Abstract
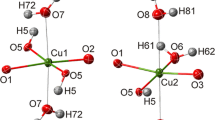
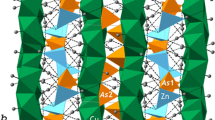
Hydrogen bonding in the Cu5(PO4)2(OH)4 polymorphs pseudomalachite, ludjibaite and reichenbachite has been studied by low-temperature single-crystal X-ray diffraction (XRD; pseudomalachite) and solid-state density functional theory (DFT; pseudomalachite, ludjibaite, reichenbachite) calculations. Pseudomalachite at 100 K is monoclinic, P21/c, a = 4.4436(4), b = 5.7320(5), c = 16.9300(15) Å, β = 91.008(8)°, V = 431.15(7) Å3 and Z = 2. The structure has been refined to R 1 = 0.025 for 1383 unique observed reflections with |F o| ≥ 4σF. DFT calculations were done with the CRYSTAL14 software package. For pseudomalachite, the difference between the calculated and experimental H sites does not exceed 0.152 Å. Structural configurations around hydroxyl groups in all three polymorphs show many similarities. Each OH5 group is involved in a three-center (bifurcated) hydrogen bond with the H···A distances in the range of 2.141–2.460 Å and the D–H···A angles in the range of 122.41°–139.30°, whereas each OH6 group forms a four-center (trifurcated) bond (H···A = 2.093–2.593 Å; D–H···A = 122.79°–137.71°). The crystal structures of the Cu5(PO4)2(OH)4 polymorphs are based on three-dimensional frameworks of Cu and P polyhedra. The copper-centered octahedra share edges to form two-dimensional layers parallel to (100) in all three structures. The layers have square voids above and beneath PO4 tetrahedra that link adjacent layers by sharing O atoms with two CuO6 octahedra each. From the topological point of view, none of the polymorphs can be obtained from another by a displacive transformation, and therefore pseudomalachite, ludjibaite and reichenbachite can be viewed as combinatorial polymorphs. According to information-based structural complexity considerations, the three phases are very similar in their configurational entropies and preferential crystallization of one phase over another cannot be entropy driven and is probably governed by other mechanisms that may involve such factors as structures of prenucleation clusters, chemical admixtures, etc.




Similar content being viewed by others
References
Shevchenko VY (2012) What is a chemical substance and how is it formed? Struct Chem 23:1011–1089
Shevchenko VY (2011) Structural chemistry of the nanoworld—a new page of inorganic chemistry. Glass Phys Chem 37:467–484
Shevchenko VY, Krivovichev SV, Tananaev IG, Myasoedov BF (2013) Cellular automata as models of inorganic structures self-assembly (illustrated by uranyl selenate). Glass Phys Chem 39:1–10
Shevchenko VY, Krivovichev SV, Mackay AL (2010) Cellular automata and local order in the structural chemistry of the lovozerite group minerals. Glass Phys Chem 36(1–9):2010
Elliot P, Cooper MA, Pring A (2014) Barlowite, Cu4FBr(OH)6, a new mineral isotructural with claringbullite: description and crystal structure. Mineral Mag 78:1755–1762
Ma Z, Li G, Chukanov NV, Poirier G, Shi N (2014) Tangdanite, a new mineral species from the Yunnan Province, China and the discreditation of ‘clinotyrolite’. Mineral Mag 78:559–569
Rieck B, Pristacz H, Giester G (2015) Colinowensite, BaCuSi2O6, a new mineral from the Kalahari Manganese Field, South Africa and new data on wesselsite, SrCuSi4O10. Mineral Mag 79:1769–1778
Pekov IV, Zubkova NV, Yapaskurt VO, Kartashov PM, Polekhovsky YS, Murashko MN, Pushcharovsky DY (2014) Koksharovite, CaMg2Fe4 3+(VO4)6, and grigorievite, Cu3Fe2 3+Al2(VO4)6, two new howardevansite-group minerals from volcanic exhalations. Eur J Mineral 26:679–688
Vergasova LP, Semenova TF, Krivovichev SV, Filatov SK, Zolotarev AA, Ananiev VV (2014) Nicksobolevite, Cu7(SeO3)2O2Cl6, a new complex copper oxoselenite chloride from Tolbachik fumaroles, Kamchatka peninsula, Russia. Eur J Mineral 26:439–449
Chukanov NV, Zubkova NV, Möhn G, Pekov IV, Pushcharovsky DY, Zadov AE (2015) Chanabayaite, Cu2(N3C2H2)Cl(NH3, Cl, H2O, □)4, a new mineral containing triazolate anion. Geol Ore Dep 57:712–720
