Abstract
Cyanobacteria are prokaryotic photosynthetic microorganisms that can generate, in addition to biomass, useful chemicals and proteins/enzymes, essentially from sunlight, carbon dioxide, and water. Selected aspects of cyanobacterial production (isoprenoids and high-value proteins) and scale-up methods suitable for product generation and downstream processing are addressed in this review. The work focuses on the challenge and promise of specialty chemicals and proteins production, with isoprenoid products and biopharma proteins as study cases, and the challenges encountered in the expression of recombinant proteins/enzymes, which underline the essence of synthetic biology with these microorganisms. Progress and the current state-of-the-art in these targeted topics are emphasized.
Similar content being viewed by others
Preface – why cyanobacteria?
Cyanobacteria, also known as blue–green algae, are a group of photosynthetic microorganisms that can use the energy of sunlight to convert carbon dioxide (CO2) and water (H2O) into valuable products (Jansson 2012; Berla et al. 2013; Savakis and Angermayr 2013; Santos-Merino et al. 2019). Advantages afforded by cyanobacteria include the extensive genomic information from a number of species, ease and precise manipulation of cyanobacterial genomes, including double homologous recombination to enable insertion of transgenes or multigene operons and antibiotic selectable markers, or deletion of specific endogenous genes from the cellular DNA (Satta et al. 2023). Important practical features of these microorganisms include the much faster growth rate relative to plants (Melis 2009) and the ability to avoid contaminants in mass liquid cultures (Chaves et al. 2015; Xie et al. 2017).
Significant advances in cyanobacterial cell factories were recently realized, including genetically engineered strains to perform more efficiently under bright sunlight and high biomass density, strains able to use high concentrations of CO2 to support high rates of photosynthesis, and also be modified to direct their metabolism toward the production of specific high-value proteins and chemicals (Bentley and Melis 2012; Kirst et al. 2014; Yu et al. 2015, Jaiswal et al. 2018; Betterle et al. 2020; Włodarczyk et al. 2020). Progress in this field has also enabled the development of tools for a more efficient and cost-effective engineering of these microorganisms for specific biotechnological applications (Albers et al. 2015; Santos-Merino et al. 2019), although the development of such tools in cyanobacteria lags behind those in conventional heterotrophic microorganisms (Cheng et al. 2023).
Recently, CRISPR-based tools have gained attention in cyanobacterial research for their ability to target polyploid genomes in these organisms and to increase the efficiency of cyanobacterial transformation (Behler et al. 2018; Sengupta et al. 2022; Patel et al. 2023). Moreover, a further advancement of CRISPR, CRISPRi, which was based on the use of a deactivated Cas enzyme, was described as an efficient method for functional studies based on gene silencing (Gordon et al. 2016; Yao et al. 2016; Liu et al. 2020).
A major area of progress has been the optimization of cyanobacterial growth capacity. This includes identification of the genetic determinants required for the rapid growth of cyanobacterial strains (Yu et al. 2015; Mills et al. 2022), as well as for enhancing their natural transformation ability (Wendt et al. 2022). These efforts have resulted in faster growth rates and higher biomass yields, which can translate into greater yields of heterologous product synthesis and lower production costs.
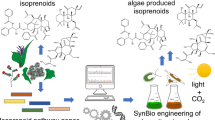
While much of the work on cyanobacterial cell factories has been conducted in the laboratory, there is now interest in scaling up production to pilot or industrial levels (Novoveská et al. 2023). Such effort would require optimization of the cultivation conditions, development of efficient downstream processing, and innovative product isolation approaches (Pandey et al. 2022). Nevertheless, the above-mentioned advances in cyanobacterial synthetic biology have enabled the engineering of modified strains that can direct metabolism toward a variety of products with commercial significance. Metabolically engineered cyanobacteria have successfully produced compounds such as ethanol, butanol, lactic acid, ethylene, terpene hydrocarbons, and other interesting products (Satta et al. 2023). Other efforts have included synthesis and accumulation of biopharmaceutical proteins, isoprenoid pathway enzymes, chemicals like plant essential oils, and potentially biofuels (Fig. 1).
Overall, advances in cyanobacterial cell factories are opening up new possibilities for sustainable production of a variety of valuable compounds. Continued R&D in this field is likely to lead to further breakthroughs in years to come that could have a significant impact on this technology and its applications in the bioeconomy.
Isoprenoid products
The simplest terpene hydrocarbons are the hemiterpene isoprene (2-methyl-1,3-butadiene; C5H8) and monoterpenes (C10H16), as exemplified by the acyclic myrcene, monocyclic phellandrene, and bicyclic pinene (Fig. 2). However, there are many other monoterpenes, identified from a variety of plants, as essential oils and fragrance chemicals, including camphene, sabinene, δ-3-carene, α-terpinene, p-cymene, limonene, β-ocimene, γ-terpinene, and terpinolene (Gomes et al. 2013), among others. Whereas, isoprene and monoterpenes have received the majority of attention in the cyanobacterial literature (Pattanaik and Lindberg 2015; Kant et al. 2023), higher-order sesquiterpenes (C15H24), diterpenes (C20H32), and triterpenes (C30H50) have also been investigated as putative biotechnology products of cyanobacterial cell factories (Rautela and Kumar 2022). Moreover, oxygenated / hydroxylated terpenes, e.g., patchoulol (C15H25OH; Dienst et al. 2020) and geranyllinalool (C20H33OH; Formighieri and Melis 2017), have been the subject of research and development, as possible cell factory products. Lastly, renewable carotenoids production has also been of interest in the field (Liu et al. 2019; Menin et al. 2019; Diao et al. 2020).
Isoprene
Isoprene is a volatile 5-carbon organic compound that is naturally generated by a variety of organisms, notably terrestrial herbaceous and deciduous plants (Loreto and Sharkey 1990; Sharkey et al. 2005, 2007; Guenther et al. 2006; Harrison et al. 2013), some types of bacteria (Kuzma and Fall 1993; Fall and Copley 2000; Bäck et al. 2010), and even fungi and humans (Mochalski et al. 2023). Of interest is the case of terrestrial plants, in which expression of the endogenous isoprene synthase gene is induced upon heat stress, resulting in substantial amounts of photosynthetic substrate conversion to isoprene (Sharkey et al. 2007). The latter readily diffuses through the chloroplasts, cell membranes, and walls and exits the leaves through the stomata, acting as a thermotolerance mechanism for the plant. The thermotolerance interpretation is based on the observation that isoprene-emitting leaves are able to quickly recover after heat stress, suggesting a mitigation of heat damage (Velikova and Loreto 2005).
A growing interest in using cyanobacteria as a heterologous source of isoprene has followed the pioneering work of Lindberg et al. (2010). Conferring heterologous isoprene production to cyanobacteria is complex and involves multiple steps. First, the genes responsible for isoprene synthesis were identified, cloned, codon-optimized, and genetically installed into the cyanobacterial genome (Lindberg et al. 2010; Bentley and Melis 2012; Bentley et al. 2014). The expression of these genes, at the protein level, was verified and maximized (Chaves et al. 2017) in order to enhance the yield of isoprene production, without negatively affecting rates of cellular photosynthesis and cell fitness. Moreover, altering the methylerythritol 4-phosphate (MEP) pathway and, specifically, the dimethylallyl diphosphate (DMAPP) to isopentenyl diphosphate (IPP) ratio toward more DMAPP and less IPP has helped to increase the yield of isoprene, as it increased the substrate (DMAPP) from which isoprene is generated (Chaves et al. 2016; Gao et al. 2016; Chaves and Melis 2018a).
