Abstract
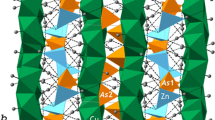
Hydrogen bonding in euchroite has been studied by means of low-temperature single-crystal X-ray diffraction (XRD) and solid-state density functional theory (DFT) calculations. The mineral is orthorhombic, P212121, a = 10.0350(8), b = 10.4794(8), c = 6.1075(5) Å, V = 642.27(9) Å3, and Z = 4. The structure has been refined to R 1 = 0.036 for 2436 unique observed reflections with |F o| ≥ 4σ F . DFT calculations were performed with the CRYSTAL14 software package. The basic features of the crystal structure of euchroite are the same as described by previous authors. There are two symmetrically-independent Cu sites octahedrally coordinated by O atoms. The CuO6 octahedra are strongly distorted containing four short (1.927–2.012 Å) and two long (2.360–2.797 Å) bonds each, in agreement with the expected Jahn-Teller distortion of an octahedrally-coordinated Cu2+ cation. There is one symmetrically-independent As site that is tetrahedrally coordinated by four O atoms to form an arsenate oxyanion, AsO4 3−. The structure is based upon chains of edge-sharing CuO6 octahedra running parallel to [001]. The chains are linked by AsO4 tetrahedra into a three-dimensional framework, which is stabilized by hydrogen bonds formed from OH and H2O groups. The coordinates of H atoms determined by single-crystal X-ray diffraction and those calculated using DFT are very similar. The distance Δ between experimental and theoretical H positions does not exceed 0.250 Å, except for the H72 site, for which Δ = 0.609 Å. The hydrogen bonding scheme in euchroite is rather complex and involves a combination of relatively strong two-center hydrogen bonds as well as few three-center (bifurcated) hydrogen bonds. The largest difference between the XRD and DFT results involves the H72 atom of the H2O7 molecule and can be assigned to the effect of temperature, which favors a strong linear hydrogen bond at 0 K (calculated) and a bifurcated three-center bond at 100 K (measured). The Cu-H2O configurations are bent for the H2O7 and H2O6 groups and are almost planar for the H2O8 groups.




Similar content being viewed by others
References
Adams RD, Layland R, Payen C (1995) Cu4(AsO4)2(O): a new copper arsenate with unusual low temperature magnetic properties. Inorg Chem 34:5397–5398
Agilent Technologies (2012) CrysAlisPro, Version 1.171.36.20
Berry LG (1951) Observations on conichalcite, cornwallite, euchroite, liroconite and olivenite. Am Mineral 36:484–503
Breithaupt A (1823) Vollständige Charakteristik des mineral-systems, 2nd edn. Arnoldsche Buchhandlung, Dresden
Bryantsev VS, Diallo MS, van Duin ACT, Goddard WA III (2008) Hydration of copper (II): new insights from density functional theory and the COSMO solvation model. J Phys Chem A 112:9104–9112
Chu S, McQueen TM, Chisnell R, Freedman DE, Müller P, Lee YS, Nocera DG (2010) A Cu2+ (S = 1/2) kagomé antiferromagnet: MgxCu4-x(OH)6Cl2. J Am Chem Soc 132:5570–5571
Chukanov NV, Britvin SN, Möhn G, Pekov IV, Zubkova NV, Nestola F, Kasatkin AV, Dini M (2015a) Shilovite, natural copper(II) tetrammine nitrate, a new mineral species. Mineral Mag 79:613–623
Chukanov NV, Aksenov SM, Rastsvetaeva RK, Lyssenko KA, Belakovskiy DI, Färber G, Möhn G, Van KV (2015b) Antipinite, KNa3Cu2(C2O4)4, a new mineral species from a guano deposit at Pabellón de Pica, Chile. Mineral Mag 79:1111–1121
Colman RH, Ritter C, Wills AS (2008) Towards perfection: Kapellasite, Cu3Zn(OH)6Cl2, a new model S = 1/2 kagomé antiferromagnet. Chem Mater 20:6897–6899
Colman RH, Sinclair A, Wills AS (2010) Comparisons between haydeeite, α-Cu3Mg(OD)6Cl2, and kapellasite, α-Cu3Zn(OD)6Cl2, isostructural S = 1/2 kagomé magnets. Chem Mater 22:5774–5779
