Abstract
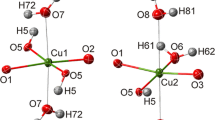
Philipsburgite, Cu5Zn((As,P)O4)2(OH)6·H2O, from the Middle Pit, Gold Hill Mine, Tooele Co., Utah, USA, was studied by single-crystal X-ray diffraction and scanning electron microscopy. The empirical formula of the studied sample is (Cu4.69Zn1.23)(As0.86P0.18O4)2(OH)5.61·H2O, which agrees well with the previous reports on the mineral. Philipsburgite is monoclinic, P21/c, a = 12.385(6), b = 9.261(4), c = 10.770(5) Å, β = 97.10(1)o, V = 1225.7(9) Å3 (at 100 K), and Z = 4. The crystal structure was refined to R1 = 0.046 for 2563 unique observed reflections with |Fo| ≥ 4σF. The crystal structure of philipsburgite is isotypic to that of kipushite and can be considered as a complex three-dimensional framework consisting of two types of layers stacked parallel to the a-axis. The A-type layer is formed by the edge-sharing Jahn–Teller-distorted Cuφ6 octahedra [φ = O2−, (OH)−, H2O]. Two adjacent octahedral layers are linked via (As2O4) tetrahedra. The B-type layer is built by corner-sharing (ZnO4) and (As1O4) tetrahedra and is formed by the four- and eight-membered tetrahedral rings. The A:B ratio of the A and B layers is equal to 2:1. The hydrogen bonding network in philipsburgite is rather complex and consists of two- and three-center hydrogen bonds. The As1 site accommodates ca. 18% of P and is a preferable position for the P substitution in philipsburgite. The observed selectivity of the As1 site for P may indicate that, for the intermediate compositions with the P:As ratios close to 1:1, there is a fully ordered species with P prevalent at the As1 site and As prevalent at the As2 site. The intermediate composition would, therefore, be Cu5Zn(AsO4)(PO4)(OH)6·H2O and such a mineral can be considered as a separate species, according to the rules of the International Mineralogical Association (IMA). Philipsburgite should be considered as structurally complex with the Shannon information contents of 4.954 bits/atom and 614.320 bits/cell. The obvious reason for the structural complexity of the mineral is its modularity, i.e., the presence of two structurally distinct modules, the octahedral–tetrahedral (A) and tetrahedral (B) layers.


Similar content being viewed by others
References
Agilent Technologies (2012) CrysAlisPro, Version 1.171.36.20
Braithwaite RSW, Ryback R (1988) Philipsburgite from the Caldbeck Fells and kipushite from Montana, and their infrared spectra. Mineral Mag 52:529–533
Buttgenbach H (1926) Description d’un minéral du Katanga. Acad Roy Belg Sci 1926:905–913
Ciesielczuk J, Janeczek J, Dulski M, Krzykawski T (2016) Pseudomalachite–cornwallite and kipushite–philipsburgite solid solutions: chemical composition and Raman spectroscopy. Eur J Mineral 28:555–569
Danisi RM, Armbruster T, Lazic B (2012) In situ dehydration behavior of zeolite-like cavansite: a single-crystal X-ray study. Amer Mineral 97:1874–1880
Danisi RM, Armbruster T, Lazic B, Vulic P, Kaindl R, Dimitrijevic R, Kahlenberg V (2013) In situ dehydration behavior of veszelyite (Cu,Zn)2Zn(PO4)(OH)3.2H2O: a single-crystal X-ray study. Am Mineral 98:1261–1269
Effenberger H, Mereiter K, Pimminger M, Zemann J (1982) Machatschkiite: crystal structure and revision of the chemical formula. Tscherm Mineral Petrog Mitt 30:145–155
Evans HT (1973) The crystal structures of cavansite and pentagonite. Amer Mineral 58:412–424
Ghose S, Leo SR, Wan C (1974) Structural chemistry of copper and zinc minerals: Part I. Veszelyite, (Cu,Zn)2ZnPO4(OH)3·2H2O: a novel type of sheet structure and crystal chemistry of copper–zinc substitution. Am Mineral 59:573–581
Hathaway BJ (1987) Copper. Comprehensive Coordination Chemistry, In: Wilkinson G, ed. Pergamon, Oxford. Vol. 5, pp. 533–774
Jahn HA, Teller E (1937) Stability of polyatomic molecules in degenerate electronic states. I. Orbital degeneracy. Proc R Soc A161:220–235
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, New York
Krivovichev SV (2005) Topology of microporous structures. Rev Mineral Geochem 57:17–68
Krivovichev SV (2013) Structural complexity of minerals: information storage and processing in the mineral world. Mineral Mag 77:275–326
Krivovichev SV (2017) Hydrogen bonding and structural complexity of the Cu3(AsO4)(OH)3 polymorphs (clinoclase, gilmarite): a theoretical study. J Geosci 62(2):79–85
Krivovichev SV (2018) Ladders of information: what contributes to the structural complexity of inorganic crystals. Z Kristallogr, https://doi.org/10.1515/zkri-2017-2117
Krivovichev SV, Zolotarev AA, Pekov IV (2016) Hydrogen bonding system in euchroite, Cu2(AsO4)(OH)(H2O)3: low-temperature crystal-structure refinement and solid-state density functional theory modeling. Mineral Petrol 110:877–883
