Abstract
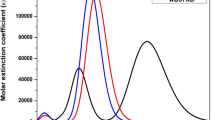
In this work, the newly designed phenothiazine-based organic dye (PT-BTBA, PT-EBTBA, and PT-EBTEBA) derivatives were screened and investigated for dye-sensitized solar cell (DSSC) application. The literature dye of SB covers the electron-donor (D) in phenothiazine and cyanoacrylic acid in electron-acceptor (A) based on D-A structure. In order to improve the π-conjugation and acceptor group effects on the SB dye were theoretically investigated. The effect of D-π-A designed dyes on the optical absorption spectra and photovoltaic (PV) parameters was implemented by the density functional theory (DFT) and time-dependent DFT (TD-DFT) calculations. Also, the hybrid functionals were initially evaluated to establish an accurate methodology for calculating the first-singlet absorption peak of SB dye. Consequently, TD-CAM-B3LYP functional and 6-311++G(d,p) theory were well matched with the literature data. According to this result, phenothiazine-4-((7-ethynylbenzo[c][1,2,5]thiadiazol-4-yl)ethynyl)benzoic acid (PT-EBTEBA) dye has the strong group for more red-shifted and successful electron injected into the conduction band edge of TiO2 surface. It is expected to provide some theoretical guidance on designing photosensitive with new metal-free organic dyes for use in DSSCs yielding highly efficient performance.






Similar content being viewed by others
References
O’Regan B, Gratzel M (1991) A low-cost, high-efficiency solar cell based on dye sensitized colloidal TiO2 films. Nature 353:737–740
Prima EC, Yuliarto B, Suendo V (2014) Improving photochemical properties of Ipomea pescaprae, Imperata cylindrica (L.) Beauv, and Paspalum conjugatum Berg as photosensitizers for dye sensitized solar cells. J Mater Sci Mater Electron 25:4603–4611
Green MA, Emery K, Hishikawa Y, Warta W (2010) Solar cell efficiency tables (version 36). Prog Photovolt Res Appl 18:346
Mishra A, Fischer MK, Bäuerle P (2009) Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew Chem Int Ed 48:2474–2499
Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H (2010) Dye-sensitized solar cells. Chem Rev 110:6595–6663
Yen YS, Chou HH, Chen YC, Hsu CY, Lin JT (2012) Recent developments in molecule-based organic materials for dye-sensitized solar cells. J Mater Chem 22:8734–8747
Wu Y, Zhu W (2013) Organic sensitizers from D-π-A to D-A-π-A: effect of the internal electron-withdrawing units on molecular absorption, energy levels and photovoltaic performances. Chem Soc Rev 42:2039–2058
Arunkumar A, Prakasam M, Anbarasan PM (2017) Influence of donor substitution at D-π-A architecture in efficient sensitizers for dye-sensitized solar cells: first principle study. Bull Mater Sci 40:1389–1396
Kitamura T, Ikeda M, Shigaki K, Inoue T, Anderson NA, Ai X, Lian T, Yanagida S (2004) Phenyl-conjugated oligoene sensitizers for TiO2 solar cells. Chem Mater 16:1806–1812
Sayama K, Hara K, Mori N, Satsuki M, Suga S, Tsukagoshi S, Abe Y, Sugihara H, Arakawa H (2000) Photosensitization of a porous TiO2 electrode with merocyanine dyes containing a carboxyl group and a long alkyl chain. Chem Commun 13:1173–1174
Ehret A, Stuhl L, Spitler MT (2001) Spectral sensitization of TiO2 nanocrystalline electrodes with aggregated cyanine dyes. J Phys Chem B 105:9960–9965
Wang ZS, Li FY, Huang CH (2000) Highly efficient sensitization of nanocrystalline TiO2 films with styryl benzothiazolium propylsulfonate. Chem Commun 20:2063–2064
Liang M, Xu W, Cai F, Chen P, Peng B, Chen J, Li Z (2007) New triphenylamine-based organic dyes for efficient dye-sensitized solar cells. J Phys Chem C 111:4465–4472
Velusamy M, Justin Thomas KR, Lin JT, Hsu YC, Ho KC (2005) Organic dyes incorporating low-band-gap chromophores for dye-sensitized solar cells. Org Lett 7:1899–1902
Tsai MS, Hsu YC, Lin JT, Chen HC, Hsu CP (2007) Organic dyes containing 1 H-phenanthro [9, 10-d] imidazole conjugation for solar cells. J Phys Chem C 111:18785–18793
Justin Thomas KR, Hsu YC, Lin JT, Lee KM, Ho KC, Lai CH, Cheng YM, Chou PT (2008) 2,3-Disubstituted thiophene-based organic dyes for solar cells. Chem Mater 20:1830–1840
Hara K, Sato T, Katoh R, Furube A, Yoshihara T, Murai M, Kurashige M, Ito S, Shinpo A, Suga S, Arakawa H (2005) Novel conjugated organic dyes for efficient dye-sensitized solar cells. Adv Funct Mater 15:246–252
Arunkumar A, Shanavas S, Anbarasan PM (2018) First-principles study of efficient phenothiazine-based D-π-A organic sensitizers with various spacers for DSSCs. J Comput Electron 17:1410–1420
