Abstract
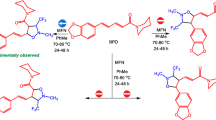
A comparative study of the kinetic resolution (KR) of racemic 6-substituted 2-methyl-1,2,3,4-tetrahydroquinolines with acyl chlorides of (S)-naproxen, N-phthaloyl-(S)-leucine, and (R)-O-phenyllactic acid was carried out. The selectivity factors in the KR of racemic amines with acyl chlorides of (S)-naproxen and (R)-O-phenyllactic acid were shown to be approximately the same and higher than those for the KR of N-phthaloyl-(S)-leucyl chloride. The reasons for the stereodifferentiation in the KR of racemic tetrahydroquinaldines containing groups of different electronic properties by acyl chlorides of three different chiral acids were explained using the DFT method. The conditions for stabilizing π—π interactions of aromatic fragments of the reagents, which do not occur in the same form in the transition state and lead to the minor diastereoisomeric product, are created in the transition state of the faster acylation reaction with (S)-naproxen and (R)-O-phenyllactic acyl chlorides. In the case of the KR of 2-methyl-1,2,3,4-tetrahydroquinoline and 2-methyl-6-methoxy-1,2,3,4-tetrahydroquinoline with N-phthaloyl-(S)-leucyl chloride, the acylation diastereoselectivity is determined, most likely, by conformational factors. The individual (S)-enantiomer of 2-methyl-6-methoxy-1,2,3,4-tetrahydroquinoline of high optical purity was synthesized using the KR of the racemate with (S)-naproxen acyl chloride.
Similar content being viewed by others
References
M. R. Maddani, J.-C. Fiaud, H. B. Kagan, in Separation of Enantiomers: Synthetic Methods, Ed. M. Todd, Wiley-VCH, Weinheim, 2014, p. 13.
M. Breuer, K. Ditrich, T. Habicher, B. Hauer, M. Kebeler, R. Stürmer, T. Zelinski, Angew. Chem., Int. Ed., 2004, 43, 788; DOI: https://doi.org/10.1002/anie.200300599.
C. E. Müller, P. R. Schreiner, Angew. Chem., Int. Ed., 2011, 50, 6012; DOI: https://doi.org/10.1002/anie.201006128.
V. P. Krasnov, D. A. Gruzdev, G. L. Levit, Eur. J. Org. Chem., 2012, 1471; DOI: https://doi.org/10.1002/ejoc.201101489.
G. L. Levit, D. A. Gruzdev, V. P. Krasnov, Adv. Org. Synth., 2018, 12, 151; DOI: https://doi.org/10.2174/9781681086804118120006.
V. P. Krasnov, G. L. Levit, M. A. Korolyova, I. M. Bukrina, L. Sh. Sadretdinova, I. N. Andreeva, V. N. Charushin, O. N. Chupakhin, Russ. Chem. Bull., 2004, 53, 1253; DOI: https://doi.org/10.1023/B:RUCB.0000042282.29765.2f.
D. A. Gruzdev, G. L. Levit, V. P. Krasnov, E. N. Chulakov, L. Sh. Sadretdinova, A. N. Grishakov, M. A. Ezhikova, M. I. Kodess, V. N. Charushin, Tetrahedron: Asymmetry, 2010, 21, 936; DOI: https://doi.org/10.1016/j.tetasy.2010.05.013.
E. N. Chulakov, D. A. Gruzdev, G. L. Levit, L. Sh. Sadret-dinova, V. P. Krasnov, V. N. Charushin, Russ. Chem. Bull., 2011, 60, 948; DOI: https://doi.org/10.1007/s11172-011-0149-0.
G. L. Levit, D. A. Gruzdev, V. P. Krasnov, E. N. Chulakov, L. Sh. Sadretdinova, M. A. Ezhikova, M. I. Kodess, V. N. Charushin, Tetrahedron: Asymmetry, 2011, 22, 185; DOI: https://doi.org/10.1016/j.tetasy.2010.12.017.
