Abstract
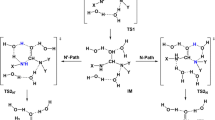
Stereospecific reactions of dimethyl (2S,4RS)-4-bromo-N-phthaloylglutamate with KOH, triethylamine, and piperidine affording dimethyl (Z)-1-phthalimidocyclopropane-1,2-dicarboxylate were studied in terms of the density functional theory. The two-stage E1cB mechanism of HBr elimination involving the formation of an intermediate carban-ion upon deprotonation at the C(2) atom was established by the nudged elastic band method. Calculations of the reactions with all bases, carried out with inclusion of the solvent effect using ethanol as the model solvent, demonstrated that the E1cB mechanism of 1,3-elimination of HBr is more preferable than the SN2 mechanism of nucleophilic substitution of bromine at the C(4) atom. Contrary to this, calculations of the reaction with piperidine using benzene as the model solvent revealed that the SN2 mechanism is slightly more preferable than the ElcB one. High stereoselectivity of the 1,3-elimination of bromine with respect to the (Z)-isomer is due to noncovalent repulsive interactions between the methoxycarbonyl groups in the transition state leading from the carbanion to the minor (E)-diastereomer of 1-phthalimidocyclopropane-1,2-dicarboxylic acid.
Similar content being viewed by others
References
Y.-Y. Fan, X.-H. Gao, J.-M. Yue, Sci. China Chem., 2016, 59, 1126; DOI: https://doi.org/10.1007/s11426-016-0233-1.
P. Keglevich, A. Keglevich, L. Hazai, G. Kalaus, C. Szántay, Curr. Org. Chem., 2014, 18, 2037; DOI: https://doi.org/10.2174/1385272819666140721190257.
The Drugbank, Chemical Structure Search (accessed December 12, 2022); https://go.drugbank.com/unearth/q&searcher=drug&query=cyclopropyl.
A. Reichelt, S. F. Martin, Acc. Chem. Res., 2006, 39, 433; DOI: https://doi.org/10.1021/ar030255s.
F. Gnad, O. Reiser, Chem. Rev., 2003, 103, 1603; DOI: https://doi.org/10.1021/cr010015v.
F. Brackmann, A. de Meijere, Chem. Rev., 2007, 107, 4493; DOI: https://doi.org/10.1021/cr078376j.
J. Salaün, in Small Ring Compounds in Organic Synthesis VI, Topics in Current Chemistry, Ed. A. de Meij ere, 2000, 207, p. 1; DOI: https://doi.org/10.1007/3-540-48255-5_1.
M. Ya. Mel’nikov, E. M. Budynina, O. A. Ivanova, I. V. Trushkov, Mendeleev Commun., 2011, 21, 293; DOI: https://doi.org/10.1016/j.mencom.2011.11.001.
A. E. Vartanova, A. Yu. Plodukhin, M. A. Boichenko, V. V. Shorokhov, S. S. Zhokhov, I. V. Trushkov, O. A. Ivanova, Russ. Chem. Bull., 2022, 71, 2431; DOI: https://doi.org/10.1007/s11172-022-3671-3.
V. P. Krasnov, M. A. Korolyova, G. L. Levit, Russ. Chem. Rev., 2003, 72, 343; DOI: https://doi.org/10.1070/RC2003v072n04ABEH000750.
V. A. Smit, A. D. Dil’man, Osnovy sovremennogo or-ganicheskogo sinteza [Foundations of Modern Organic Synthesis], BINOM. Laboratoriya znaniy, Moscow, 2009, p. 447 (in Russian).
O. G. Kulinkovich, Cyclopropanes in Organic Synthesis, J. Wiley & Sons, Inc, Hoboken, New Jersey, 2015, p. 65.
V. D. Gvozdev, K. N. Shavrin, M. P. Egorov, O. M. Nefedov, Russ. Chem. Bull., 2021, 70, 2051; DOI: https://doi.org/10.1007/s11172-021-3318-9.