Interest in cyanobacterial isoprene is based on the potential for constitutive production by these photosynthetic microorganisms (Englund et al. 2018; Chaves and Melis 2018b; Ko et al. 2019; Sethia et al. 2019; Zhou et al. 2021; Yadav et al. 2023; Yahya et al. 2023) and the potential to provide a sustainable and renewable source of this important compound for use in a variety of applications. These include the production of synthetic rubber, plastics, adhesives, pesticides, medicines, fragrances, and biofuel derivatives. In this respect, isoprene is in high demand in the commercial sector, with more than one million tonnes, currently produced from fossil fuel resources and commercially consumed annually (Whited et al. 2010; Morais et al. 2015).
With current technology, yield of isoprene production in cyanobacterial cell factories has reached 12.5 mg per g biomass (Chaves and Melis 2018a,b). Flux through the native methylerythritol phosphate isoprenoid pathway naturally consumes about 50 mg of photosynthetically generated metabolites per g biomass produced, which is needed to satisfy the endogenous isoprenoid needs of the cell (Lindberg et al. 2010; Melis 2012; 2013). Hence, the above-mentioned currently achieved isoprene yield of 12.5 mg per g biomass suggested that the installed heterologous pathway to isoprene constitutively achieved flux equal to about 25% of that in the native isoprenoid pathway. Heterologous carbon flux to isoprene was in addition to that consumed for the synthesis of native isoprenoids, based on the observation that isoprene production did not impede cell growth.
However, flux through the isoprenoid biosynthetic pathway need not be limited to the above-mentioned yields. There are examples in the literature, e.g., the green colonial microalga Botryococcus braunii variety Showa, whereby carbon partitioning through the isoprenoid biosynthetic pathway consumes ~ 45% of the cellular endogenous metabolites, most of which is invested in the synthesis and extracellular accumulation of the biologically aberrant triterpenoid botryococcene (Melis 2013). Thus, it is theoretically possible to enhance the carbon partitioning and flux of the cyanobacterial isoprenoid biosynthetic pathway to achieve far greater than present product yields.
Monoterpenes, higher-order Isoprenoids, and other cyanobacterial bio-products
Cyanobacterial limonene (Davies et al. 2014; Jongedijk et al. 2016; Lin et al. 2021; Li et al. 2022) and β-phellandrene production (Bentley et al. 2013; Formighieri and Melis 2015; 2016; Xie et al. 2017; Lin and Pakrasi 2019; Betterle and Melis 2019) have attracted attention in the field. Production of sesquiterpenes (C15H24) like farnesene (Lee et al. 2017), bisabolene (Davies et al. 2014), and caryophyllene (Reinsvold et al. 2011) have also been reported.
Several reviews on the cyanobacterial production of other useful chemicals (Ducat et al. 2011; Machado and Atsumi 2012; Ungerer et al. 2012; Savakis and Hellingwerf 2015; Pattanaik and Lindberg 2015; Davies et al. 2015; Woo 2017; Wang et al. 2020; Rodrigues and Lindberg 2021b; Rautela and Kumar 2022; Barone et al. 2023) amplify the breadth and depth of this growing field. These will not be discussed further in the present “Perspectives.” The interested reader is referred to the relevant review articles cited in this section.
Spontaneous product separation from the biomass and the liquid culture
Critical issues in the field of isoprenoids production include the yield of the process. Inherent limitations including (i) flux of endogenous metabolites, comprising the products of photosynthesis, to the isoprenoid biosynthetic pathway (Melis 2013) and (ii) product separation from the biomass and the liquid culture. As discussed above, flux of endogenous metabolites through the MEP pathway for the synthesis of all cellular isoprenoids is typically limited to about 5% of total cellular carbon (Lindberg et al. 2010). Efforts to enhance this highly regulated flux in cyanobacteria included installation of the heterologous mevalonic acid pathway in isoprene and β-phellandrene competent Synechocystis sp. PCC 6803 (hereafter referred to as Synechocystis), a property that substantially enhanced the yield of isoprene (Bentley et al. 2014) and β-phellandrene accumulation (Formighieri and Melis 2015; 2016; Betterle and Melis 2019).
The interplay between biomass accumulation and heterologous product generation is also of interest and pertinent to cell factories. In theory, biomass accumulation and heterologous product generation (biofuels or chemicals) are assumed to be competing processes (Melis 2012). In this respect, it is intriguing that preliminary observations have shown enhancement in the rate of light-saturated photosynthesis, when a heterologous process adds a new metabolic sink, e.g., when isoprene or β-phellandrene biosynthetic pathways are installed, without a negative effect on the rate of biomass accumulation (Bentley et al. 2013). This was interpreted to mean that the rate of photosynthesis under saturating illumination is sink limited by the rate of biomass accumulation, rather than by the rate of the light reactions and that photosynthesis can be enhanced when additional carbon-sinks are installed. Thus, a heterologous sink that is separate and apart from the cellular biomass may hold promise in terms of enhancing the light-saturated rate of photosynthesis.
Spontaneous product diffusion (efflux) from the cells and exclusion from the liquid phase of the culture confers a substantial advantage with respect to downstream processing, as it simplifies product isolation and lowers the cost of production. Moreover, it alleviates product inhibition of cellular endogenous metabolism and mitigates potentially adverse effects on cell vitality and growth. In general, product separation from the cells and from the liquid culture proved to be necessary for continuous substrate flux through the heterologous pathway, ensuring product accumulation, while preserving cell fitness. Heterologous products trapped inside the transformant cells are typically detrimental to cell fitness and tend to inhibit cell growth and productivity. Examples of the latter in cyanobacteria include efforts to make botryococcene, a C30H50 a biologically aberrant triterpene analogous to squalene, and cannabidiol, or CBD oil, a C21H30O2 hybrid of the fatty acid and isoprenoid biosynthetic pathways. Both of these heterologous products proved to be too large for spontaneous diffusion and exclusion from the cells, resulting in cell growth inhibition and loss of productivity (unpublished results). Low amounts of the natural triterpene squalene (C30H50), however, accumulated in transformant Synechocystis, showing that squalene was tolerated and was not detrimental to growth, in spite of the intracellular sequestration of this compound (Englund et al. 2014; Choi et al. 2017; Pattanaik et al. 2020).
Of interest is the case of geranyllinalool, a C20H33OH diterpenol useful as a fragrance and a feedstock for the synthesis of the antimalarial drug “teprenone.” Geranyllinalool in Synechocystis displayed limited spontaneous diffusion from the cells (Formighieri and Melis 2017). It was primarily sequestered inside the transformant cells, corresponding to 60–70% of the total heterologous product, instead of being entirely exuded, as the case is with shorter heterologous terpene hydrocarbons. Extraction of geranyllinalool necessitated disruption of the cells in order to enable release and quantitative isolation of this chemical product. Moreover, geranyllinalool accumulation in the cells caused a mild inhibitory effect on cell fitness and a decline in biomass growth rate such that the duplication time of geranyllinalool-producing Synechocystis transformants was 1.4-fold longer than that of the control. The remaining 30–40% of the geranyllinalool product, which diffused out of the cells, was found to float on the surface of sealed liquid cultures (Formighieri and Melis 2017).
