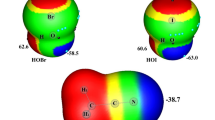
Abstract
The most stable structures of 32 complexes containing oxyanions with HOH···A− are optimized according to density functional theory M06-2X with basis set 6-311++G(d,p). Thermodynamic characteristics of the formation of complexes are calculated with allowance for the basic superposition error (BSSE), and correlations with the enthalpy of formation of ion-molecular hydrogen bonds (ΔHIog) (Johansen’s formula) are found. The contribution from enthalpy ΔHIog to the enthalpy of formation of HOH···A− complexes changes in the range of 12 to 54%. Molecular graphs of complexes are calculated according to QTAIM and the densities of the full energy of electrons at critical point (3, −1) of O–H···O bonds are analyzed. As the values of ΔHIog grow in the range of 28.0 to 58.4 kJ/mol, the contribution from the covalent constituent to the formation of O–H···O bonds increases as a result of stronger polarization interaction between anions and water molecules.



Similar content being viewed by others
REFERENCES
K. A. Peterson, B. C. Shepler, D. Figgen, and H. Stoll, J. Phys. Chem. A 110, 13877 (2006).
K. H. Goh, T. T. Lim, and Z. L. Dong, Water Res. 42, 1343 (2008). doi 10.1016/j.watres.2007.10.043
O. N. Gruba and A. G. Ryabukhin, in Proceedings of the 19th International Conference on Chemical Thermodynamics in Russia RCCT-2013, Moscow, p. 118.
G. P. Mikhailov and V. V. Lazarev, Russ. J. Phys. Chem. A 90, 1367 (2016).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, V. F. Robb, J. R. Cheeseman, J. A. Montgomery, T. Vreven, Jr., K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, et al., Gaussian 03 (Gaussian Inc., 2003).
A. V. Iogansen, Spectrochim. Acta, Part A 55, 1585 (1999). doi 10.1016/S1386-1425(98)00348-5
R. F. W. Bader, Atoms in Molecules. A Quantum Theory (Clarendon, Oxford, 1990).
T. A. Keith, AIMAll, Version 10.05.04. http://aim. tkgristmill.com.5
I. Rozas, I. Alkorta, and J. Elguero, J. Am. Chem. Soc. 122, 11154 (2000).
S. J. Grabowski, Chem. Rev. 111, 2597 (2011). doi 10.12691/ajmo-5-1-3
ACKNOWLEDGMENTS
This work was supported by the RF Ministry of Education and Science, project no. 16.1969.2017/4.6.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Bannov
Rights and permissions
About this article
Cite this article
Mikhailov, G.P. Thermodynamics of the Formation of Complexes and the Hydrogen Bonds between Oxyanions and Water. Russ. J. Phys. Chem. 92, 1861–1864 (2018). https://doi.org/10.1134/S0036024418100199
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024418100199