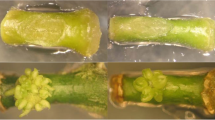
Abstract
The aim of this study was to evaluate the influence of ethylene-releasing (ACC, Ethephon, Methionine) and -inhibiting (cobalt chloride, silver thiosulfate) compounds on ethylene production and shoot organogenesis of nodal segments, where buds were completely removed, from mature tissues of Citrus limon, Fino 49 and Verna 51 cultivars. The addition of ACC to the culture medium produced a very significant decrease of the regeneration. These results were directly related to the ethylene levels measured in the atmosphere inside the tube. Similar results were observed with ethephon and methionine; the gradual increase in ethylene levels in the tubes, with increasing ethylene-releasing compounds in the culture medium, agrees with the decrease in the regeneration rate observed, but the effect was lower than with ACC. When cobalt chloride (CoCl2) was added to the culture medium, contrary to what was expected, the regeneration decreased in both cultivars and this decreasing was not related with the increase in ethylene production. These observations matched the occurrence of yellowish necrotic explants increasing the concentration of cobalt chloride, probably because of a toxic effect on lemon explants. The increase of silver thiosulfate (STS) in the culture medium enhanced the regeneration percentage in both Verna 51 and Fino 49 cultivars. Nevertheless, ethylene levels increased proportionally with the STS concentration and the regeneration rate. These results may be explained since the effects produced by the high ethylene levels measured in STS experiments were blocked by Ag+ ions, thereby increasing the regeneration percentage. Media to which STS was added produced the highest regeneration percentages. The results obtained in this study showed that ethylene plays an important role in the organogenesis of mature explants of C. limon.



Similar content being viewed by others
Abbreviations
- ACC:
-
1-Aminocyclopropane-1-carboxilic acid
- BA:
-
N6-benzyladenine
- DKW:
-
Driver and Kuniyuki Walnut Medium (Driver and Kuniyuki, 1984)
- GA:
-
Gibberellic acid
- IBA:
-
Indole-3-butyric acid
- LSD:
-
Least significant difference test
- STS:
-
Silver thiosulfate
References
Abeles FB, Morgan PW, Saltveir ME Jr (1992) Ethylene in plant biology. Academic Press, New York
Arigita L, Sánchez Tamés R, González A (2003) 1-Methylcyclopropene and ethylene as regulators of in vitro organogenesis in kiwi explants. Plant Growth Regul 40:59–64
Ashraf R, Ali TA (2007) Effect of heavy metals on soil microbial community and mung beans seed germination. Pak J Bot 39(2):629–636
Auboiron E, Carron C, Michaux-Ferrière N (1990) Influence of atmospheric gases, particularly ethylene, on somatic embryogenesis of Hevea brasiliensis. Plant Cell Tissue Organ Cult 21:31–37
Biddington NL (1992) The influence of ethylene in plant tissue culture. Plant Growth Regul 11:173–187
Brar MS, Moore MJ, Al-Khayri JM, Morelock TE, Anderson EJ (1999) Ethylene inhibitors promote in vitro regeneration of cowpea (Vigna unguiculata L.) In Vitro Cell Dev Biol Plant 35:222–225
Buddendorf-Joosten JMC, Woltering EJ (1994) Components of the gaseous environment and their effects on plant growth and development in vitro. Plant Growth Regul 15:1–16
Chae SC, Park SU (2012) Improved shoot organogenesis of Echinacea angustifolia DC treated with ethylene inhibitors. Life Sci J 9(4):1725–1728
Chae SC, Kim HH, Park SU (2012) Ethylene inhibitors enhance shoot organogenesis of gloxinia (Sinningia speciosa). Sci World J 2012:859381. doi:10.1100/2012/859381
Chi GL, Pua EC, Goh CJ (1991) Role of ethylene on de novo shoot regeneration from cotyledonary explants of Brassica campestris ssp. pekinensis (Lour) Olsson in vitro. Plant Physiol 96:178–183
Dias LCC, Ribeiro DM, Santa-Catarina C, Barros RS, Floh EIS, Otoni WC (2010) Ethylene and polyamine interactions in morphogenesis of Passiflora cincinnata: effects of ethylene biosynthesis and action modulators, as well as ethylene scavengers. Plant Growth Regul 62:9–19
Dimasi-Theriou K, Economou AS (1995) Ethylene enhances shoot formation in cultures of the peach rootstock GF-677 (Prunus persica x P. amygdalus). Plant Cell Rep 15:87–90
