of their origin (E+ or E−). Finally, after 4 weeks of acclimatization, the plantlets regenerated from E+ seeds reached a greater weight than the ones from E− seeds, (461 ± 64 vs. 172 ± 25 mg). These results indicate that the use of endophyte-infected (E+) seeds enhances significantly B. auleticus micropropagation and promotes growth of the regenerated plantlets.


Similar content being viewed by others
References
Agarwal T, Gupta AK, Patel AK, Shekhawat NS (2015) Micropropagation and validation of genetic homogeneity of Alhagimaurorum using SCoT, ISSR and RAPD markers. Plant Cell Tiss Organ Cult 120:313–323
Ahmed MdR, Anis M, Alatar AA, Faisal M (2017) In vitro clonal propagation and evaluation of genetic fidelity using RAPD and ISSR marker in micropropagated plants of Cassia alata L.: a potential medicinal plant. Agroforest Syst 91:637–647
Arrieta AM, Iannone LJ, Scervino JM, Vignale MV, Novas MV (2015) A foliar endophyte increases the diversity of phosphorus-solubilizing rhizospheric fungi and mycorrhizal colonization in the wild grass Bromus auleticus. Fungal Ecol 17:146–154
Ayala W, Bemhaja M, Cotro B, Do Canto J, García J, Olmos F, Real D, Rebuffo M, Reyno R, Rossi C (2010) Forrajeras; catálogo de cultivares 2010. INIA, Montevideo
Aygun A, Dumanoglu H (2015) In vitro shoot proliferation and in vitro and ex vitro root formation of Pyruselaea grifolia Pallas. Front Plant Sci 6:225
Clark E, White J, Patterson R (1983) Improved histochemical techniques for the detection of Acremonium coenophialum in tall fescue and methods of in vitro culture of the fungus. J Microbiol Methods 1:149–155
Clay K (1987) Effects of fungal endophytes on the seed and seedling biology of Lolium perenne and Festuca arundinacea. Oecologia 73:358–362
Clay K, Schardl C (2002) Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am Nat 160:99–127
Condón F, Jaurena M, Reyno R, Otaño C, Lattanzi FA (2017) Spatial analysis of genetic diversity in a comprehensive collection of the native grass Bromus auleticus Trinius (ex Nees) in Uruguay. Grass Forage Sci 72:723–733
De Battista JP, Bacon CW, Severson R, Plattner RD, Bouton JH (1990) Indole acetic acid production by the fungal endophyte of tall fescue. Agron J 82:878–880
Gasser M, Ramos J, Vegetti A, Tivano JC (2005) Digestión de láminas foliares de Bromus auleticus Trin. ex Nees sometidas a diferentes tiempos de incubación ruminal. Agricultura Técnica 65:48–54
Giri CC, Praveena M (2015) In vitro regeneration, somatic hybridization and genetic transformation studies: an appraisal on biotechnological interventions in grasses. Plant Cell Tiss Org Cult 120:843–860
Gutiérrez HF, Pensiero JF, Zabala JM (2015) Effect of population combinations on the reproductive success and germination of seeds of Bromus auleticus (Poaceae). Grass Forage Sci 70:176–184
Gwinn K, Gavin A (1992) Relationship between endophyte infestation level of tall fescue seed lots and Rhizoctonia zeae seedling disease. Plant Dis 76:911–914
Iannone LJ, Cabral D (2006) Effects of the Neotyphodium endophyte status on plant performance of Bromus auleticus, a wild native grass from South America. Symbiosis 41:61–69
Iannone LJ, Cabral D, Schardl CL, Rossi MS (2009) Phylogenetic divergence, morphological and physiological differences distinguish a new Neotyphodium endophyte species in the grass Bromus auleticus from South America. Mycologia 101:340–351
Iannone LJ, Pinget AD, Nagabhyru P, Schardl CL, De Battista JP (2012) Beneficial effects of Neotyphodium tembladerae and Neotyphodium pampeanum on a wild forage grass. Grass Forage Sci 67:382–390
Iannone LJ, Vignale MV, Pinget AD, Re A, Mc Cargo PD, Novas MV (2017) Seed-transmitted Epichloë sp. endophyte alleviates the negative effects of head smut of grasses (Ustilago bullata) on Bromus auleticus. Fungal Ecol 29:45–51
Ishikawa M, Robertson AJ, Gusta LV (1990) Effect of temperature, light, nutrients and dehardening on abscisic acid induced cold hardiness in Bromus inermis Leyss suspension cultured cells. Plant Cell Physiol 31:51–59
Ishikawa M, Suzuki M, Nakamura T, Kishimoto T, Robertson AJ, Gusta LV (2006) Effect of growth phase on survival of bromegrass suspension cells following cryopreservation and abiotic stresses. Ann Bot 97:453–459
Johnson L, de Bonth ACM, Briggs LR et al (2013) The exploitation of Epichloë endophytes for agricultural benefit. Fungal Divers 60:171–188
Lee KW, Choi GJ, Kim KY, Ji HC, Park HS, Yoon SH, Lee SH (2009) High frequency plant regeneration from mature seed derived callus of Italian ryegrass (Lolium multiflorum) cultivars. Afr J Biotechnol 8:6828–6833
Liu P, Zhang ZX, Yuan JG, Xi JB, Du XL, Yang ZY (2006) Callus induction and plant regeneration in eleven perennial ryegrass cultivars. Biotechnol Equip 20:30–37
Mc Cargo PD (2015) Evolución y diversidad de endófitos Epichloë de la forrajera nativa Bromus auleticus. Tesis Doctoral, p 136
Millot JC (1999) Bromus auleticus Trinius. Otra gramínea forrajera perenne invernal. Revista Oficial del Instituto Nacional de Semillas 2:25
Millot JC (2001) Bromus auleticus: una nueva especie domesticada. Documento de recursos fitogenéticos PROCISUR, Diálogo LVI, Montevideo, 3–6