Pekov IV, Zubkova NV, Belakovskiy DI, Yapaskurt VO, Vigasina MF, Sidorov EG, Pushcharovsky DYu (2015) New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. IV. Shchurovskyite, K2CaCu6O2(AsO4)4 and dmisokolovite, K3Cu5AlO2(AsO4)4. Mineral Mag 79:1737–1753
Pekov IV, Siidra OI, Chukanov NV, Yapaskurt VO, Britvin SN, Krivovichev SV, Schüller W, Ternes B (2015) Engelhauptite, KCu3(V2O7)(OH)2Cl, a new mineral species from Eifel, Germany. Mineral Petrol 209:705–711
Pekov IV, Siidra OI, Chukanov NV, Yapaskurt VO, Belakovskiy DI, Murashko MN, Sidorov EG (2014) Kaliochalcite, KCu2(SO4)2[(OH)(H2O)], a new tsumcorite-group mineral from the Tolbachik volcano, Kamchatka, Russia. Eur J Mineral 26:597–604
Mills SJ, Kampf AR, Christy AG, Housley RM, Thorne B, Chen YS, Steele IM (2014) Favreauite, a new selenite mineral from the El Dragón mine, Bolivia. Eur J Mineral 26:771–781
Chukanov NV, Britvin SN, Möhn G, Pekov IV, Zubkova NV, Nestola F, Kasatkin AV, Dini M (2015) Shilovite, natural copper(II) tetramine nitrate, a new mineral species. Mineral Mag 79:613–623
Goldsmith JR (1953) A “simplexity principle” and its relation to “ease” of crystallization. J Geol 61:439–451
Hausmann JFL (1813) Handbuch der Mineralogie. Bd. 3. Bandenhoed u. Ruprecht, Göttingen, pp 1036–1041
Berry LG (1950) Pseudomalachite and cornetite. Am Mineral 35:365–385
Ghose S (1962) Crystal structure of pseudomalachite, Cu5(PO4)2(OH)4. Naturwiss 49:324–325
Ghose S (1963) The crystal structure of pseudomalachite, Cu5(PO4)2(OH)4. Acta Crystallogr 16:124–128
Shoemaker GL, Anderson JB, Kostiner E (1977) Refinement of the crystal structure of pseudomalachite. Am Mineral 62:1042–1048
Anderson JB, Shoemaker GL, Kostiner E, Ruszala FA (1977) The crystal structure of synthetic Cu5(PO4)2(OH)4, a polymorph of pseudomalachite. Am Mineral 62:115–121
Shoemaker GL, Kostiner E (1981) Polymorphism in Cu5(PO4)2(OH)4. Am Mineral 66:176–181
Shoemaker GL, Anderson JB, Kostiner E (1981) The crystal structure of a third polymorph of Cu5(PO4)2OH)4. Am Mineral 66:169–175
Sieber NHW, Tillmanns E, Medenbach O (1987) Hentschelite, CuFe2(PO4)2(OH)2, a new member of the lazulite group, and reichenbachite, Cu5(PO4)2(OH)4, a polymorph of pseudomalachite, two new copper phosphate minerals from Reichenbach, Germany. Am Mineral 72:404–408
Piret P, Deliens M (1988) Description de la ludjibaite, un polymorphe de la pseudomalachite, Cu5(PO4)2(OH)4. Bull Mineral 111:167–171
Hyrsl J (1991) Three polymorphs of Cu5(PO4)2(OH)4 from Lubietova, Czechoslovakia. N Jb Mineral Mh 1991:281–287
Braithwaite RSW, Ryback G (1994) Reichenbachite from Cornwall and Portugal. Mineral Mag 58:449–451
Frost RL, Williams PA, Martens W, Kloprogge JT, Leverett P (2002) Raman spectroscopy of the basic copper phosphate minerals cornetite, libethenite, pseudomalachite, reichenbachite and ludjibaite. J Raman Spectr 33:260–263
Frost RL, Kloprogge T, Williams PA, Martens W, Johnson TE, Leverett P (2002) Vibrational spectroscopy of the basic copper phosphate minerals: pseudomalachite, ludjibaite and reichenbachite. Spectrochim Acta 58A:2861–2868
Martens W, Frost RL (2003) An infrared spectroscopic study of the basic copper phosphate minerals: cornetite, libethenite, and pseudomalachite. Am Mineral 88:37–46
Martens WN, Frost RL, Williams PA (2003) The basic copper phosphate minerals pseudomalachite, ludjibaite and reichenbachite: an infrared emission and Raman spectroscopic study. N Jb Mineral Mh 2003:337–362
Kharbish S, Andras P, Luptakova J, Milovska S (2014) Raman spectra of oriented and non-oriented Cu hydroxy-phosphate minerals: libethenite, cornetite, pseudomalachite, reichenbachite and ludjibaite. Spectrochim Acta 130A:152–163
Majzlan J, Zittlau A, Grevel KD, Schliesser J, Woodfield BF, Dachs E, Števko M, Plášil J, Milovská S (2016) Thermodynamic properties and phase equilibria of the secondary copper minerals libethenite, olivenite, pseudomalachite, kröhnkite, cyanochroite, and devilline. Can Mineral. doi:10.3749/canmin.1400066
Kikuchi H, Nguyen TTY, Fujii Y, Matsuo A, Kindo K (2013) Magnetic properties of the frustrated magnet Cu5(PO4)3(OH)4 on a peculiar spin network composed of pentagons and triangles. J Korean Phys Soc 62:2037–2040