G. W. Cannon, R. C. Ellis, J. R. Leal, Org. Synth., 1951, 31, 74; DOI: https://doi.org/10.15227/orgsyn.031.0074.
N. Vignola, B. List, J. Am. Chem. Soc., 2004, 126, 450; DOI: https://doi.org/10.1021/ja0392566.
T. Selvi, K. Srinivasan, J. Org. Chem., 2014, 79, 3653; DOI: https://doi.org/10.1021/jo402848v.
J. P. Phelan, S. B. Lang, J. S. Compton, C. B. Kelly, R. Dykstra, O. Gutierrez, G. A. Molander, J. Am. Chem. Soc., 2018, 140, 8037; DOI: https://doi.org/10.1021/jacs.8b05243.
H. Pellissier, A. Lattanzi, R. Dalpozzo, Asymmetric Synthesis of Three-Membered Rings, Wiley-VCH, Weinheim, Germany, 2017, p. 147.
V. Kh. Kravtsov, V. N. Biyushkin, T. I. Malinovskii, V. P. Krasnov, T. V. Matveeva, Dokl. Chem., 1990, 311, 622.
V. P. Krasnov, M. A. Koroleva, T. V. Matveeva, E. A. Zhdanova, A. N. Grishakov, N. A. Kluev, Russ. Chem. Bull., 2001, 50, 644; DOI: https://doi.org/10.1023/A:1011356727378.
V. P. Krasnov, A. Yu. Vigorov, I. A. Nizova, T. V. Matveeva, A. N. Grishakov, I. V. Bazhov, A. A. Tumashov, M. A. Ezhikova, M. I. Kodess, Eur. J. Org. Chem., 2007, 4257; DOI: https://doi.org/10.1002/ejoc.200700346.
I. M. Bukrina, V. P. Krasnov, V. Kh. Kravtsov, V. N. Biyushkin, L. V. Alekseeva, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1989, 38, 1910; DOI: https://doi.org/10.1007/bf00957790.
V. P. Krasnov, I. M. Bukrina, E. A. Zhdanova, M. I. Kodess, M. A. Korolyova, Synthesis, 1994, 961; DOI: https://doi.org/10.1055/s-1994-25614.
I. A. Nizova, V. P. Krasnov, O. V. Korotovskikh, L. V. Alekseeva, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1989, 38, 2545; DOI: https://doi.org/10.1007/BF00962442.
A. Yu. Vigorov, I. A. Nizova, G. L. Levit, T. V. Matveeva, L. Sh. Sadretdinova, O. I. Nazarov, N. S. Kovalev, D. A. Bakulin, D. V. Kurkin, I. N. Tyurekov, V. P. Krasnov, Russ. Chem. Bull., 2022, 71, 2636; DOI: https://doi.org/10.1007/s11172-022-3693-x.
A. Yu. Vigorov, V. P. Krasnov, I. A. Nizova, L. Sh. Sadretdinova, G. L. Levit, T. V. Matveeva, P. A. Slepukhin, D. A. Bakulin, N. S. Kovalyov, I. N. Tyurenkov, V. N. Charushin, Dokl. Chem., 2020, 494, 131; DOI: https://doi.org/10.1134/S0012500820090049.
A. Yu. Vigorov, I. A. Nizova, K. E. Shalunova, A. N. Grishakov, L. Sh. Sadretdinova, I. N. Ganebnykh, M. A. Ezhikova, M. I. Kodess, V. P. Krasnov, Russ. Chem. Bull., 2011, 60, 873; DOI: https://doi.org/10.1007/s11172-011-0137-4.
V. P. Krasnov, I. A. Nizova, A. Yu. Vigorov, T. V. Matveeva, G. L. Levit, P. A. Slepukhin, M. A. Ezhikova, M. I. Kodess, Eur. J. Org. Chem., 2008, 1802; DOI: https://doi.org/10.1002/ejoc.200701154.