Chen R, Yang X, Tian H, Sun L (2007) Tetrahydroquinoline dyes with different spacers for organic dye-sensitized solar cells. J Photochem Photobiol A Chem 189:295–300
Wang ZS, Hara K, Dan-oh Y, Kasada C, Shinpo A, Suga S, Arakawa H, Sugihara H (2005) Photophysical and (photo) electrochemical properties of a coumarin dye. J Phys Chem B 109:3907–3914
Hara K, Sato T, Katoh R, Furube A, Ohga Y, Shinpo A, Suga S, Sayama K, Sugihara H, Arakawa H (2003) Molecular design of coumarin dyes for efficient dye-sensitized solar cells. J Phys Chem B 107:597–606
Hara K, Kurashige M, Dan-oh Y, Kasada C, Shinpo A, Suga S, Sayama K, Arakawa H (2003) Design of new coumarin dyes having thiophene moieties for highly efficient organic-dye-sensitized solar cells. New J Chem 27:783–785
Hara H, Sayama K, Ohga Y, Shinpo A, Suga S, Arakawa H (2001) A coumarin-derivative dye sensitized nanocrystalline TiO2 solar cell having a high solar-energy conversion efficiency up to 5.6%. Chem Commun 6:569–570
Ito S, Zakeeruddin SM, Humphry-Baker R, Liska P, Charvet R, Comte P, Nazeeruddin MK, Péchy P, Takata M, Miura H, Uchida S (2006) High-efficiency organic-dye-sensitized solar cells controlled by nanocrystalline-TiO2 electrode thickness. Adv Mater 18:1202–1205
Schmidt-Mende L, Bach U, Humphry-Baker R, Horiuchi T, Miura H, Ito S, Uchida S, Grätzel M (2005) Organic dye for highly efficient solid-state dye-sensitized solar cells. Adv Mater 17:813–815
Horiuchi T, Miura H, Uchida S (2004) Highly efficient metal-free organic dyes for dye-sensitized solar cells. J Photochem Photobiol A 164:29–32
Horiuchi T, Miura H, Sumioka K, Uchida S (2004) High efficiency of dye-sensitized solar cells based on metal-free indoline dyes. J Am Chem Soc 126:12218–12219
Kim D, Lee JK, Kang SO, Ko J (2007) Molecular engineering of organic dyes containing N-aryl carbazole moiety for solar cell. Tetrahedron 63:1913–1922
Kar S, Roy JK, Leszczynski J (2017) In silico designing of power conversion efficient organic lead dyes for solar cells using todays innovative approaches to assure renewable energy for future. NPJ Comput Mater 3:22
Kim S, Lee JK, Kang SO, Ko J, Yum JH, Fantacci S, De Angelis F, Di Censo D, Nazeeruddin MK, Grätzel M (2006) Molecular engineering of organic sensitizers for solar cell applications. J Am Chem Soc 128:16701–16707
Liu P, Fu JJ, Guo MS, Zuo X, Liao Y (2013) Effect of the chemical modifications of thiophene-based N3 dyes on the performance of dye-sensitized solar cells: a density functional theory study. Comput Theor Chem 1015:8–14
Arunkumar A, Deepana M, Shanavas S, Acevedo R, Anbarasan PM (2019) Computational investigation on series of metal-free sensitizers in tetrahydroquinoline with different π-spacer groups for DSSCs. ChemistrySelect 4:4097–4104
Arunkumar A, Shanavas S, Acevedo R, Anbarasan PM (2020) Computational analysis on D-π-A based perylene organic efficient sensitizer in dye-sensitized solar cells. Opt Quant Electron 52:1–3
Arunkumar A, Shanavas S, Acevedo R, Anbarasan PM (2020) Quantum chemical investigation of modified coumarin-based organic efficient sensitizers for optoelectronic applications. Eur Phys J D 74:1–8
Arunkumar A, Shanavas S, Acevedo R, Anbarasan PM (2020) Acceptor tuning effect on TPA-based organic efficient sensitizers for optoelectronic applications-quantum chemical investigation. Struct Chem 31:1029–1042
Feng J, Jiao Y, Ma W, Nazeeruddin MK, Grätzel M, Meng S (2013) First principles design of dye molecules with ullazine donor for dye sensitized solar cells. J Phys Chem C 117:3772–3778
Cao D, Peng J, Hong Y, Fang X, Wang L, Meier H (2011) Enhanced performance of the dye-sensitized solar cells with phenothiazine-based dyes containing double D-A branches. Org Lett 13:1610–1613
Yang L, Yao Z, Liu J, Wang J, Wang P (2016) A systematic study on the influence of electron-acceptors in phenanthrocarbazole dye-sensitized solar cells. ACS Appl Mater Interfaces 8:9839–9848
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627
Arunkumar A, Anbarasan PM (2018) Highly efficient organic indolocarbazole dye in different acceptor units for optoelectronic applications - a first principle study. Struct Chem 29:967–976
Meng S, Kaxiras E, Nazeeruddin MK, Grätzel M (2011) Design of dye acceptors for photovoltaics from first-principles calculations. J Phys Chem C 115:9276–9282
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57
Perdew JP, Burke K, Wang Y (1996) Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys Rev B 54:16533
Lin YS, Li GD, Mao SP, Chai JD (2013) Long-range corrected hybrid density functionals with improved dispersion corrections. J Chem Theor Comput 9:263–272