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Nagabhyru P, Dinkins RD, Wood CL, Bacon CW, Schardl CL (2013) Tall fescue endophyte effects on tolerance to water-deficit stress. BMC Plant Biol 13:1
Nakamura T, Ishikawa M (2006) Transformation of suspension cultures of bromegrass (Bromus inermis Leyss) by Agrobacterium tumefaciens. Plant Cell Tiss Org Cult 84:293–299
Nakamura T, Yazaki J, Kishimoto N, Kikuchi S, Robertson AJ, Gusta LV, Ishikawa M (2013) Comparison of long-term up-regulated genes during induction of freezing tolerance by cold and ABA in bromegrass cell cultures revealed by microarray analyses. Plant Growth Regul 71:113–136
Novas MV, Gentile A, Cabral D (2003) Comparative study of growth parameters on diaspores and seedlings between populations of Bromus setifolius from Patagonia, differing in Neotyphodium endophyte infection. Flora 198:421–426
Novas MV, Iannone LJ, Godeas AM, Scervino JM (2011) Evidence for leaf endophytes regulation on root symbionts: effect of Neotyphodium endophytes on the pre-infective state of mycorrhizal fungi. Symbiosis 51(1):19–28
Ran Y, Patron N, Yu Q, Georges S, Mason J, Spangenberg G (2014) Agrobacterium-mediated transformation of Lolium rigidum Gaud. Plant Cell Tiss Organ Cult 118:67–75
Regalado JJ, Vignale MV, Novas MV, Pitta-Alavarez SI, Iannone L (2017) Epichloë occultans improve the Lolium multiflorum micropropagation. Plant Cell Tiss Organ Cult 130:37–46
Scheffer-Basso SM, Fávero F, Jouris C, Dall’Agnol M (2009) Selection of Bromus auleticus populations: a winter perennial grass. R Bras Zootec 38:249–255
Song G, Walworth A, Hancock JF (2012) Factors influencing Agrobacterium-mediated transformation of switchgrass cultivars. Plant Cell Tiss Organ Cult 108:445–453
Tanino KR, Chen THH, Fuchigami LH, Weiser CJ (1991) Abscisic acid-induced cellular alterations during the induction of freezing tolerance in bromegrass cells. J Plant Physiol 137:619–624
Thomas J, Ajay D, Kumar RR, Mandal AKA (2010) Influence of beneficial microorganisms during in vivo acclimatization of in vitro-derived tea (Camellia sinensis) plants. Plant Cell Tiss Organ Cult 101:365–370
Torres MS, White JF Jr, Zhang X, Hinton DM, Bacon CW (2012) Endophyte mediated adjustments in host morphology and physiology and effects on host fitness traits in grasses. Fungal Ecol 5:322–330
Verma P, Khan SA, Mathur AK, Shanker K, Kalra A (2015) Fungal endophytes enhanced the growth and production kinetics of Vinca minor hairy roots and cell suspensions grown in bioreactor. Plant Cell Tiss Organ Cult 118:257–268
Vignale MV, Astiz-Gassó MM, Novas MV, Iannone LJ (2013) Epichloid endophytes confer resistance to the smut Ustilago bullata in the wild grass Bromus auleticus (Trin.). Biol Control 67:1–7
Vignale MV, Iannone LJ, Pinget AD, De Battista JP, Novas MV (2016) Effect of epichloid endophytes and soil fertilization on arbuscular mycorrhizal colonization of a wild grass. Plant Soil 405:279–287
Vignale MV, Iannone LJ, Scervino JM, Novas MV (2017) Epichloë exudates promote in vitro and in vivo arbuscular mycorrhizal fungi development and plant growth. Plant Soil https://doi.org/10.1007/s11104-017-3173-5
Wang X, Yam TW, Meng Q, Zhu J, Zhang P, Wu H, Wang J, Zhao Y, Song X (2016) The dual inoculation of endophytic fungi and bacteria promotes seedlings growth in Dendrobium catenatum (Orchidaceae) under in vitro culture conditions. Plant Cell Tiss Organ Cult 126:523–531
Wattanasiri C, Walton PD (1993) Effects of growth regulators on callus cell growth, plant regeneration, and somaclonal variation of smooth bromegrass (Bromus inermis Leyss). Euphytica 69:77–82
Xia C, Zhang X, Christensen MJ, Nan Z, Li C (2016) Epichloë endophyte affects the ability of powdery mildew (Blumeria graminis) to colonise drunken horse grass (Achnatherum inebrians). Fungal Ecol 16:26–34
Acknowledgements
This work was supported by funding from Agencia Nacional de Promoción Científica y Tecnológica (PICT Joven 2016-0487, PICT 2016-0877, PICT 2014-3315), CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina) Grant PIP 11220150100956CO and from Universidad de Buenos Aires UBACyT (20020150100067BA and 20020150200075BA).
Author information
Authors and Affiliations
Contributions
RJJ and PASI designed the micropropagation experiments. RJJ and BV executed the micropropagation experiments. VMV checked endophytic status of plant material. NMV and ILJ provided the plant material. RJJ wrote the manuscript. VMV, NMV and ILJ reviewed the manuscript. PASI reviewed the English of the manuscript. ILJ supervised the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Fredy Altpeter.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Regalado, J.J., Berdion, V., Vignale, M.V. et al. The presence of Epichloë sp. in Bromus auleticus (Trin.) seeds enhances micropropagation and growth of micropropagated plantlets from these seeds. Plant Cell Tiss Organ Cult 135, 279–286 (2018). https://doi.org/10.1007/s11240-018-1462-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-018-1462-1