Of interest is also the application of dodecane (5% (v/v)) as a non-miscible organic layer on the surface of liquid cultures, designed to help extract from cells and contain medium- and larger-size isoprenoids, such as limonene and bisabolene (Davies et al. 2014; Lee et al. 2017; Rodrigues and Lindberg 2021a). Product titers were reportedly higher, when a dodecane overlay was applied during culturing, suggesting either that dodecane traps large quantities of volatile limonene and α-bisabolene that would otherwise be lost to evaporation and/or that continuous product removal in dodecane alleviates product feedback inhibition, thus promoting higher rates of synthesis.
In summary, spontaneous or assisted (via dodecane) product diffusion out of the cells and exclusion from the liquid phase of the culture affords a substantial advantage in commercialization as (i) it alleviates product inhibition of cellular endogenous metabolism and (ii) it lowers the cost of downstream processing, as it helps to avoid the need for culture dewatering, cell fractionation, and product extraction from the cells.
Provision of carbon dioxide (CO2)
In addition to cultivation conditions, such as growth temperature and nutrient availability (Rodrigues et al. 2023), provision of CO2 (Bentley and Melis 2012) and sunlight utilization efficiency (Melis 2009; Kirst et al. 2014; Hu et al. 2023) are pivotal in efforts to maximize productivity of photosynthetic microorganisms. Bubbling cultures with air, which contains a mere 0.04% CO2, poses a stark limitation to the capacity of photosynthesis for cell growth and productivity. This limitation can be alleviated via the use of closed and sealed photobioreactors or other closed cultivation systems, designed specifically for the provision and containment of high amounts of carbon dioxide [100% gaseous CO2] to the culture, while at the same time affording containment of volatile isoprenoid products. Of interest in this respect is the design and use of “the Bentley bottle” (Fig. 3, Bentley and Melis 2012), comprising a custom-made fed-batch bioreactor for diffusion-based CO2/O2 gas exchange and volatile hydrocarbons production and containment. A gas stream comprising 100% CO2 was slowly fed into this gaseous/aqueous two-phase bioreactor to flush the aqueous phase and to fill the reactor headspace, upon which the vessel was sealed. Efficient and spontaneous uptake by diffusion and assimilation of headspace CO2 by the cells occurred concomitantly with the exchange of photosynthetically produced O2 and isoprene or β-phellandrene accumulation during photoautotrophic growth. Accumulation of isoprenoids took place in the sealed bioreactor headspace (Bentley and Melis 2012). The principle of the gaseous/aqueous two-phase bioreactor was successfully tested in an expanded 1200-L pilot bioreactor under ambient conditions in the greenhouse, where the beginning composition of the gaseous phase comprised 100% CO2 (Fig. 4a). An illustration of productivity under these conditions was provided upon culture inoculation on 2018-04-06 (Fig. 4b) and the attainment of high cell density in the photobioreactor on 2018-04-13 (Fig. 4c), which showed that growth of cyanobacteria can be robust in the presence of sufficient amounts of CO2 and bright sunlight.
The “Bentley bottle,” a lab-designed 1-L sealed bioreactor for diffusion-based delivery of CO2 and O2 gas exchange occurring concomitantly with terpene hydrocarbons production and release/accumulation. A 100% CO2 gas stream was slowly bubbled into the gaseous/aqueous two-phase medium, calibrated to flush the liquid phase and fill the headspace prior to sealing the reactor. Efficient and spontaneous uptake and assimilation of headspace CO2 by the cells occurred in the fully sealed reactor by diffusion and was concomitantly replaced by photosynthetically produced O2 and hydrophobic isoprenoids during cell photoautotrophic growth. The latter (O2 and hydrophobic isoprenoids) accumulated in the reactor headspace. This device was successfully tested with isoprene, β-phellandrene, and geranyllinalool, as the target isoprenoid products. Schematic adapted from Bentley and Melis (2012)
A 1,200-L tubular modular pilot photobioreactor layout in the greenhouse was designed to support cell growth and product generation / accumulation. A control box enabled culture manipulations, including addition of nutrients and removal of culture samples from the reactor. a Shown is the gaseous/aqueous two-phase configuration with growth media present but prior to cyanobacterial inoculation. This set-up employed an approximately 50:50 partition between the aqueous and gaseous phases. The latter was filled with a stream of 100% CO2. However, the gaseous/aqueous partition ratio could vary depending on organism and growth conditions. b View of the reactor described above, immediately following inoculation with a starter culture. In a sealed reactor, this configuration permitted spontaneous diffusion-driven CO2 uptake from the gaseous phase and its replacement by O2 and by the isoprenoid products generated from the photosynthesis of the cells in the aqueous phase. c View of the reactor seven days after inoculation. With ambient sunlight and sufficient CO2 substrate, cells grew quickly, resulting in a high-density biomass in the reactor, as evidenced from the high optical density of the culture. Abundance of CO2 (100% provision) and bright sunlight helped the rate of cyanobacterial growth and productivity
Sunlight utilization efficiency
Cyanobacterial culture productivities can also be improved upon an increase in sunlight utilization efficiency. Cyanobacterial cell factories are converting the energy of sunlight in photosynthesis and storing it as biomass, biofuel, and target chemical or protein/enzyme of interest. The mechanics of this operation require, in addition to non-limiting amounts of CO2, ability to absorb all incident sunlight and also ability to convert it in the form of useful photochemistry. The former is satisfied by a sufficiently high density of biomass to ensure absorption of all incident irradiance, which is easily attained. The latter, however, is severely compromised because photosynthetic organisms assemble large arrays of light-absorbing antenna molecules, leading to excessive absorption of sunlight at the surface of the culture, frequently way beyond what they can use biochemically, resulting in a wasteful dissipation of excitation and in a lower than expected culture photochemical productivity. In consequence, cells deeper in such cultures are shaded and do not perform to capacity. A genetic tendency of photosynthetic organisms to assemble large arrays of light-absorbing antenna molecules in their photosystems is a survival strategy and a competitive advantage in the wild (Melis 2009), where sunlight is often limiting (Kirk 1994). Maximum competition of photosynthetic organisms in the wild requires capturing more light for self, even if wasted, and preventing light capture by competing neighbors (Melis 2009). Obviously, this property is detrimental to the yield and productivity in a dense monoculture.