D. A. Gruzdev, G. L. Levit, V. P. Krasnov, Tetrahedron: Asymmetry, 2012, 23, 1640; DOI: https://doi.org/10.1016/j.tetasy.2012.11.001.
E. N. Chulakov, G. L. Levit, A. A. Tumashov, L. Sh. Sadret-dinova, V. P. Krasnov, Chem. Heterocycl. Compd., 2012, 48, 724; DOI: https://doi.org/10.1007/s10593-012-1051-x.
D. A. Gruzdev, G. L. Levit, M. I. Kodess, V. P. Krasnov, Chem. Heterocycl. Compd., 2012, 48, 748; DOI: https://doi.org/10.1007/s10593-012-1053-8.
D. A. Gruzdev, E. N. Chulakov, G. L. Levit, M. A. Ezhikova, M. I. Kodess, V. P. Krasnov, Tetrahedron: Asymmetry, 2013, 24, 1240; DOI: https://doi.org/10.1016/j.tetasy.2013.07.024.
D. A. Gruzdev, S. A. Vakarov G. L. Levit, V. P. Krasnov, Chem. Heterocycl. Compd., 2014, 49, 1795; DOI: https://doi.org/10.1007/s10593-014-1432-4.
S. A. Vakarov, D. A. Gruzdev, E. N. Chulakov, L. Sh. Sadretdinova, M. A. Ezhikova, M. I. Kodess, G. L. Levit, V. P. Krasnov, Chem. Heterocycl. Compd., 2014, 50, 838; DOI: https://doi.org/10.1007/s10593-014-1538-8.
S. A. Vakarov, D. A. Gruzdev, E. N. Chulakov, L. Sh. Sadret-dinova, A. A. Tumashov, M. G. Pervova, M. A. Ezhikova, M. I. Kodess, G. L. Levit, V. P. Krasnov, V. N. Charushin, Tetrahedron: Asymmetry, 2016, 27, 1231; DOI: https://doi.org/10.1016/j.tetasy.2016.10.004.
D. A. Gruzdev, E. N. Chulakov, L. Sh. Sadretdinova, G. L. Levit, V. P. Krasnov, V. N. Charushin, Dokl. Chem., 2018, 483, 293; DOI: https://doi.org/10.1134/S0012500818120017.
S. A. Vakarov, D. A. Gruzdev, G. L. Levit, V. P. Krasnov, V. N. Charushin, O. N. Chupakhin, Russ. Chem. Rev., 2019, 88, 1063; DOI: https://doi.org/10.1070/RCR4893.
Sh.-M. Lu, C. Bolm, Adv. Synth. Catal., 2008, 350, 1101; DOI: https://doi.org/10.1002/adsc.200800068.
V. A. Potemkin, V. P. Krasnov, G. L. Levit, E. V. Bartoshevich, I. N. Andreeva, M. B. Kuzminsky, N. A. Anikin, V. N. Charushin, O. N. Chupakhin, Mendeleev Commun., 2004, 14, 69; DOI: https://doi.org/10.1070/MC2004v014n02ABEH001887.
M. A. Korolyova, S. A. Vakarov, D. N. Kozhevnikov, D. A. Gruzdev, G. L. Levit, V. P. Krasnov, Eur. J. Org. Chem., 2018, 4577; DOI: https://doi.org/10.1002/ejoc.201800656.
S. A. Vakarov, M. A. Korolyova, D. A. Gruzdev, M. G. Pervova, G. L. Levit, V. P. Krasnov, Russ. Chem. Bull., 2019, 68, 1257; DOI: https://doi.org/10.1007/s11172-019-2550-z.
