Abstract
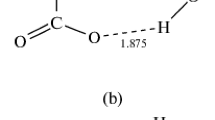
The theoretical study of the complexes produced from the interactions between hypohalous acids (HOX; X = F, Cl, Br, and I) and acetonitrile (AN) was carried out at the MP2/aug-cc-pVTZ level of theory. Three different conformations were produced, namely, A, B, and C, in which conformations B and C were stabilized by only the N···H HB and the N···X XB interactions, respectively. In contrast, conformation A was stabilized by two O···C TB and H···X HB interactions through a cyclic structure. The characteristics of product complexes were analyzed by various methods such as molecular electrostatic potential (MEP), spectroscopy, binding energy (ΔE0), quantum theory of atoms in molecules (QTAIM), natural bond orbital (NBO), energy decomposition analysis (EDA), electron density differences (EDD), and non-covalent interaction (NCI) index.







Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Scheiner S (2013) The Pnicogen bond: its relation to hydrogen, halogen, and other noncovalent bonds. Acc Chem Res 46:280–288. https://doi.org/10.1021/ar3001316
Müller-Dethlefs K, Hobza P (2000) Noncovalent interactions: a challenge for experiment and theory. Chem Rev 100:143–168. https://doi.org/10.1021/cr9900331
Alkorta I, Blanco F, Solimannejad M, Elguero J (2008) Competition of hydrogen bonds and halogen bonds in complexes of hypohalous acids with nitrogenated bases. J Phys Chem A 112. https://doi.org/10.1021/jp806101t
Dannenberg JJ (1998) An Introduction to hydrogen bonding By George A. Jeffrey (University of Pittsburgh). Oxford University Press: New York and Oxford. 1997. ix + 303 pp. $60.00. ISBN 0–19–509549–9. J Am Chem Soc 120:5604–5604. https://doi.org/10.1021/ja9756331
Jeffrey GA, Saenger W (1991) Hydrogen bonding in biological structures. Springer, Berlin Heidelberg, Berlin, Heidelberg
Scheiner S (2013) Detailed comparison of the pnicogen bond with chalcogen, halogen, and hydrogen bonds. Int J Quantum Chem 113:1609–1620. https://doi.org/10.1002/qua.24357
Czyznikowska Z (2009) On the importance of electrostatics in stabilization of stacked guanine-adenine complexes appearing in B-DNA crystals. Journal of Molecular Structure: Theochem 895. https://doi.org/10.1016/j.theochem.2008.10.040
Moradkhani M, Naghipour A, Abbasi Tyula Y (2023) Competition and interplay between Hydrogen, Tetrel, and Halogen bonds from interactions of COCl2 and HX (X = F, Cl, Br, and I). Comput Theor Chem 1223:114099. https://doi.org/10.1016/j.comptc.2023.114099
Desiraju GR, Steiner T (2001) The weak hydrogen bond: in structural chemistry and biology. Int Union Crystal
Clark T, Hennemann M, Murray JS, Politzer P (2007) Halogen bonding: the σ-hole. J Mol Model 13:291–296. https://doi.org/10.1007/s00894-006-0130-2
Auffinger P, Hays FA, Westhof E, Ho PS (2004) Halogen bonds in biological molecules. Proc Natl Acad Sci USA 101. https://doi.org/10.1073/pnas.0407607101
Murray JS, Lane P, Politzer P (2007) A predicted new type of directional noncovalent interaction. In: Int J Quantum Chem
Murray JS, Concha MC, Lane P, Hobza P, Politzer P (2008) Blue shifts vs red shifts in σ-hole bonding. In: J Mol Model
Politzer P, Murray JS, Clark T (2013) Halogen bonding and other σ-hole interactions: a perspective
Politzer P, Lane P, Concha MC, Ma Y, Murray JS (2007) An overview of halogen bonding. J Mol Model 13:305–311. https://doi.org/10.1007/s00894-006-0154-7
Murray JS, Lane P, Clark T, Politzer P (2007) σ-hole bonding: molecules containing group VI atoms. J Mol Model 13:1033–1038. https://doi.org/10.1007/s00894-007-0225-4
Murray JS, Lane P, Politzer P (2008) Simultaneous σ-hole and hydrogen bonding by sulfur- and selenium-containing heterocycles. Int J Quantum Chem 108:2770–2781. https://doi.org/10.1002/qua.21753