Minimizing, or truncating, the light-harvesting antennae of photosynthesis to maximize culture productivity, i.e., application of the so-called “Truncated Light-harvesting Antenna” (TLA) concept, successfully increased solar energy conversion efficiencies of photosynthesis in tobacco (Kirst et al. 2017; 2018), green microalgae (Polle et al. 2003), and cyanobacteria (Kirst et al. 2014). Generation of TLA strains in all classes of photosynthetic organisms helps to alleviate excess absorption of bright sunlight and the ensuing wasteful dissipation of excitation energy and to maximize solar-to-product energy conversion efficiency and photosynthetic productivity in high-density mass cultivations (Melis 2009). The TLA concept may find application in the commercial exploitation of cyanobacteria for the generation of biomass, biofuels, and chemical feedstocks, as well as nutraceuticals and pharmaceutical proteins. This concept was validated in a side-by-side greenhouse cultivation of Synechocystis wild-type and a phycocyanin-deficient (TLA) strain, grown in 13-L volume, 12-inch-diameter carboys in the greenhouse (Fig. 5a). Rates of cell growth for the TLA strains (0.058 g L−1 d−1) were faster than those for the WT (0.035 g L−1 d−1) by about 66%, consistent with the lab findings by Kirst et al. (2014). In spite of the greater biomass accumulating in the TLA carboys, cultures in the latter can be seen to having a lighter coloration with an apparent greater transmittance of sunlight through them, as compared to the dark blue–green coloration of the wild type (Fig. 5a). Such differences in culture coloration could also be discerned in early-stage growth in the lab (Fig. 5b). These results with TLA cyanobacteria corroborate similar findings with TLA Dunaliella salina (Melis et al. 1999) in which cells with a smaller than wild-type chlorophyll antenna sizes exhibited higher photosynthetic productivities and photon use efficiencies than normally pigmented cells, while displaying greater transmittance of light through a high-density culture.
Comparative growth and biomass accumulation of wild-type and Truncated Light-harvesting Antenna (TLA) strains of Synechocystis 6803 lacking phycocyanin. a 13-L carboys with a 12-inch (30 cm) diameter were inoculated with Synechocystis wild-type and a phycocyanin-deficient (TLA) mutant, followed by growth in BG-11 inorganic nutrients under ambient conditions in the greenhouse. b Early stages of growth for the wild-type and two TLA strains of Synechocystis under artificial illumination in the lab
Biopharmaceutical and other recombinant protein synthesis
The success of synthetic biology approaches with cyanobacteria and other systems requires overexpression of pathway enzymes and proteins of interest in order to attain higher yields and lower costs. For example, yield of the constitutive generation of isoprenoid compounds in cyanobacteria strongly depends on the concentration of the pathway-catalyzing recombinant enzymes (Chaves et al. 2017; Betterle and Melis 2018; Lim et al. 2020). However, heterologous proteins are almost always not well tolerated by the recipient cell and either pellet as inclusion bodies or are degraded by the cellular proteasome. This is a barrier to the useful application of cyanobacteria in synthetic biology, as it has resulted in low steady-state levels of recombinant proteins (Demain and Vaishnav 2009; Surzycki et al. 2009; Tran et al. 2009; Sebesta and Peebles 2020; Coragliotti et al. 2011; Gregory et al. 2013; Jones and Mayfield 2013; Rasala and Mayfield 2015; Baier et al. 2018; Dyo and Purton 2018), often much less than 0.1% of the cell protein content. This drawback limits carbon partitioning toward the heterologous pathway or target protein and has a negative impact on rates and yield of product biosynthesis. Thus, true overexpression of recombinant proteins functioning in heterologous biosynthetic pathways and systems has been a barrier and a problem that we are only now beginning to overcome in this field (Formighieri and Melis 2015; Zhang et al. 2021; Hidalgo Martinez et al. 2022).
In general, there is a need to develop methods that will systematically and reliably over-express eukaryotic, including plant and human, proteins in photosynthetic microorganisms. However, the question of recombinant pathway enzymes or biopharma protein overexpression in photosynthetic microorganisms has been neglected, in spite of its central importance to synthetic biology. The problem is exacerbated because of the frequent assumption in the field that a strong promoter will automatically translate in gene overexpression (Formighieri and Melis 2014), when, in practice, SDS-PAGE and Coomassie stain measurements fail to detect presence of the transgenic protein and only sensitive Western blot analysis (Bentley et al. 2013) can offer evidence of low-level protein presence. Zhang et al. (2021) reported that, in cyanobacteria and presumably other photosynthetic microorganisms, eukaryotic plant- and animal-origin recombinant proteins are unstable and they are degraded by the host cell. The low steady-state level of such proteins, therefore, reflects the balance between the rate of synthesis and degradation, explaining why strong promoters for the expression of transgenes may be necessary but not sufficient for true protein overexpression and accumulation.
“Fusion constructs as protein overexpression vectors” proved to be unparalleled in their ability to cause substantial accumulation of recombinant proteins from plants, animals, and bacteria, as soluble and functional proteins in unicellular cyanobacteria (Formighieri and Melis 2015; 2016; Chaves et al. 2017; Betterle et al. 2020; Hidalgo Martinez et al. 2022; Hidalgo Martinez and Melis 2023). Stable recombinant protein expression reached levels in the range of 10 − 20% of the total cellular protein with this approach. Typically, the target heterologous protein “P” of interest was expressed as a fusion with the abundant CpcB β-subunit (Fig. 6) or as a fusion with the also abundant CpcA α-subunit of phycocyanin (Hidalgo Martinez and Melis 2023), which are placed in the leader sequence position. A broad range of recombinant proteins have been successfully tested, including proteins of the plant isoprenoid biosynthetic pathway, i.e., the isoprene synthase (ISPS), β-phellandrene synthase (PHLS), geranyl diphosphate synthase (GPPS), and geranyllinalool synthase (GLS), the human interferon α-2 protein (IFN), and the bacterial Clostridium tetani tetanus toxin fragment C (TTFC). Such fusion constructs had no adverse effect on cell fitness but they did cause rearrangement in the protein profile of phycocyanin and the phycobilisome in the transgenic cells. This is exemplified in the SDS-PAGE results of Fig. 7 where, in the wild type (WT), the Coomassie-stained dominant proteins are the CpcB 19 kDa β-subunit and CpcA 15 kDa α-subunit of phycocyanin. The latter appear to be missing from the CpcB*P fusion protein transformant (MT). Instead, the dominant protein is now the CpcB*PHLS fusion or CpcB*ISPS fusion, both migrating to about 83 kDa, clearly seen and easily quantifiable from the SDS-PAGE and Coomassie strain analysis of total protein cell extracts (Formighieri and Melis 2015; Chaves et al. 2017; Betterle et al. 2020). An initial hypothesis for such overexpression was that CpcB*P and CpcA*P fusion proteins somehow accumulate in a soluble and stable form in the cytosol of the cyanobacteria, retaining the activity of the trailing heterologous “P” protein of interest. However, work by Hidalgo Martinez and coauthors (Hidalgo Martinez et al. 2022) revealed a substantially different and previously unobvious picture, in which the CpcB*P and CpcA*P proteins assembled as functional (α,β*P)3 or (α*P,β)3 heterohexameric disks (Fig. 8), where α is the CpcA α-subunit, β is the CpcB β-subunit of phycocyanin, and *P denotes the target recombinant protein in fusion with either the native CpcB or CpcA.