H. B. Bürgi, J. D. Dunitz, E. Shefter, J. Am. Chem. Soc., 1973, 95, 5065; DOI: https://doi.org/10.1021/ja00796a058.
S. L. Cockroft, J. Perkins, C. Zonta, H. Adams, S. E. Spey, C. M. R. Low, J. G. Vinter, K. R. Lawson, C. J. Urch, C. A. Hunter, Org. Biomol. Chem., 2007, 5 1062; DOI: https://doi.org/10.1039/617576g.
W. Wang, W. Zeng, Y. G. Zhou, Tetrahedron: Asymmetry, 2007, 18, 1103; DOI: https://doi.org/10.1016/j.tetasy.2007.04.028.
A. Nose, T. Kudo, Chem. Pharm. Bull., 1984, 32, 2421; DOI: https://doi.org/10.1248/cpb.32.2421.
M. A. Amin, M. A. Camerino, S. J. Mountford, X. Ma, D. T. Manallack, D. K. Chalmers, M. Wills, P. E. Thompson, Tetrahedron, 2019, 75, 130591; DOI: https://doi.org/10.1016/j.tet.2019.130591.
A. Pedretti, L. Villa, G. Vistoli, J. Mol. Graph., 2002, 21, 47; DOI: https://doi.org/10.1016/S1093-3263(02)00123-7.
A. Pedretti, L. Villa, G. Vistoli, J. Comput.-Aided Mol. Des., 2004, 18, 167; DOI: https://doi.org/10.1023/B:JCAM.0000035186.90683.f2.
F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73; DOI: https://doi.org/10.1002/wcms.81.
F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2017, 8, e1327; DOI: https://doi.org/10.1002/wcms.1327.
S. Grimme, J. Comput. Chem., 2006, 27, 1787; DOI: https://doi.org/10.1002/jcc.20495.
S. Grimme, J. Antony, S. Ehrlich, H. Krieg, J. Chem. Phys., 2010, 132, 154104; DOI: https://doi.org/10.1063/1.3382344.
S. Grimme, S. Ehrlich, L. Goerigk, J. Comput. Chem., 2011, 32, 1456; DOI: https://doi.org/10.1002/jcc.21759.
S. Grimme, A. Hansen, J. G. Brandenburg, C. Bannwarth, Chem. Rev., 2016, 116, 5105; DOI: https://doi.org/10.1021/acs.chemrev.5b00533.
E. Caldeweyher, C. Bannwarth, S. Grimme, J. Chem. Phys. 2017, 147, 034112; DOI: https://doi.org/10.1063/1.4993215.
S. Grimme, Chem. Eur. J., 2012, 18, 9955; DOI: https://doi.org/10.1002/chem.201200497.
A. D. Becke, J. Chem. Phys., 1993, 98, 1372; DOI: https://doi.org/10.1063/1.464304.
A. D. Becke, J. Chem. Phys., 1993, 98, 5648; DOI: https://doi.org/10.1063/1.464913.
F. Weigend, R. Ahlichs, J. Phys. Chem. Chem. Phys., 2005, 7, 3297; DOI: https://doi.org/10.1039/B508541A.
H. Kruse, S. Grimme, J. Chem. Phys., 2012, 126, 154101; DOI: https://doi.org/10.1063/1.3700154.
A. Fernández-Ramos, B. A. Ellingson, R. Maena-Paneda, J. M. C. Marques, D. G. Truhlar, Theor. Chem. Acc., 2007, 118, 813; DOI: https://doi.org/10.1007/s00214-007-0328-0.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences V. N. Charushin on the occasion of his 70th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 5, pp. 890–899, May, 2021.
Rights and permissions
About this article
Cite this article
Chulakov, E.N., Korolyova, M.A., Sadretdinova, L.S. et al. Kinetic resolution of racemic 6-substituted 1,2,3,4-tetrahydroquinaldines with chiral acyl chlorides. Experiment and quantum chemical simulation. Russ Chem Bull 70, 890–899 (2021). https://doi.org/10.1007/s11172-021-3164-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-021-3164-9