Desiraju GR, Ho PS, Kloo L, Legon AC, Marquardt R, Metrangolo P, Politzer P, Resnati G, Rissanen K (2013) Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl Chem 85:1711–1713. https://doi.org/10.1351/PAC-REC-12-05-10
Bouchmella K, Boury B, Dutremez SG, van der Lee A (2007) Molecular assemblies from imidazolyl-containing haloalkenes and haloalkynes: competition between halogen and hydrogen bonding. Chem Eur J 13:6130–6138. https://doi.org/10.1002/chem.200601508
Bauzá A, Ramis R, Frontera A (2014) Computational study of anion recognition based on tetrel and hydrogen bonding interaction by calix[4]pyrrole derivatives. Comput Theor Chem 1038:67–70. https://doi.org/10.1016/j.comptc.2014.04.010
Grabowski SJ (2014) Tetrel bond-σ-hole bond as a preliminary stage of the SN2 reaction. Phys Chem Chem Phys 16. https://doi.org/10.1039/c3cp53369g
Robertazzi A, Platts JA, Gamez P (2014) Anion{dot operator}{dot operator}{dot operator}Si interactions in an inverse sandwich complex: a computational study. Chemphyschem 15. https://doi.org/10.1002/cphc.201400018
Bauzá A, Mooibroek TJ, Frontera A (2013) Tetrel-bonding interaction: rediscovered supramolecular force? Angew Chemie - Int Ed 52. https://doi.org/10.1002/anie.201306501
Garcia RR, Solomon S (1994) A new numerical model of the middle atmosphere 2. Ozone and related species. J Geophys Res 99. https://doi.org/10.1029/94jd00725
Molina MJ, Rowland FS (1974) Stratospheric sink for chlorofluoromethanes: chlorine atomc-atalysed destruction of ozone. Nature 249. https://doi.org/10.1038/249810a0
Moradkhani M, Naghipour A, Tyula YA, Abbasi S (2023) Competition of hydrogen, tetrel, and halogen bonds in COCl2-HOX (X = F, Cl, Br, I) complexes. J Mol Graph Model 122:108482. https://doi.org/10.1016/j.jmgm.2023.108482
Zabardasti A, Abbasi Tyula Y, Goudarziafshar H (2017) Theoretical investigation of molecular interactions between sulfur ylide and hypohalous acids (HOX, X═F, Cl, Br, and I). J Sulfur Chem 38:119–133. https://doi.org/10.1080/17415993.2016.1246551
Zabardasti, Abedien, Yunes Abbasi Tyula, and Hamid Goudarziafshar (2017) "Interplay between N··· H, N··· X and π··· X interactions in the complex pairing of pyrazine with hypohalous acids: A NBO and QTAIM (quantum theory of atoms in molecules) analysis." Bull Chem Soc Ethiop 31(2):241–252
Tang Q, Guo Z, Li Q (2014) A quantum chemical study of the structures, stability, and spectroscopy of halogen- and hydrogen-boned complexes between cyanoacetaldehyde and hypochlorous acids. Spectrochim Acta A Mol Biomol Spectrosc 121:157–163. https://doi.org/10.1016/j.saa.2013.10.088
Kakanejadifard A, Japelaghi S, Ghasemian M, Zabardasti A (2015) Theoretical study of molecular interactions of sulfoximine with hypohalous acids HOF, HOCl, and HOBr. Struct Chem 26:23–33. https://doi.org/10.1007/s11224-014-0461-z
Li Q, Xu X, Liu T, Jing B, Li W, Cheng J, Gong B, Sun J (2010) Competition between hydrogen bond and halogen bond in complexes of formaldehyde with hypohalous acids. Phys Chem Chem Phys 12:6837. https://doi.org/10.1039/b926355a
Wolf ME, Zhang B, Turney JM, Schaefer HF (2019) A comparison between hydrogen and halogen bonding: the hypohalous acid–water dimers, HOX⋯H 2O (X = F, Cl, Br). Phys Chem Chem Phys 21:6160–6170. https://doi.org/10.1039/C9CP00422J
Frisch MJ, Trucks GW, Schlegel HB et al (2009) Gaussian 09 (Revision A.02), Gaussian, Inc., Wallingford, CT
Note on an approximation treatment for many-electron systems (1934) Møller, Chr., Plesset, M.S. Phys Rev 46:618–622. https://doi.org/10.1103/PhysRev.46.618
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566. https://doi.org/10.1080/00268977000101561
Bulat FA, Toro-Labbé A, Brinck T, Murray JS, Politzer P (2010) Quantitative analysis of molecular surfaces: areas, volumes, electrostatic potentials and average local ionization energies. J Mol Model 16:1679–1691. https://doi.org/10.1007/s00894-010-0692-x
