Abstract
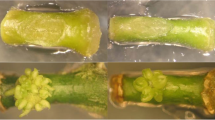
The influence of ethylene on shoot and root formation from petunia leaf explants was studied in cultures in test tubes placed in 51 glass jars. Reduction of the endogenously produced ethylene by inclusion of ethysorb (KMnO4), an ethylene absorbent, caused a decrease of the number of shoots. On the other hand, supplementing the cultures with ethylene (0.01–10 ppm) caused a marked increase of the number of shoots without, however, any effect on the length and fresh weight. Ethylene treatments (1 ppm) were found to be most effective when they were applied in the second week of culturing of petunia explants. Addition of Co++ to the medium resulted in a reduction of the endogenously produced ethylene and concomitantly reduced shoot formation. Similarly, inclusion of Ag+, an inhibitor of ethylene action, resulted in poor shoot formation. Ethylene also appeared to play a role on rooting of petunia microshoots in vitro in an auxin-free medium. Ethylene at a concentration of 10 ppm induced adventitious root formation considerably, whereas at low levels (0.01–1 ppm) it had no influence on rooting.
Similar content being viewed by others
References
Beyer EMJr (1976) A potent inhibitor of ethylene action in plants. Plant Physiol. 58: 268–271.
Biondi S, Inglesias I, Diaz T & Bagni N (1990) Polyamines and ethylene during rooting of wild cherry shoot cultures. In: Abstracts VIIth Intl. Congress on Plant Tissue and Cell Culture, Amsterdam, June 24–29, 1990: 277.
Coleman WK, Huxter TJ, Reid DM & Thorpe TA (1980) Ethylene as endogenous inhibitor of root regeneration in tomato leaf discs cultured in vitro. Physiol. Plant. 48: 519–525.
DeProft MP, Maene LJ & Debergh PC (1985) Carbon dioxide and ethylene evolution in the culture atmosphere of Magnolia cultured in vitro. Physiol. Plant. 65: 375–379.
Economou AS & Read PE (1982) Effect of NAA on shoot production in vitro from BA-treated petunia leaf explants. J. Amer. Soc. Hort. Sci. 107: 504–506.
Gavinlertvatana P, Read PE, Wilkins HF & Heins R (1982) Ethylene levels in flask atmospheres of Dahlia pinata Cav. leaf segments and callus cultured in vitro. J. Amer. Soc. Hort. Sci. 107: 3–6.
Geneve RL, Hackett WP & Swanson BT (1990) Ethylene production in deblated petioles from the juvenile and mature phases of English ivy in relation to adventitious root initiation. J. Amer. Soc. Hort. Sci. 115: 123–127.
Horner M, McComb JA, McComb AJ & Street HE (1977) Ethylene production and plantlet formation by Nicotiana anthers cultured in the presence and absence of charcoal. J. Exp. Bot. 28: 1365–1372.
Huxter TJ, Reid DM & Thorpe TA (1979) Ethylene production by tobacco (Nicotiana tabacum) callus. Physiol. Plant. 46: 374–380.
Koves E & Szabo M (1987) Ethylene production in habituated and auxin-requiring tobacco callus cultures. Does ethylene play a role in the habituation? Physiol. Plant. 69: 351–355.
Krishnamoorthy HN (1970) Promotion of rooting in mung bean hypocotyl cuttings with ethrel an ethylene releasing compound. Plant Cell Physiol. 11: 979–982.
Krishnamoorthy HN (1972) The effects of ethrel, auxin and maleic hydrazide on the rooting of mung bean hypocotyl cuttings. Z. Pflanzenphysiol. 66: 273–274.
Kumar PP, Reid DM & Thorpe TA (1987) The role of ethylene and carbon dioxide in differentiation of shoot buds in excised cotyledons of Pinus radiata in vitro. Physiol. Plant. 69: 244–252.
LaRue TAG & Gamborg OL (1971) Ethylene production by plant cell cultures: Variations in production growing cycle and in different plant species. Plant Physiol. 48: 394–398.
Lau O & Yang SF (1976) Inhibition of ethylene production by cobaltous ion. Plant Physiol. 58: 114–117.
Linkins AE, Lewis LN & Palmer RL (1973) Hormonally induced changes in stem and petiole anatomy and cellulase enzyme patterns in Phaseolus vulgaris. Plant Physiol. 52: 554–560.
Mensuali-Sodi A, Tognoni F & Serra G (1990) Effects of ethylene on in vitro culture of tomato cotyledonary explants. In: Abstracts VIIth Intl. Congress on Plant Tissue and Cell Culture, Amsterdam, June 24–29, 1990: 282.
Moncousin C, Favre J-M & Gaspar T (1989) Early changes in auxin and ethylene production in vine cuttings before adventitious rooting. Plant Cell Tiss. Org. Cult. 19: 243–256.
Mudge K (1989) Effect of ethylene on rooting. In: Davis TD, Haissig BE & Sankhla N (Eds) Adventitious Root Formation in Cuttings (pp 150–161). Dioscorides Press, Portland.
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497.
Panizza M, Mensuali-Sodi A & Tognoni F (1988) In vitro propagation of lavandin: Ethylene production during plant development. Acta Hortic. 227: 334–339.
Pua EC, Chi GL & Barfield DG (1990) Effect of ethylene inhibitors on plant regeneration from seedling explants and somatic embryos of recalcitrant genotypes in Cruciferae. In: Abstracts VIIth Intl. Congress on Plant Tissue and Cell Culture, Amsterdam, June 24–29, 1990: 284.
Rao PS, Handro W & Harada H (1973) Hormonal control of differentiation of shoots, roots and embryos in leaf and stem cultures of Petunia inflata and Petunia hybrida Physiol. Plant. 28: 458–463.
Robbins JA, Kay SJ & Dirr MA (1983) Enhanced rooting of wounded mung bean cuttings by wounding and ethephon. J. Amer. Soc. Hort. Sci. 108: 325–329.
Roy BN, Basu R & Bose TK (1972) Interaction of auxins with growth-retarding,-inhibiting, and ethylene producing chemicals in rooting of cuttings. Plant Cell Physiol. 12: 1123–1127.
Sauerbrey E, Grossmann K & Jung J (1988) Ethylene production by sunflower cell suspensions. Plant Physiol. 87: 510–513.
Thomas DS & Murashige T (1979) Volatile emissions of plant tissue cultures. In Vitro 15: 654–658.
VanAartrijk J, Blom-Barnhoorn GJ & Bruinsma J (1985) Adventitious bud formation from bulb-scale explants of Lilium speciosum Thunb in vitro: Effects of aminoethoxyvinyl-glycine, 1-aminocyclopropane-1-carboxylic acid, and ethylene. J. Plant Physiol. 117: 401–410.
Williams J, Pink DAC & Biddington NL (1990) Effect of silver nitrate on long-term culture and regeneration of callus from Brassica oleracea var. ‘gemmifera’. Plant Cell Tiss. Org. Cult. 21: 61–66.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dimasi-Theriou, K., Economou, A.S. & Sfakiotakis, E.M. Promotion of petunia (Petunia hybrida L.) regeneration in vitro by ethylene. Plant Cell Tiss Organ Cult 32, 219–225 (1993). https://doi.org/10.1007/BF00029846
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00029846