V. P. Krasnov, M. A. Koroleva, Russ. Chem. Bull., 1995, 44, 631; DOI: https://doi.org/10.1007/BF00698492.
M. A. Korolyova, A. Yu. Vigorov, V. P. Krasnov, Russ. Chem. Bull., 2022, 71, 1135; DOI: https://doi.org/10.1007/s11172-022-3513-3.
E. N. Chulakov, M. A. Korolyova, L. Sh. Sadretdinova, A. A. Tumashov, M. I. Kodess, G. L. Levit, V. P. Krasnov, Russ. Chem. Bull., 2021, 70, 890; DOI: https://doi.org/10.1007/s11172-021-3164-9.
M. A. Korolyova, S. A. Vakarov, D. N. Kozhevnikov, D. A. Gruzdev, G. L. Levit, V. P. Krasnov, Eur. J. Org. Chem., 2018, 33, 4577; DOI: https://doi.org/10.1002/ejoc.201800656.
D. A. Gruzdev, S. A. Vakarov, M. A. Korolyova, E. V. Bartashevich, A. A. Tumashov, E. N. Chulakov, M. A. Ezhikova, M. I. Kodess, G. L. Levit, V. P. Krasnov, Org. Biomol. Chem., 2022, 20, 862; DOI: https://doi.org/10.1039/d1ob02099d.
A. D. Becke, J. Chem. Phys., 1993, 98, 1372; DOI: https://doi.org/10.1063/1.464304.
A. D. Becke, J. Chem. Phys., 1993, 98, 5648; DOI: https://doi.org/10.1063/1.464913.
F. Weigend, R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297; DOI: https://doi.org/10.1039/B508541A.
V. Barone, M. Cossi, J. Phys. Chem. A, 1998, 102, 1995; DOI: https://doi.org/10.1021/JP9716997.
S. Grimme, S. Ehrlich, L. Goerigk, J. Comput. Chem., 2011, 32, 1456; DOI: https://doi.org/10.1002/jcc.21759.
S. Grimme, A. Hansen, J. G. Brandenburg, C. Bannwarth, Chem. Rev., 2016, 116, 5105; DOI: https://doi.org/10.1021/acs.chemrev.5b00533.
H. Kruse, S. Grimme, J. Chem. Phys., 2012, 136, 154101; DOI: https://doi.org/10.1063/1.3700154.
F. Neese, J. Comput. Chem., 2003, 24, 1740; DOI: https://doi.org/10.1002/jcc.10318.
A. Pedretti, A. Mazzolari, S. Gervasoni, L. Fumagalli, G. Vistoli, Bioinformatics, 2021, 37, 1174; DOI: https://doi.org/10.1093/bioinformatics/btaa774.
J. Gasteiger, M. Marsili, Tetrahedron, 1980, 36, 3219; DOI: https://doi.org/10.1016/0040-4020(80)80168-2.
F. Neese, F. Wennmohs, U. Becker, C. Riplinger, J. Chem. Phys., 2020, 152, 224108; DOI: https://doi.org/10.1063/5.0004608.
V. Asgeirsson, B. O. Birgisson, R. Bjornsson, U. Becker, F. Neese, C. Riplinger, H. Jónsson, J. Chem. Theory Comput., 2021, 17, 4929; DOI: https://doi.org/10.1021/acs.jctc.1c00462.
K. Ishida, K. Morokuma, A. Komornicki, J. Chem. Phys., 1977, 66, 2153; DOI: https://doi.org/10.1063/1.434152.
S. Grimme, J. Chem. Phys., 2006, 124, 034108; DOI: https://doi.org/10.1063/1.2148954.
A. Fernández-Ramos, B. A. Ellingson, R. Meana-Paneda, J. M. C. Marques, D. G. Truhlar, Theor. Chem. Acc., 2007, 118, 813; DOI: https://doi.org/10.1007/s00214-007-0328-0.