Ordon P, Tachibana A (2005) Investigation of the role of the C-PCM solvent effect in reactivity indices. J Chem Sci 117:583–589
Arunkumar A, Anbarasan PM (2019) Optoelectronic properties of a simple metal-free organic sensitizer with different spacer groups: quantum chemical assessments. J Electron Mater 48:1522–1530
O’boyle NM, Tenderholt AL, Langner KM (2008) Cclib: a library for package-independent computational chemistry algorithms. J Comput Chem 29:839–845
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RJ, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian Inc., Wallingford, CT, USA
Jungsuttiwong S, Tarsang R, Sudyoadsuk T, Promarak V, Khongpracha P, Namuangruk S (2013) Theoretical study on novel double donor-based dyes used in high efficient dye-sensitized solar cells: the application of TDDFT study to the electron injection process. Org Electron 14:711–722
Asbury JB, Wang YQ, Hao E, Ghosh HN, Lian T (2001) Evidences of hot excited state electron injection from sensitizer molecules to TiO2 nanocrystalline thin films. Res Chem Intermed 27:393–406
Gong J, Liang J, Sumathy K (2012) Review on dye-sensitized solar cells (DSSCs): fundamental concepts and novel materials. Renew Sust Energ Rev 16:5848–5860
Gadisa A, Svensson M, Andersson MR, Inganäs O (2004) Correlation between oxidation potential and open-circuit voltage of composite solar cells based on blends of polythiophenes/fullerene derivative. Appl Phys Lett 84:1609–1611
Zhang J, Li HB, Sun SL, Geng Y, Wu Y, Su ZM (2012) Density functional theory characterization and design of high-performance diarylamine-fluorene dyes with different π spacers for dye-sensitized solar cells. J Mater Chem 22:568–576
Chen SL, Yang LN, Li ZS (2013) How to design more efficient organic dyes for dye-sensitized solar cells? Adding more sp2-hybridized nitrogen in the triphenylamine donor. J Power Sources 223:86–93
Peach MJ, Benfield P, Helgaker T, Tozer DJ (2008) Excitation energies in density functional theory: an evaluation and a diagnostic test. J Chem Phys 128:044118
Fitri A, Benjelloun AT, Benzakour M, Mcharfi M, Hamidi M, Bouachrine M (2014) Theoretical design of thiazolothiazole-based organic dyes with different electron donors for dye-sensitized solar cells. Spectrochim Acta A Mol Biomol Spectrosc 132:232–238
Yang Z, Wang D, Bai X, Shao C, Cao D (2014) Designing triphenylamine derivative dyes for highly effective dye-sensitized solar cells with near-infrared light harvesting up to 1100 nm. RSC Adv 4:48750–48757
Islam A, Sugihara H, Arakawa H (2003) Molecular design of ruthenium(II) polypyridyl photosensitizers for efficient nanocrystalline TiO2 solar cells. J Photochem Photobiol A Chem 158:131–138
Li M, Kou L, Diao L, Zhang Q, Li Z, Wu Q, Lu W, Pan D, Wei Z (2015) Theoretical study of WS-9-based organic sensitizers for unusual vis/NIR absorption and highly efficient dye-sensitized solar cells. J Phys Chem C 119:9782–9790
Sang-aroon W, Saekow S, Amornkitbamrung V (2012) Density functional theory study on the electronic structure of Monascus dyes as photosensitizer for dye-sensitized solar cells. J Photochem Photobiol A 236:35–40
Li Y, Pullerits T, Zhao M, Sun M (2011) Theoretical characterization of the PC60BM: PDDTT model for an organic solar cell. J Phys Chem C 115:21865–21873
Nithya R, Senthilkumar K (2014) Theoretical studies on the quinoidal thiophene based dyes for dye sensitized solar cell and NLO applications. Phys Chem Chem Phys 16:21496–21505
Availability of data and material
All the data and electronic materials are available for Gaussian program.
Code availability
Chemdraw, Gaussian 09w, Gaussview, and Gausssum.
Funding
The Researchers Supporting Project at King Saud University, Riyadh, Saudi Arabia, provided funding this research (2020/130).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the revised final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The phenothiazine-based organic dyes are designed and investigated.
• The absorption wavelength of PT-EBTEBA showed better dye than the SB and other molecules.
Supplementary information
Fig. S0
Dipole moment of the SB and designed molecules are calculated by B3LYP/6-31G(d,p) level of theory. (DOC 670 kb)
Rights and permissions
About this article
Cite this article
Munusamy, A.P., Ammasi, A., Shajahan, S. et al. Quantum chemical investigation on D-π-A-based phenothiazine organic chromophores with spacer and electron acceptor effects for DSSCs. Struct Chem 32, 2199–2207 (2021). https://doi.org/10.1007/s11224-021-01787-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-021-01787-x