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926. https://doi.org/10.1021/cr00088a005
Keith TA (2011) “AIMAll (Version 11.08. 23).” TK Gristmill Software, Overland Park, KS, USA
ADF2013, SCM, Theoretical chemistry; Vrije Universiteit: Amsterdam, The Netherlands. Available at https://www.scm.com
Lu T (2014) "Multiwfn." Software manual. Version 3, no. 6
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Humphrey W, Dalke A, Schulten K (1996) VMD: Visual molecular dynamics. J Mol Graph 14:33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Popelier PLA (1998) Characterization of a dihydrogen bond on the basis of the electron density. J Phys Chem A 102. https://doi.org/10.1021/jp9805048
Lipkowski P, Grabowski SJ, Robinson TL, Leszczynski J (2004) Properties of the C-H⋯H dihydrogen bond: an ab initio and topological analysis. J Phys Chem A 108. https://doi.org/10.1021/jp048562i
Lu YX, Zou JW, Wang YH, Jiang YJ, Yu Q (2007) Sen: Ab initio investigation of the complexes between bromobenzene and several electron donors: some insights into the magnitude and nature of halogen bonding interactions. J Phys Chem A 111. https://doi.org/10.1021/jp0740954
Koch U, Popelier PLA (1995) Characterization of C-H-O hydrogen bonds on the basis of the charge density. J Phys Chem 99. https://doi.org/10.1021/j100024a016
Wang C, Mo Y (2019) Classical electrostatic interaction is the origin for blue-shifting halogen bonds. Inorg Chem 58. https://doi.org/10.1021/acs.inorgchem.9b00875
Inscoe B, Rathnayake H, Mo Y (2021) Role of charge transfer in halogen bonding. J Phys Chem A. 125. https://doi.org/10.1021/acs.jpca.1c01412
Khodiev MK, Holikulov UA, ISSAOUI N, Al-Dossary OM, Bousiakoug LG, Lavrik NL (2023) Estimation of electrostatic and covalent contributions to the enthalpy of H-bond formation in H-complexes of 1,2,3-benzotriazole with proton-acceptor molecules by IR spectroscopy and DFT calculations. J King Saud Univ Sci 35. https://doi.org/10.1016/j.jksus.2022.102530
Ahmad G, Rasool N, Qamar MU, Alam MM, Kosar N, Mahmood T, Imran M (2021) Facile synthesis of 4-aryl-N-(5-methyl-1H-pyrazol-3-yl)benzamides via Suzuki Miyaura reaction: antibacterial activity against clinically isolated NDM-1-positive bacteria and their docking studies. Arab J Chem 14. https://doi.org/10.1016/j.arabjc.2021.103270
Khan P, Jamshaid M, Tabassum S, Perveen S, Mahmood T, Ayub K, Yang J, Gilani MA (2021) Exploring the interaction of ionic liquids with Al12N12 and Al12P12 nanocages for better electrode-electrolyte materials in super capacitors. J Mol Liq 344. https://doi.org/10.1016/j.molliq.2021.117828
Bader RFW (1985) Atoms in molecules. Acc Chem Res 18:9–15. https://doi.org/10.1021/ar00109a003
Singh H (2023) A DFT insight into structure, NBO, NCI, QTAIM, vibrational, and NLO properties of cationic amino acid ionic liquid [Pro-H] + BF4−. Struct Chem. https://doi.org/10.1007/s11224-023-02195-z
Author information
Authors and Affiliations
Contributions
Mohammadmehdi Moradkhani: Writing – original draft, Formal analysis, Software, Investigation, Methodology, Conceptualization, Writing – review & editing. Ali Naghipour: Validation, Supervision, Project administration. Yunes Abbasi Tyula: Writing – original draft, Investigation, Formal analysis, Methodology, Writing – review & editing.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moradkhani, M., Naghipour, A. & Tyula, Y.A. Investigation of structural, spectral, and electronic properties of complexes resulting from the interaction of acetonitrile and hypohalous acids. Struct Chem (2023). https://doi.org/10.1007/s11224-023-02243-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11224-023-02243-8