Schematic of DNA maps of the cpc operon in wild-type and Synechocystis fusion construct transformants. (Upper) The native cpc operon, as it occurs in wild-type Synechocystis, comprising the cpcB (phycocyanin β-subunit) and cpcA (phycocyanin α-subunit) DNA, as well as DNA encoding for the phycocyanin rod linker CpcC1, CpcC2, and CpcD proteins. This DNA operon configuration and sequence is referred to as the wild type (WT). (Lower). Replacement of the cpcB gene with fusion DNA construct cpcB*P, where P encodes for a protein of interest. The cpcB*P fusion construct is followed by DNA encoding the chloramphenicol (cmR) resistance cassette in an operon configuration. This transgenic DNA configuration enabled substantial accumulation of the recombinant CpcB*P fusion protein
Protein expression analysis of Synechocystis wild-type (WT) and fusion construct transformants (MT) harboring the CpcB*PHLS or CpcB*ISPS encoding recombinant DNA. Total cellular protein extracts were resolved by SDS-PAGE and visualized by Coomassie stain. Two different versions of the fusion construct were used comprising the CpcB*PHLS or CpcB*ISPS, both of which migrate to about 83 kDa, yielding similar results. Note the equivalent amounts of the Rubisco large subunit (RbcL) in wild type and mutant, migrating to about 56 kDa, suggesting about equal loading of WT and MT proteins. Also note the presence of the CmR protein, migrating to about 23 kDa, and the absence of the CpcB protein from the ~ 19 kDa electrophoretic mobility position, as the latter is migrating to ~ 83 kDa in the MT mutants. Sample loading corresponds to 0.25 mg of chlorophyll per lane
Schematic of the heterohexameric structure of recombinant protein fusions with the CpcB β-subunit of phycocyanin. Shown are the (α,β*PHLS)3CpcG1, (α,β*IFN)3CpcG1, and (α,β*TTFC)3CpcG1 heterohexameric complex configurations with the lavender β-phellandrene synthase, (left panel), the human interferon (middle), and the tetanus toxin fragment C (right panel). A similar configuration resulted when these heterologous proteins were fused to the CpcA α-subunit of phycocyanin. The CpcG1 linker protein (denoted by G) occupies the disk center of the respective complexes and serves to functionally link the modified phycocyanin disk to the allophycocyanin core cylinders in Synechocystis. Assembly of the native α,β heterohexameric complex and its functional association with the allophycocyanin core cylinders suggested that the corresponding heterologous fusion proteins localize away from the disk center and are likely placed at the periphery or emanate radially from the (α,β*PHLS)3, (α,β*IFN)3, and (α,β*TTFC)3 disks, thus being exposed to the medium
The work of Hidalgo Martinez et al. (2022; 2023) further showed that the (α,β*P)3 and (α*P,β)3 heterohexameric disks are functionally attached to the Synechocystis allophycocyanin core cylinders, via the native CpcG1 linker protein, and efficiently absorb and transfer excitation energy from the assembled (α,β*P)3 or (α*P,β)3 heterohexameric phycocyanin subunits to the PSII reaction centers, thereby enhancing the rate of charge separation and photochemical electron transfer in the cellular thylakoid membrane. This discovery demonstrated that cyanobacterial cells can tolerate heterologous recombinant proteins, when the latter are in a fusion construct configuration with essential cellular proteins, e.g., phycocyanin. The latter, in this case, is needed by the cell for light absorption and excitation energy transfer to a photosystem, thus enabling the substantial and stable accumulation of the associated transgenic proteins (Hidalgo Martinez and Melis 2023).
Outlook
In recent years, the field of cyanobacterial biotechnology has emerged as a transformative process, receiving considerable attention due to its potential to revolutionize synthetic biology with the generation of sustainable and renewable products. Cyanobacteria are adept at dual roles as photocatalysts and cellular processors, exhibit the remarkable ability to both photosynthesize, and convert inorganic carbon into an array of valuable bio-products. An aspect that sets this technology apart is its carbon-negative footprint, making it a tool in the mitigation of climate change. Moreover, its versatility and easiness to use allows for cost-effective product generation, with applicability spanning across the globe, offering a distributed source of employment and economic growth, particularly benefiting developing countries.
Cyanobacteria cell factories hold promise in several key areas with far-reaching societal impacts. These include the synthesis of chemical compounds essential for the flavor and fragrance industries, as well as providing feedstock for the synthetic chemistry industry. Furthermore, their potential extends to the production of specialty enzymes and proteins, catering to the biopharmaceutical sector’s diverse needs, encompassing diagnostics, antigens, antibodies, growth factors, and more. Additionally, cyanobacteria offer a sustainable alternative to conventional plastics through the production of thermoplastics, like the biodegradable polyhydroxybutyrate (PHB), thus addressing the global plastic pollution crisis.
However, to fully harness the potential of cyanobacterial cell factories, significant research efforts are required. Overcoming challenges such as enhancing the simultaneous overexpression of multiple heterologous enzymes to optimize the redirection of endogenous substrates toward desired products is paramount. Moreover, increasing the yield of specialty enzymes and recombinant biopharmaceutical proteins to levels exceeding the current 10–15% of the total cellular protein content is a critical milestone. Achieving these advances will facilitate the seamless transition of cyanobacterial biotechnology from the research laboratory to the private sector, unlocking a sustainable future for both industry and the environment.
Data availability
All data and material are available from the corresponding author.
Abbreviations
- AP:
-
Allophycocyanin
- PC:
-
Phycocyanin
- cpcA :
-
Gene encoding the PC α-subunit
- cpcB :
-
Gene encoding the PC β-subunit
- cpcG1 :
-
Gene encoding the proximal PC-AP linker protein;
- Synechocystis :
-
Synechocystis sp. PCC 6803
- PBS:
-
Phycobilisome
- PSII:
-
Photosystem II
- Chl:
-
Chlorophyll
- WT:
-
Wild type
References
Albers SC, Gallegos VA, Peebles CAM (2015) Engineering of genetic control tools in Synechocystis sp. PCC 6803 using rational design techniques. J Biotechnol 216:36–46. https://doi.org/10.1016/j.jbiotec.2015.09.042