Majzlan J, Drahota P, Filippi M (2014) Parageneses and crystal chemistry of arsenate minerals. Rev Mineral Geochem 79:17–184
Majzlan J, Števko M, Dachs E, Benisek A, Plášil A, Sejkora J (2017) Thermodynamics, stability, crystal structure, and phase relations among euchroite, Cu2(AsO4)(OH)·3H2O, and related minerals. Eur J Mineral 29:5–16
Minceva-Stefanova J (2001) Arsenate minerals diversity in oxidation zones of the polymetallic stratabound deposits in Western Balkan Mountain. C R Acad Bulg Sci 54(6):39
Panikorovskii TL, Krivovichev SV, Yakovenchuk VN, Ivanyuk GYu (2017) The crystal structure of epifanovite. Zap Ross Mineral Obshch 146(3):39–50
Peacor DR, Dunn PJ, Ramik RA, Sturman BD, Zeihen LG (1985) Philipsburgite, a new copper zinc arsenate hydrate related to kipushite, from Montana. Can Mineral 23:255–258
Pekov IV, Zubkova NV, Yapaskurt VO, Polekhovsky YS, Vigasina MF, Belakovskiy DI, Britvin SN, Sidorov EG, Pushcharovsky DY (2016a) New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. VI. Melanarsite, K3Cu7Fe3+O4(AsO4)4. Mineral Mag 80:855–867
Pekov IV, Lykova IS, Koshlyakova NN, Belakovskiy DI, Vigasina MF, Turchkova AG, Britvin SN, Sidorov EG, Scheidl KS (2016b) Zincobradaczekite, IMA 2016-041. CNMNC Newsletter No. 33, October 2016. Mineral Mag 80:1135–1144
Pekov IV, Zubkova NV, Agakhanov AA, Yapaskurt VO, Belakovskiy DI, Britvin SN, Sidorov EG, Pushcharovsky DY (2017a) Axelite, IMA2017-015a. CNMNC Newsletter No 38:August 2017. Mineral Mag 81:1033–1038
Pekov IV, Zubkova NV, Agakhanov AA, Sidorov EG, Ksenofontov DA, Britvin SN, Pushcharovsky DY (2017b) Alumoedtollite, IMA2017-020. CNMNC Newsletter No. 38, August 2017. Mineral Mag 81:1033–1038
Pekov IV, Zubkova NV, Agakhanov AA, Pautov LA, Vigasina MF, Sidorov EG, Ksenofontov DA, Britvin SN, Pushcharovsky DY (2016c) Edtollite, IMA 2016-010. CNMNC Newsletter No. 31, June 2016. Mineral Mag 80:691–697
Piret P, Deliens M, Piret-Meunier J (1985) Occurrence and crystal structure of kipushite, a new copper–zinc phosphate from Kipushi, Zaire. Can Mineral 23:35–42
Plášil J, Kasatkin AV, Škoda R, Škácha P (2014a) Klajite, MnCu4(AsO4)2(AsO3OH)2(H2O)10, from Jachymov (Czech Republic): the second world occurrence. Mineral Mag 78:119–129
Plášil J, Sejkora J, Škoda R, Novák M, Kasatkin AV, Škácha P, Veselovský F, Fejfarová K, Ondruš P (2014b) Hloušekite, (Ni,Co)Cu4(AsO4)2(AsO3OH)2(H2O)9, a new member of the lindackerite supergroup from Jáchymov, Czech Republic. Mineral Mag 78:1341–1353
Schnorrer G, Poeverlein R (2007) Die Minerale des Martinstollens am Weißen Schrofen. Der Aufschluss 58:27–40
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64:112–116
Shirose Y, Uehara S (2011) Philipsburgite from the Yamato mine, Yamaguchi Prefecture, Japan. J Mineral Petrol Sci 106:153–157
Vrtiška L, Sejkora J, Malíková R (2016) Philipsburgit z Krásna u Horního Slavkova, Slavkovský les (Česká republika). Bull Mineral-Petrol Odd Nár Muz (Praha) 24:243–251
Walenta K (1977) Machatschkiit, ein neues Arsenatmineral aus der Grube Anton im Heubachtal bei Schiltach (Schwarzwald. Bundesrepublik Deutschland). Tscherm Mineral Petrogr Mitt 24:125–132
Yakovenchuk VN, Pakhomovsky YA, Konopleva NG, Panikorovskii TL, Mikhailova JA, Bocharov VN, Krivovichev SV, Ivanyuk GYu (2017) Epifanovite, NaCaCu5(PO4)4[AsO2(OH)2]·7H2O, a new mineral from the Kester deposit (Sakha-Yakutia, Russia). Zap Ross Mineral Obshch 146(3):30–39 (in Russian)
Yakovenchuk VN, Pakhomovsky YA, Konopleva NG, Panikorovskii TL, Bazai A, Mikhailova JA, Bocharov VN, Ivanyuk GYu, Krivovichev SV (2018) Batagayite, CaZn2(Zn,Cu)6(PO4)4(PO3OH)3·12H2O, a new phosphate mineral from Këster tin deposit (Yakutia, Russia): occurrence and crystal structure. Mineral Petrol https://doi.org/10.1007/s00710-017-0551-x (in press)
Acknowledgements
We are grateful to Justyna Ciesielczuk and anonymous referee for useful comments on the manuscript and interesting discussion. This work was supported by the Russian Science Foundation (Grant 14-17-00071). The experiments were carried out using facilities of XRD and Geomodel Resource Centers of St. Petersburg State University. The contribution of Fersman Mineralogical Museum is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Krivovichev, S.V., Zhitova, E.S., Ismagilova, R.M. et al. Site-selective As–P substitution and hydrogen bonding in the crystal structure of philipsburgite, Cu5Zn((As,P)O4)2(OH)6·H2O. Phys Chem Minerals 45, 917–923 (2018). https://doi.org/10.1007/s00269-018-0972-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-018-0972-z