Dovesi R, Orlando R, Erba A, Zicovich-Wilson CM, Civalleri B, Casassa S, Maschio L, Ferrabone M, De La Pierre M, D’Arco P, Noel Y, Causa M, Rerat M, Kirtman B (2014) CRYSTAL14: a program for ab initio investigation of crystalline solids. Int J Quantum Chem 114:1287–1317
Eby RK, Hawthorne FC (1989) Euchroite, a heteropolyhedral framework structure. Acta Crystallogr C 45:1479–1482
Elliott P, Cooper MA, Pring A (2014) Barlowite, Cu4FBr(OH)6, a new mineral isostructural with claringbullite: description and crystal structure. Mineral Mag 78:1755–1762
Finney JJ (1966) Refinement of the crystal structure of euchroite, Cu2(AsO4)(OH)·3H2O. Acta Crystallogr 21:437–440
Frost RL, Bahfenne S (2010) Thermal analysis and hot-stage Raman spectroscopy of the basic copper arsenate mineral. Euchroite. J Therm Anal Calorim 100:89–94
Frost RL, Čejka J, Sejkora J, Plašil J, Bahfenne S, Palmer S (2010) Raman spectroscopy of the basic copper arsenate mineral: euchroite. J Raman Spectrosc 41:571–575
Giuseppetti G (1963) Crystal structure of euchroite, Cu2(AsO4)(OH).3H2O. Period Mineral 32:131–156
Haidinger W (1825) Notice respecting euchroite, a new mineral species. Edinburgh J Sci 2:133–135
Jahn HA, Teller E (1937) Stability of polyatomic molecules in degenerate electronic states. I. Orbital degeneracy. Proc Roy Soc A 161:220–235
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, New York Oxford
Jeffrey GA, Saenger W (1994) Hydrogen bonding in biological structures. Springer-Verlag, Berlin Heidelberg
Kikuchi H, Fujii Y, Takahashi D, Azuma M, Shimakawa Y, Taniguchi T, Matsuo A, Kindo K (2011) Spin gapped behavior of a frustrated delta chain compound euchroite. J Phys Conf Ser 320:012045/1–012045/6
Magalhäes MCF, Pedrosa de Jesus JD, Williams PA (1988) The chemistry of formation of some secondary arsenate minerals of copper(II), zinc(II), and lead(II). Mineral Mag 52:679–690
Mills SJ, Kampf AR, Christy AG, Housley RM, Thorne B, Chen YS, Steele IM (2014a) Favreauite, a new selenite mineral from the El Dragón mine, Bolivia. Eur J Mineral 26:771–781
Mills SJ, Kampf AR, Christy AG, Housley RM, Rossman GR, Reynolds RE, Marty J (2014b) Bluebellite and mojaveite, two new minerals from the central Mojave Desert, California, USA. Mineral Mag 78:1325–1340
Mills SJ, Christy AG, Schnyder C, Favreau G, Price JR (2014c) The crystal structure of camerolaite and structural variation in the cyanotrichite family of merotypes. Mineral Mag 78:1527–1552
Mills SJ, Christy AG, Colombo F, Price JR (2015) The crystal structure of cyanotrichite. Mineral Mag 79:321–335
Milman V, Winkler B (2001) Prediction of hydrogen positions in complex structures. Z Kristallogr 216:99–104
Minčeva-Stefanova J (1999) Paragenetic typization of copper arsenate minerals based on their Cu:[AsO4] ratio. Dokl Bulg Akad Nauk 52:55–58
Modig K, Pfrommer BG, Halle B (2003) Temperature-dependent hydrogen-bond geometry in liquid water. Phys Rev Lett 90:075502
Peintinger MF, Oliveira DV, Bredow T (2013) Consistent Gaussian basis sets of triple-zeta valence with polarization quality for solid-state calculations. J Comput Chem 34:451–459
Pekov IV, Siidra OI, Chukanov NV, Yapaskurt VO, Belakovskiy DI, Murashko MN, Sidorov EG (2014a) Kaliochalcite, KCu2(SO4)2[(OH)(H2O)], a new tsumcorite-group mineral from the Tolbachik volcano, Kamchatka, Russia. Eur J Mineral 26:597–604
Pekov IV, Zubkova NV, Yapaskurt VO, Kartashov PM, Polekhovsky YS, Murashko MN, Pushcharovsky DY (2014b) Koksharovite, CaMg2Fe3+ 4(VO4)6, and grigorievite, Cu3Fe3+ 2Al2(VO4)6, two new howardevansite-group minerals from volcanic exhalations. Eur J Mineral 26:667–677
Pekov IV, Zubkova NV, Yapaskurt VO, Belakovskiy DI, Vigasina MF, Sidorov EG, Pushcharovsky DY (2014c) New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. II. Ericlaxmanite and kozyrevskite, two natural modifications of Cu4O(AsO4)2. Mineral Mag 78:1553–1569