Dimasi-Theriou K, Economou AS, Sfakiotakis EM (1993) Promotion of petunia (Petunia hybrid L.) regeneration in vitro by ethylene. Plant Cell Tissue Organ Cult 32:219–225
Driver JA, Kuniyuki AH (1984) In vitro propagation of Paradox walnut rootstock. Hortscience 19:507–550
Druart PH (1988) Regulation of axillary branching in micropropagation of woody fruit species. Acta Hortic 227:369–380
Enviromental Protection Agengy (1995) Reregistration eligibility decision (RED): ethephon. Prevention, pesticides and toxic substances (7508W), EPA 738-R-95-003. http://www.epa.gov. Accessed 27 April 2016
Fehér A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult 74:201–228
Gaspar T, Kevers C, Penel C, Greppin H, Reid DM, Thorpe TA (1996) Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cell Dev Biol Plant 32:272–289
George EF (2007) Plant propagation by tissue culture. Part 1: the technology. Exegetics Limited, Edington
Goh CJ, Ng SK, Lakshmanan P, Loh CS (1997) The role of ethylene on direct shoot bud regeneration from mangosteen (Garcinia mangostana L.) leaves cultured in vitro. Plant Sci 124:193–202
González A, Rodríguez R, Sánchez Tames R (1991) Ethylene and in vitro rooting of hazelnut (Corylus avellana) cotyledons. Physiol Plant 81:227–233
González A, Arigita L, Majada J, Sánchez Tamés R (1997) Ethylene involvement in in vitro organogenesis and plant growth of Populus tremula L. Plant Growth Regul 22:1–6
Goudey JS, Saini HS, Spencer MS (1987) Uptake and fate of ethephon ([2-chloroethyl] phosphonic acid) in dormant weed seeds. Plant Physiol 85:155–157
Hansen H, Grossmann K (2000) Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol 124(3):1437–1448
Harpaz-Saad S, Yoon GM, Mattoo AK, Kieber JJ (2012) The formation of ACC and competition between polyamines and ethylene for SAM. In: McManus MT (ed) Annual plant reviews volume 44: the plant hormone ethylene. Wiley-Blackwell, Oxford
Huxter TJ, Thorpe TA, Reid DM (1981) Shoot initiation in light- and dark grown tobacco callus: the role of ethylene. Physiol Plant 53:319–326
Imaseki H (1999) Control of ethylene synthesis and metabolism. In: Hooykaas PJJ, Hall MA, Ibbenga KR (eds) Biochemistry and molecular biology of plant hormones. Elsevier, Amsterdam, pp 209–245
Ishizaki T, Komai F, Masuda K, Megumi C (2000) Exogenous ethylene enhances formation of embryogenic callus and inhibits embryogenesis in cultures of explants of spinach roots. J Am Soc Hortic Sci 125:21–24
Kochba J, Spiegel-Roy P, Neumann H, Saad S (1978) Stimulation of embryogenesis in citrus ovular callus by ABA, ethephon, CCC and Alar and its suppression by GA. Z Pflanzenphysiol 89:427–432
Kumar PP, Lakshmanan P, Thorpe TA (1998) Regulation of morphogenesis in plant tissue culture by ethylene. In Vitro Cell Dev Biol Plant 34(2):94–103
Kumar V, Ramakrishna A, Ravishankar GA (2007) Influence of different ethylene inhibitors on somatic embryogenesis and secondary embryogenesis from Coffea canephora P ex Fr. In Vitro Cell Dev Biol Plant 43:602–607
Kumar V, Parvatam G, Ravishankar GA (2009) AgNO3: a potential regulator of ethylene activity and plant growth modulator. Electron J Biotechnol 12(2):8–9
Lau OL, Yang SF (1976) Stimulation of ethylene production in the mung bean hypocotyls by cupric ion, calcium ion, and kinetin. Plant Physiol 57:88–92
Lu J, Vahala J, Pappinen A (2011) Involvement of ethylene in somatic embryogenesis in Scots pine (Pinus sylvestris L.) Plant Cell Tissue Organ Cult 107:25–33
Marutani-Hert M, Evens TJ, McCollum GT, Niedz RP (2011) Bud emergence and shoot growth from mature citrus nodal stem segments. Plant Cell Tissue Organ Cult 106:81–91
Mohiuddin AKM, Chowdhury MKU, Abdullah ZC, Napis S (1997) Influence of silver nitrate (ethylene inhibitor) on cucumber in vitro shoot regeneration. Plant Cell Tissue Organ Cult 51:75–78
Moshkov IE, Novikova GV, Hall MA, George EF (2008) Plant growth regulators III: ethylene. In: George EF, Hall MA, Klerk GJD (eds) Plant propagation by tissue culture, vol 1. Springer, Berlin, pp 239–248
Nair PMG, Chung IM (2015) Physiological and molecular level studies of the toxicity of silver nanoparticles in germinating seedlings of mung bean (Vigna radiata L.). Acta Physiol Plant 37(1):1719. doi:10.1007/s11738-014-1719-1
Navarro-García N, Morte A, Pérez-Tornero O (2016) In vitro adventitious organogenesis and histological characterization from mature nodal explants of Citrus limon. In Vitro Cell Dev Biol Plant 52(2):161–173