Bäck J, Aaltonen H, Hellén H, Kajos MJ, Patokoski J, Taipale R, Pumpanen J, Heinonsalo J (2010) Variable emissions of microbial volatile organic compounds (MVOCs) from root-associated fungi isolated from Scots pine. Atmos Environ 44:3651–3659. https://doi.org/10.1016/j.atmosenv.2010.06.042
Baier T, Kros D, Feiner RC, Lauersen KJ, Müller KM, Kruse O (2018) Engineered fusion proteins for efficient protein secretion and purification of a human growth factor from the green microalga Chlamydomonas reinhardtii. ACS Synth Biol 7:2547–2557. https://doi.org/10.1021/acssynbio.8b00226
Barone GD, Cernava T, Ullmann J, Liu J, Lio E, Germann AT, Nakielski A, Russo DA, Chavkin T, Knufmann K, Tripodi F, Coccetti P, Secundo F, Fu P, Pfleger B, Axmann IM, Lindblad P (2023) Recent developments in the production and utilization of photosynthetic microorganisms for food applications. Heliyon 9(4):e14708. https://doi.org/10.1016/j.heliyon.2023.e14708
Behler J, Vijay D, Hess WR, Akhtar MK (2018) CRISPR-based technologies for metabolic engineering in cyanobacteria. Trends Biotechnol 36(10):996–1010. https://doi.org/10.1016/j.tibtech.2018.05.011
Bentley FK, Melis A (2012) Diffusion-based process for carbon dioxide uptake and isoprene emission in gaseous/aqueous two-phase photobioreactors by photosynthetic microorganisms. Biotech Bioeng 109:100–109. https://doi.org/10.1002/bit.23298
Bentley FK, García-Cerdán JG, Chen H-C, Melis A (2013) Paradigm of monoterpene (β-phellandrene) hydrocarbons production via photosynthesis in cyanobacteria. BioEnergy Res 6:917–929. https://doi.org/10.1007/s12155-013-9325-4
Bentley FK, Zurbriggen A, Melis A (2014) Heterologous expression of the mevalonic acid pathway in cyanobacteria enhances endogenous carbon partitioning to isoprene. Mol Plant 7:71–86. https://doi.org/10.1093/mp/sst134
Berla BM, Saha R, Immethun CM, Maranas CD, Moon TS, Pakrasi HB (2013) Synthetic biology of cyanobacteria: unique challenges and opportunities. Front Microbiol 4:246. https://doi.org/10.3389/fmicb.2013.00246
Betterle N, Melis A (2018) Heterologous leader sequences in fusion constructs enhance expression of geranyl diphosphate synthase and yield of β-phellandrene production in cyanobacteria (Synechocystis). ACS Synth Biol 7(3):912–921. https://doi.org/10.1021/acssynbio.7b00431
Betterle N, Melis A (2019) Photosynthetic generation of heterologous terpenoids in cyanobacteria. Biotechnol Bioeng 116:2041–2051. https://doi.org/10.1002/bit.26988
Betterle N, Hidalgo Martinez D, Melis A (2020) Cyanobacterial production of biopharmaceutical and biotherapeutic proteins. Front Plant Sci 11:237. https://doi.org/10.3389/fpls.2020.00237
Chaves JE, Melis A (2018a) Biotechnology of cyanobacterial isoprene production. Appl Microbiol Biotechnol 102(15):6451–6458. https://doi.org/10.1007/s00253-018-9093-3
Chaves JE, Melis A (2018b) Engineering isoprene synthesis in cyanobacteria. FEBS Lett 592(12):2059–2069. https://doi.org/10.1002/1873-3468.13052
Chaves JE, Kirst H, Melis A (2015) Isoprene production in Synechocystis under alkaline and saline growth conditions. J Appl Phycol 27:1089–1097. https://doi.org/10.1007/s10811-014-0395-2
Chaves JE, Rueda Romero P, Kirst H, Melis A (2016) Role of isopentenyl-diphosphate isomerase in heterologous cyanobacterial (Synechocystis) isoprene production. Photosynth Res 130:517–527. https://doi.org/10.1007/s11120-016-0293-3
Chaves JE, Rueda-Romero P, Kirst H, Melis A (2017) Engineering isoprene synthase expression and activity in cyanobacteria. ACS Synth Biol 6(12):2281–2292. https://doi.org/10.1021/acssynbio.7b00214
Cheng J, Zhang K, Hou Y (2023) The current situations and limitations of genetic engineering in cyanobacteria: a mini review. Mol Biol Rep 50:5481–5487. https://doi.org/10.1007/s11033-023-08456-8
Choi SY, Wang JY, Kwak HS, Lee SM, Um Y, Kim Y, Sim SJ, Choi JI, Woo HM (2017) Improvement of squalene production from CO2 in Synechococcus elongatus PCC 7942 by metabolic engineering and scalable production in a photobioreactor. ACS Synth Biol 6(7):1289–1295. https://doi.org/10.1021/acssynbio.7b00083
Coragliotti AT, Beligni MV, Franklin SE, Mayfield SP (2011) Molecular factors affecting the accumulation of recombinant proteins in the Chlamydomonas reinhardtii chloroplast. Mol Biotechnol 48:60–75. https://doi.org/10.1007/s12033-010-9348-4
Davies FK, Work VH, Beliaev AS, Posewitz, (2014) Engineering limonene and bisabolene production in wild type and a glycogen-deficient mutant of Synechococcus sp. PCC 7002. Front Bioeng Biotechnol 2:21. https://doi.org/10.3389/fbioe.2014.00021
Davies FK, Jinkerson RE, Posewitz MC (2015) Toward a photosynthetic microbial platform for terpenoid engineering. Photosynth Res 123:265–284. https://doi.org/10.1007/s11120-014-9979-6
Demain AL, Vaishnav P (2009) Production of recombinant proteins by microbes and higher organisms. Biotechnol Adv 27:297–306. https://doi.org/10.1016/j.biotechadv.2009.01.008
Diao J, Song X, Zhang L, Cui J, Chen L, Zhang W (2020) Tailoring cyanobacteria as a new platform for highly efficient synthesis of astaxanthin. Metab Eng 61:275–287. https://doi.org/10.1016/j.ymben.2020.07.003
Dienst D, Wichmann J, Mantovani O, Rodrigues JS, Lindberg P (2020) High density cultivation for efficient sesquiterpenoid biosynthesis in Synechocystis sp. PCC 6803. Sci Rep 10:5932. https://doi.org/10.1038/s41598-020-62681-w
Ducat DC, Way JC, Silver PA (2011) Engineering cyanobacteria to generate high-value products. Trends Biotechnol 29(2):95–103. https://doi.org/10.1016/j.tibtech.2010.12.003
Dyo YM, Purton S (2018) The algal chloroplast as a synthetic biology platform for production of therapeutic proteins. Microbiology 164:113–121. https://doi.org/10.1099/mic.0.000599
Englund E, Pattanaik B, Ubhayasekera SJK, Stensjö K, Bergquist J, Lindberg P (2014) Production of squalene in Synechocystis sp. PCC 6803. PLoS ONE 9(3):e90270. https://doi.org/10.1371/journal.pone.0090270
Englund E, Shabestary K, Hudson EP, Lindberg P (2018) Systematic overexpression study to find target enzymes enhancing production of terpenes in Synechocystis PCC 6803, using isoprene as a model compound. Metab Eng 49:164–177. https://doi.org/10.1016/j.ymben.2018.07.004
Fall R, Copley SD (2000) Bacterial sources and sinks of isoprene, a reactive atmospheric hydrocarbon. Environ Microbiol 2:123–130. https://doi.org/10.1046/j.1462-2920.2000.00095.x
Formighieri C, Melis A (2014) Regulation of β-phellandrene synthase gene expression, recombinant protein accumulation, and monoterpene hydrocarbons production in Synechocystis transformants. Planta 240:309–324. https://doi.org/10.1007/s00425-014-2080-8