G. A. Zhurko, Chemcraft, graficheskaya programma dlya Windows dlya obrabotki kvantovo-khimicheskikh raschetov [Chemcraft — A Graphical Program for Windows to Work with Results of Quantum Chemical Calculations], Ivanovo, 2005 (in Russian).
O. S. Tee, J. A. Altmann, K. Yates, J. Am. Chem. Soc., 1974, 96, 3141; DOI: https://doi.org/10.1021/ja00817a021.
Y. Liu, T. S. Sorensen, F. Sun, Can. J. Chem., 1993, 71, 258; DOI: https://doi.org/10.1139/v93-037.
F. G. Bordwell, B. B. Jarvis, J. Am. Chem. Soc., 1973, 95, 3585; DOI: https://doi.org/10.1021/ja00792a021.
F. G. Bordwell, E. Doomes, J. Org. Chem., 1974, 39, 2531; DOI: https://doi.org/10.1021/jo00931a015.
F. M. Bickelhaupt, MassSpectrom. Rev., 2001, 20, 347; DOI: https://doi.org/10.1002/mas.10007.
E. Mosconi, F. De Angelis, L. Belpassi, F. Tarantelli, S. Alunni, Eur. J. Org. Chem., 2009, 32, 5501; DOI: https://doi.org/10.1002/ejoc.200900906.
D. K. Mandal, Stereochemistry and Organic Reactions: Conformation, Configuration, Stereoelectronic Effects and Asymmetric Synthesis, Elsevier—Academic Press— SPi Global, Kolkata, India, 2021, p. 223; DOI: https://doi.org/10.1016/C2020-0-01299-5.
D. E. Ortega, R. Ormazábal-Toledo, R. Contreras, R. A. Matute, Org. Biomol. Chem., 2019, 17, 9874; DOI: https://doi.org/10.1039/c9ob02004g.
Z. S. Jia, J. Rudzinski, P. Paneth, A. Thibblin, J. Org. Chem., 2002, 67, 177; DOI: https://doi.org/10.1021/jo0159340.
L. P. Wolters, Y. Ren, F. M. Bickelhaupt, ChemistryOpen, 2014, 3, 29; DOI: https://doi.org/10.1002/open.201300043.
J. K. Laerdahl, P. U. Civcir, L. Bache-Andreassen, E. Uggerud, Org. Biomol. Chem., 2006, 4, 135; DOI: https://doi.org/10.1039/b513315g.
M. Bortoli, L. P. Wolters, L. Orian, F. M. Bickelhaupt, J. Chem. Theory Comput., 2016, 12, 2752; DOI: https://doi.org/10.1021/acs.jctc.6b00253.
A. P. Bento, F. M. Bickelhaupt, J. Org. Chem., 2008, 73, 7290; DOI: https://doi.org/10.1021/jo801215z.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no competing interests.
Additional information
The authors express their gratitude to S. V. Scharf (N. N. Krasovskii Institute of Mathematics and Mechanics, Ural Branch of the Russian Academy of Sciences) for help in installing and debugging the ORCA software on the Uran supercomputer cluster.
This work was carried out within the State Assignment of the Ministry of Science and Higher Education of the Russian Federation (Reg. Nos AAAA-A19-119011790134-1 and AAAA-A19-119011790130-3).
No human or animal subjects were used in this research.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, Vol. 72, No. 9, pp. 1991–2005, September, 2023.
Rights and permissions
About this article
Cite this article
Korolyova, M.A., Vigorov, A.Y. & Krasnov, V.P. 1,3-Elimination of HBr from dimethyl (2S,4RS)-4-bromo-N-phthaloylglutamate under the action of bases: a theoretical study. Russ Chem Bull 72, 1991–2005 (2023). https://doi.org/10.1007/s11172-023-3992-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-023-3992-x