Nissen P (1994) Stimulation of somatic embryogenesis in carrot by ethylene: effects of modulators of ethylene biosynthesis and action. Physiol Plant 92:397–403
Oliveira MLP, Costa MGC, Silva CV, Otoni WC (2010) Growth regulators, culture media and antibiotics in the in vitro shoot regeneration from mature tissue of citrus cultivars. Pesqui Agropecu Bras 45:654–660
Pérez-Tornero O, Tallón CI, Porras I (2010) An efficient protocol for micropropagation of lemon (Citrus limon) from mature nodal segments. Plant Cell Tissue Organ Cult 100:263–227
Petri C, Scorza R (2010) Factors affecting adventitious regeneration from in vitro leaf explants of ‘Improved French’ plum, the most important dried plum cultivar in the USA. Ann Appl Biol 156:79–89
Ptak A, Tahchy A, Wyzgolik G, Henry M, Laurain-Mattar D (2010) Effects of ethylene on somatic embryogenesis and galanthamine content in Leucojum aestivum L. cultures. Plant Cell Tissue Organ Cult 102:61–67
Puschmann R, Ke D, Romani R (1985) Ethylene production by suspension-cultured pear fruit cells as related to their senescence. Plant Physiol 79:973–976
Saly S, Joseph C, Corbineau F, Lelu MA, Côme D (2002) Induction of secondary somatic embryogenesis in hybrid larch (Larix × leptoeuropaea) as related to ethylene. Plant Growth Regul 37:287–294
Santana-Buzzy N, Canto-Flick A, Iglesias-Andreu LG, Montalvo-Peniche MC, López-Puc G, Barahona-Pérez F (2006) Improvement of in vitro culturing of Habanero pepper by inhibition of ethylene effects. Hortscience 41:405–409
Sgamma T, Thomas B, Muleo R (2015) Ethylene inhibitor silver nitrate enhances regeneration and genetic transformation of Prunus avium (L.) cv Stella. Plant Cell Tissue Organ Cult 120:79–88
Sisler EC, Sereck M, Dupille E (1996) Comparison of cyclopropene, 1-methylcyclopropene, and 3,3-dimethylcyclopropene as ethylene antagonists in plants. Plant Growth Regul 17:1–6
Songstad DD, Duncan DR, Widholm JM (1988) Effect of 1-aminocyclopropane-1-carboxylic acid, silver nitrate, and norbornadiene on plant regeneration from maize callus cultures. Plant Cell Rep 7:262–265
Sridevi V, Giridhar P (2013) In vitro shoot growth, direct organogenesis and somatic embryogenesis promoted by silver nitrate in Coffea dewevrei. J Plant Biochem Biotechnol 23(1):112–118
Tadeo FR, Tudela D, Primo-Millo E (1995) 1-Aminocyclopropane-1-carboxylic acid-induced ethylene stimulates callus formation by cell enlargement in the cambial region of internodal explants of Citrus. Plant Sci 110:113–119
Tallón CI, Porras I, Pérez-Tornero O (2013) High efficiency in vitro organogenesis from mature tissue explants of Citrus macrophylla and C. aurantium. In Vitro Cell Dev Biol Plant 49:145–155
Trujillo-Moya C, Gisbert C (2012) The influence of ethylene and ethylene modulators on shoot organogenesis in tomato. Plant Cell Tissue Organ Cult 111:41–48
Wang KLC, Li H, Ecker JR (2002) Ethylene biosynthesis and signalling networks. Plant Cell. doi:10.1105/tpc.001768
Wang H, Alburquerque N, Burgos L, Petri C (2011) Adventitious shoot regeneration from hypocotyl slices of mature apricot (Prunus armeniaca L.) seeds: a feasible alternative for apricot genetic engineering. Sci Hortic 128:457–464
Würschum T, Tucker MR, Maurer HP, Leiser WL (2015) Ethylene inhibitors improve efficiency of microspore embryogenesis in hexaploid triticale. Plant Cell Tissue Organ Cult 122:751–757
Yasmin S, Mensuali-Sodi A, Perata P, Pucciariello C (2013) Ethylene influences in vitro regeneration frequency in the FR13A rice harbouring the SUB1A gene. Plant Growth Regul 72(1):97–103
Zhang W, Hu W, Wen CK (2010) Ethylene and its application to physiological experiments. Plant Signal Behav 5(4):453–457
Acknowledgments
The authors thank Mr. Fernando Córdoba for technical assistance in the laboratory. This work was supported by the European Regional Development Fund.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Navarro-García, N., Martínez-Romero, D. & Pérez-Tornero, O. Assessment of the impact of ethylene and ethylene modulators in Citrus limon organogenesis. Plant Cell Tiss Organ Cult 127, 405–415 (2016). https://doi.org/10.1007/s11240-016-1062-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-1062-x