Formighieri C, Melis A (2015) A phycocyanin*phellandrene synthase fusion enhances recombinant protein expression and β-phellandrene (monoterpene) hydrocarbons production in Synechocystis (cyanobacteria). Metab Eng 32:116–124. https://doi.org/10.1016/j.ymben.2015.09.010
Formighieri C, Melis A (2016) Sustainable heterologous production of terpene hydrocarbons in cyanobacteria. Photosynth Res 130:123–135. https://doi.org/10.1007/s11120-016-0233-2
Formighieri C, Melis A (2017) Heterologous synthesis of geranyllinalool, a diterpenol plant product, in the cyanobacterium Synechocystis. Appl Microbiol Biotechnol 101:2791–2800. https://doi.org/10.1007/s00253-016-8081-8
Gao X, Gao F, Liu D, Zhang H, Nie X, Yang C (2016) Engineering the methylerythritol phosphate pathway in cyanobacteria for photosynthetic isoprene production from CO2. Energy Environ Sci 9(4):1400–1411. https://doi.org/10.1039/C5EE03102H
Gomes MRF, Schuh RS, Jacques ALB, Augustin OA, Bordignon SAL, Dias DO, Kelmann RG, Koester LS, Gehring MP, Morrone FB, Campos MM, Limberger RP (2013) Citotoxic activity evaluation of essential oils and nanoemulsions of Drimys angustifolia and D. brasiliensis on human glioblastoma (U-138 MG) and human bladder carcinoma (T24) cell lines in vitro. Rev Bras 23:259–267. https://doi.org/10.1590/S0102-695X2012005000136
Gordon GC, Korosh TC, Cameron JC, Markley AL, Begemann MB, Pfleger BF (2016) CRISPR interference as a titratable, trans-acting regulatory tool for metabolic engineering in the cyanobacterium Synechococcus sp. strain PCC 7002. Metab Eng 38:170–179. https://doi.org/10.1016/j.ymben.2016.07.007
Gregory JA, Topol AB, Doerner DZ, Mayfield SP (2013) Alga-produced cholera toxin-Pfs25 fusion proteins as oral vaccines. Appl Environ Microbiol 79:3917–3925. https://doi.org/10.1128/AEM.00714-13
Guenther A, Karl T, Harley P, Wiedinmyer C, Palmer PI, Geron C (2006) Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos Chem Phys 6:3181–3210. https://doi.org/10.5194/acp-6-3181-2006
Harrison SP, Morfopoulos C, Dani KG, Prentice IC, Arneth A, Atwell BJ, Barkley MP, Leishman MR, Loreto F, Medlyn BE, Niinemets Ü, Possell M, Peñuelas J, Wright IJ (2013) Volatile isoprenoid emissions from plastid to planet. New Phytol 197:49–57. https://doi.org/10.1111/nph.12021
Hidalgo Martinez D, Melis A (2023) Cyanobacterial phycobilisomes as a platform for the stable production of heterologous enzymes and other proteins. Metab Eng 77:174–187. https://doi.org/10.1016/j.ymben.2023.04.002
Hidalgo Martinez D, Betterle N, Melis A (2022) Phycocyanin fusion constructs for heterologous protein expression accumulate as functional heterohexameric complexes in cyanobacteria. ACS Synth Biol 11(3):1152–1166. https://doi.org/10.1021/acssynbio.1c00449
Hu J, Wang D, Chen H, Wang Q (2023) Advances in genetic engineering in improving photosynthesis and microalgal productivity. Int J Mol Sci 24(3):1898. https://doi.org/10.3390/ijms24031898
Jaiswal D, Sengupta A, Sohoni S, Sengupta S, Phadnavis AG, Pakrasi HB, Wangikar PP (2018) Genome features and biochemical characteristics of a robust, fast growing and naturally transformable cyanobacterium Synechococcus elongatus PCC 11801 Isolated from India. Sci Rep 8:16632. https://doi.org/10.1038/s41598-018-34872-z
Jansson C (2012) Metabolic engineering of cyanobacteria for direct conversion of CO2 to hydrocarbon biofuels. Prog Bot 73:81–93. https://doi.org/10.1007/978-3-642-22746-2_3
Jones CS, Mayfield SP (2013) Steps toward a globally available malaria vaccine: harnessing the potential of algae for future low-cost vaccines. Bioengineered 4:164–167. https://doi.org/10.4161/bioe.22577
Jongedijk E, Cankar K, Buchhaupt M, Schrader J, Bouwmeester H, Beekwilder J (2016) Biotechnological production of limonene in microorganisms. Appl Microbiol Biotechnol 100:2927–2938. https://doi.org/10.1007/s00253-016-7337-7
Kant G, Pandey A, Shekhar H, Srivastava S (2023) Enhanced bio-synthesis of isoprene via modifying mevalonate and methylerythritol phosphate pathways for industrial application: a review. Process Biochem 130:256–271. https://doi.org/10.1016/j.procbio.2023.04.021
Kirk JTO (1994) Light and Photosynthesis in Aquatic Ecosystems, 2nd edn. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9780511623370
Kirst H, Formighieri C, Melis A (2014) Maximizing photosynthetic efficiency and culture productivity in cyanobacteria upon minimizing the phycobilisome light-harvesting antenna size. Biochim Biophys Acta—Bioenerg 10:1653–1664. https://doi.org/10.1016/j.bbabio.2014.07.009
Kirst H, Gabilly ST, Niyogi KK, Lemaux PG, Melis A (2017) Photosynthetic antenna engineering to improve crop yields. Planta 245:1009–1020. https://doi.org/10.1007/s00425-017-2659-y
Kirst H, Shen YX, Vamvaka E, Betterle N, Xu DM, Warek U, Strickland JA, Melis A (2018) Downregulation of the CpSRP43 gene expression confers a truncated light-harvesting antenna (TLA) and enhances biomass and leaf-to-stem ratio in Nicotiana tabacum canopies. Planta 248:139–154. https://doi.org/10.1007/s00425-018-2889-7
Ko SC, Lee HJ, Choi SY, Choi JI, Woo HM (2019) Bio-solar cell factories for photosynthetic isoprenoids production. Planta 249:181–193. https://doi.org/10.1007/s00425-018-2969-8
Kuzma J, Fall R (1993) Leaf isoprene emission rate is dependent on leaf development and the level of isoprene synthase. Plant Physiol 101:435–440. https://doi.org/10.1104/pp.101.2.435
Lee HJ, Lee J, Lee SM, Um Y, Kim Y, Sim SJ, Choi JI, Woo HM (2017) Direct conversion of CO2 to α-farnesene using metabolically engineered Synechococcus elongatus PCC 7942. J Agric Food Chem 65(48):10424–10428. https://doi.org/10.1021/acs.jafc.7b03625
Li M, Long B, Dai SY, Golden JW, Wang X (2022) Altered carbon partitioning enhances CO2 to terpene conversion in cyanobacteria. BioDesign Res. https://doi.org/10.34133/2022/9897425
Lim H, Park J, Woo HM (2020) Overexpression of the key enzymes in the methylerythritol 4-phosphate pathway in Corynebacterium glutamicum for improving farnesyl diphosphate-derived terpene production. J Agric Food Chem 68:10780–10786. https://doi.org/10.1021/acs.jafc.0c04307
Lin P-C, Pakrasi HB (2019) Engineering cyanobacteria for production of terpenoids. Planta 249:145–154. https://doi.org/10.1007/s00425-018-3047-y
Lin P-C, Zhang F, Pakrasi HB (2021) Enhanced limonene production in a fast-growing cyanobacterium through combinatorial metabolic engineering. Met Eng Comm 12:e00164. https://doi.org/10.1016/j.mec.2021.e00164
Lindberg P, Park S, Melis A (2010) Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metabol Engin 12:70–79. https://doi.org/10.1016/j.ymben.2009.10.001