Sumin NG, Lasheva NK (1952) Copper phosphates from Ural, Mednorudyansk mineral deposits. Trudy Mineral Muz AN SSSR 4:86–101
Popov VI, Popova VA (2015) Blinov IA (2015) Interrelations between malachite and pseudomalachite in the Mednjrudyanskoe deposit (Central Urals). Mineralogy 2:31–37 (in Russian)
Agilent Technologies (2012) CrysAlisPro, Version 1.171.36.20
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64:112–116
Dovesi R, Orlando R, Erba A, Zicovich-Wilson CM, Civalleri B, Casassa S, Maschio L, Ferrabone M, De La Pierre M, D’Arco P, Noel Y, Causa M, Rerat M, Kirtman B (2014) CRYSTAL14: a program for ab initio investigation of crystalline solids. Int J Quantum Chem 114:1287–1317
Milman V, Winkler B (2001) Prediction of hydrogen positions in complex structures. Z Kristallogr 216:99–104
Zhou B, Michaelis VK, Pan P, Yao Y, Tait KT, Hyde BC, Wren JEC, Sherriff BL, Kroeker S (2012) Crystal structure refinements of borate dimorphs inderite and kurnakovite using 11B and 25Mg nuclear magnetic resonance and DFT calculations. Am Mineral 97:1858–1865
Krivovichev SV, Zolotarev AA, Pekov IV (2016) Hydrogen bonding system in euchroite, Cu2(AsO4)(OH)(H2O)3: low-temperature crystal-structure refinement and solid-state density functional theory modeling. Mineral Petrol. doi:10.1007/s00710-016-0450-6
Peintinger MF, Oliveira DV, Bredow T (2013) Consistent Gaussian basis sets of triple-zeta valence with polarization quality for solid-state calculations. J Comput Chem 34:451–459
Ruiz E, Llunell M, Cano J, Rabu P, Drillon M, Massobrio C (2006) Theoretical determination of multiple exchange couplings and magnetic susceptibility data in inorganic solids: the prototypical case of Cu2(OH)3NO3. J Phys Chem B 110:115–118
Ruggiero MT, Erba A, Orlando R, Korter TM (2015) Origins of contrasting copper coordination geometries in crystalline copper sulfate pentahydrate. Phys Chem Chem Phys 17:31023–31029
Krivovichev SV (2012) Topological complexity of crystal structures: quantitative approach. Acta Crystallogr A 68:393–398
Krivovichev SV (2012) Information-based measures of structural complexity: application to fluorite-related structures. Struct Chem 23:1045–1052
Krivovichev SV (2013) Structural complexity of minerals: information storage and processing in the mineral world. Mineral Mag 77:275–326
Krivovichev SV (2014) Which inorganic structures are the most complex? Angew Chem Int Ed 53:654–661
Krivovichev SV (2016) Structural complexity and configurational entropy of crystalline solids. Acta Crystallogr B 72:274–276
Blatov VA, Shevchenko AP, Proserpio DM (2014) Applied topological analysis of crystal structures with the program package ToposPro. Cryst Growth Des 14:3576–3586
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, Oxford
Jahn HA, Teller E (1937) Stability of polyatomic molecules in degenerate electronic states. I. Orbital degeneracy. Proc R Soc A161:220–235
Moore PB (1975) Laueite, pseudolaueite, stewartite and metavauxite: a study in combinatorial polymorphism. N Jb Mineral Abh 133:148–159
Krivovichev SV (2009) Structural crystallography of inorganic oxysalts. Oxford University Press, Oxford
Krivovichev SV, Hawthorne FC, Williams PA (2016) Structural complexity and crystallization: the Ostwald sequence of phases in the Cu2(OH)3Cl system (botallackite–atacamite–clinoatacamite). Struct Chem. doi:10.1007/s11224-016-0792-z
Acknowledgments
This work was supported by the Russian Science Foundation (Grant 14-17-00071). X-ray diffraction measurements of pseudomalachite were done in the SPbSU X-ray Diffraction Centre.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Acad. V.Ya. Shevchenko on the occasion of his 75th birthday.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Krivovichev, S.V., Zolotarev, A.A. & Popova, V.I. Hydrogen bonding and structural complexity in the Cu5(PO4)2(OH)4 polymorphs (pseudomalachite, ludjibaite, reichenbachite): combined experimental and theoretical study. Struct Chem 27, 1715–1723 (2016). https://doi.org/10.1007/s11224-016-0820-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-016-0820-z