Pekov IV, Siidra OI, Chukanov NV, Yapaskurt VO, Britvin SN, Krivovichev SV, Schüller W, Ternes B (2015a) Engelhauptite, KCu3(V2O7)(OH)2Cl, a new mineral species from Eifel, Germany. Mineral Petrol 109:705–711
Pekov IV, Zubkova NV, Belakovskiy DI, Yapaskurt VO, Vigasina MF, Lykova IS, Sidorov EG, Pushcharovsky DY (2015b) Chrysothallite K6Cu6Tl3+Cl17(OH)4·H2O, a new mineral species from the Tolbachik volcano, Kamchatka, Russia. Mineral Mag 79:365–376
Pekov IV, Zubkova NV, Yapaskurt VO, Belakovskiy DI, Vigasina MF, Sidorov EG, Pushcharovsky DY (2015c) New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. III. Popovite, Cu5O2(AsO4)2. Mineral Mag 79:133–143
Pekov IV, Zubkova NV, Belakovskiy DI, Yapaskurt VO, Vigasina MF, Sidorov EG, Pushcharovsky DY (2015d) New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. IV. Shchurovskyite, K2CaCu6O2(AsO4)4 and dmisokolovite, K3Cu5AlO2(AsO4)4. Mineral Mag 79:1737–1753
Plášil J, Kasatkin AV, Škoda R, Škácha P (2014a) Klajite, MnCu4(AsO4)2(AsO3OH)2(H2O)10, from Jachymov (Czech Republic): the second world occurrence. Mineral Mag 78:119–129
Plášil J, Sejkora J, Škoda R, Novák M, Kasatkin AV, Škácha P, Veselovský F, Fejfarová K, Ondruš P (2014b) Hloušekite, (Ni,Co)Cu4(AsO4)2(AsO3OH)2(H2O)9, a new member of the lindackerite supergroup from Jáchymov, Czech Republic. Mineral Mag 78:1341–1353
Rieck B, Pristacz H, Giester G (2015) Colinowensite, BaCuSi2O6, a new mineral from the Kalahari Manganese field, South Africa and new data on wesselsite, SrCuSi4O10. Mineral Mag 79:1769–1778
Ruggiero MT, Erba A, Orlando R, Korter TM (2015) Origins of contrasting copper coordination geometries in crystalline copper sulfate pentahydrate. Phys Chem Chem Phys 17:31023–31029
Ruiz E, Llunell M, Cano J, Rabu P, Drillon M, Massobrio C (2006) Theoretical determination of multiple exchange couplings and magnetic susceptibility data in inorganic solids: the prototypical case of Cu2(OH)3NO3. J Phys Chem B 110:115–118
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64:112–116
Staack M, Müller-Buschbaum H (1996) Zur Kenntnis des Kupfer-Oxid-Arsenats Cu4O(AsO4)2. Z Naturforsch B 51:1279–1282
Turner MD (1825) Analysis of euchroite. Edinburgh J Sci 2:301–305
Vergasova LP, Semenova TF, Krivovichev SV, Filatov SK, Zolotarev AA Jr, Ananiev VV (2014) Nicksobolevite, Cu7(SeO3)2O2Cl6, a new complex copper oxoselenite chloride from Tolbachik fumaroles, Kamchatka peninsula, Russia. Eur J Mineral 26:439–449
Zhou B, Michaelis VK, Pan P, Yao Y, Tait KT, Hyde BC, Wren JEC, Sherriff BL, Kroeker S (2012) Crystal structure refinements of borate dimorphs inderite and kurnakovite using 11B and 25Mg nuclear magnetic resonance and DFT calculations. Am Mineral 97:1858–1865
Acknowledgments
We are very grateful to Andrew Locock and Stuart Mills for constructive remarks and for polishing the English, and to the Associate Editor Luca Bindi for the efficient handling of the manuscript. This work was supported by the Russian Science Foundation (grant 14-17-00071). X-ray diffraction measurements have been performed in St. Petersburg State University XRD Resource Centre.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: L. Bindi
Electronic supplementary material
ESM 1
(TXT 177 kb)
Rights and permissions
About this article
Cite this article
Krivovichev, S.V., Zolotarev, A.A. & Pekov, I.V. Hydrogen bonding system in euchroite, Cu2(AsO4)(OH)(H2O)3: low-temperature crystal-structure refinement and solid-state density functional theory modeling. Miner Petrol 110, 877–883 (2016). https://doi.org/10.1007/s00710-016-0450-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-016-0450-6