Liu Y, Cui Y, Chen J, Qin S, Chen G (2019) Metabolic engineering of Synechocystis sp. PCC6803 to produce astaxanthin. Algal Research 44:101679. https://doi.org/10.1016/j.algal.2019.101679
Liu D, Johnson VM, Pakrasi HB (2020) A reversibly induced CRISPRi system targeting photosystem II in the cyanobacterium Synechocystis sp. PCC 6803. ACS Synth Biol 9:1441–1449. https://doi.org/10.1021/acssynbio.0c00106
Loreto F, Sharkey TD (1990) A gas-exchange study of photosynthesis and isoprene emission in Quercus rubra L. Planta 182:523–531. https://doi.org/10.1007/BF02341027
Machado IMP, Atsumi S (2012) Cyanobacterial biofuel production. J Biotechnol 162:50–56. https://doi.org/10.1016/j.jbiotec.2012.03.005
Melis A (2009) Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency. Plant Sci 177:272–280. https://doi.org/10.1016/j.plantsci.2009.06.005
Melis A (2012) Photosynthesis-to-fuels: from sunlight to hydrogen, isoprene, and botryococcene production. Energy Environ Sci 5(2):5531–5539. https://doi.org/10.1039/c1ee02514g
Melis A (2013) Carbon partitioning in photosynthesis. Curr Opin Chem Biol 17:453–456. https://doi.org/10.1016/j.cbpa.2013.03.010
Melis A, Neidhardt J, Benemann JR (1999) Dunaliella salina (Chlorophyta) with small chlorophyll antenna sizes exhibit higher photosynthetic productivities and photon use efficiencies than normally pigmented cells. J Appl Phycol 10:515–525. https://doi.org/10.1023/A:1008076231267
Menin B, Lami A, Musazzi S, Petrova AA, Santabarbara S, Casazza AP (2019) A comparison of constitutive and inducible non-endogenous keto-carotenoids biosynthesis in Synechocystis sp. PCC 6803. Microorganisms. https://doi.org/10.3390/microorganisms7110501
Mills LA, Moreno-Cabezuelo JÁ, Włodarczyk A, Victoria AJ, Mejías R, Nenninger A, Moxon S, Bombelli P, Selão TT, McCormick AJ, Lea-Smith DJ (2022) Development of a biotechnology platform for the fast-growing cyanobacterium Synechococcus sp. PCC 11901. Biomolecules 12(7):872. https://doi.org/10.3390/biom12070872
Mochalski P, King J, Mayhew CA, Unterkofler K (2023) A review on isoprene in human breath. J Breath Res 17:037101. https://doi.org/10.1088/1752-7163/acc964
Morais ARC, Dworakowska S, Reis A, Gouveia L, Matos CT, Bogdał D, Bogel-Łukasik, (2015) Chemical and biological-based isoprene production: green metrics. Catal Today 239:38–43. https://doi.org/10.1016/j.cattod.2014.05.033
Novoveská L, Nielsen SL, Eroldoğan OT, Haznedaroglu BZ, Rinkevich B, Fazi S, Robbens J, Vasquez M, Einarsson H (2023) Overview and challenges of large-scale cultivation of photosynthetic microalgae and cyanobacteria. Mar Drugs 21(8):445. https://doi.org/10.3390/md21080445
Pandey A, Kumar S, Srivastava S (2022) Algal biomass harvesting for biofuel production, in Algal Biofuel. CRC Press, London, pp 23–65. https://doi.org/10.1201/9781003363231-2
Patel VK, Das A, Kumari R, Kajla S (2023) Recent progress and challenges in CRISPR-Cas9 engineered algae and cyanobacteria. Algal Res 71:103068. https://doi.org/10.1016/j.algal.2023.103068
Pattanaik B, Lindberg P (2015) Terpenoids and their biosynthesis in cyanobacteria. Life 5:269–293. https://doi.org/10.3390/life5010269
Pattanaik B, Englund E, Nolte N, Lindberg P (2020) Introduction of a green algal squalene synthase enhances squalene accumulation in a strain of Synechocystis sp. PCC 6803. Metab Eng Commun 10:e00125. https://doi.org/10.1016/j.mec.2020.e00125
Polle JEW, Kanakagiri S, Melis A (2003) tla1, a DNA insertional transformant of the green alga Chlamydomonas reinhardtii with a truncated light-harvesting chlorophyll antenna size. Planta 217:49–59. https://doi.org/10.1007/s00425-002-0968-1
Rasala BA, Mayfield SP (2015) Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses. Photosynth Res 123:227–239. https://doi.org/10.1007/s11120-014-9994-7
Rautela A, Kumar S (2022) Engineering plant family TPS into cyanobacterial host for terpenoids production. Plant Cell Rep 41:1791–1803. https://doi.org/10.1007/s00299-022-02892-9
Reinsvold RE, Jinkerson RE, Radakovits R, Posewitz MC, Basu C (2011) The production of the sesquiterpene β-caryophyllene in a transgenic strain of the cyanobacterium Synechocystis. J. Plant Physiol 168:848–852. https://doi.org/10.1016/j.jplph.2010.11.006
Rodrigues JS, Lindberg P (2021) Metabolic engineering of Synechocystis sp. PCC 6803 for improved bisabolene production. Metabolic Eng Commun 12:e00159. https://doi.org/10.1016/j.mec.2020.e00159
Rodrigues JS, Lindberg P (2021) Engineering cyanobacteria as host organisms for production of terpenes and terpenoids. In: Nielsen J, Lee S, Stephanopoulos G, Hudson P (eds) Cyanobacteria Biotechnology. Hoboken, Wiley on Line Library, pp 267–299. https://doi.org/10.1002/9783527824908
Rodrigues JS, Kovács L, Lukeš M, Höper R, Steuer R, Červený J, Lindberg P, Zavřel T (2023) Characterizing isoprene production in cyanobacteria—Insights into the effects of light, temperature, and isoprene on Synechocystis sp. PCC 6803. Bioresour Technol 380:129068. https://doi.org/10.1016/j.biortech.2023.129068
Santos-Merino M, Singh AK, Ducat DC (2019) New applications of synthetic biology tools for cyanobacterial metabolic engineering. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2019.00033
Satta A, Esquirol L, Ebert BE (2023) Current metabolic engineering strategies for photosynthetic bioproduction in cyanobacteria. Microorganisms 11(2):455. https://doi.org/10.3390/microorganisms11020455
Savakis P, Angermayr HKJ (2013) Synthesis of 2,3-butanediol by Synechocystis sp. PCC6803 via heterologous expression of a catabolic pathway from lactic acid- and enterobacteria. Metab Eng 20:121–130. https://doi.org/10.1016/j.ymben.2013.09.008
Savakis P, Hellingwerf KJ (2015) Engineering cyanobacteria for direct biofuel production from CO2. Curr Opin Biotechnol 33:8–14. https://doi.org/10.1016/j.copbio.2014.09.007
Sebesta J, Peebles CAM (2020) Improving heterologous protein expression in Synechocystis sp. PCC 6803 for alpha-bisabolene production. Metabolic Eng Commun 10:e00117. https://doi.org/10.1016/j.mec.2019.e00117
Sengupta A, Liu D, Pakrasi HB (2022) CRISPR-Cas mediated genome engineering of cyanobacteria. Methods Enzymol 676:403–432. https://doi.org/10.1016/bs.mie.2022.07.023
Sethia P, Ahuja M, Rangaswamy V (2019) Metabolic engineering of microorganisms to produce isoprene. J Microbial & Biochem Technology 11(4):419. https://doi.org/10.4172/1948-5948.1000419
Sharkey TD, Yeh S, Wiberley AE, Falbel TG, Gong D, Fernandez DE (2005) Evolution of the isoprene biosynthetic pathway in kudzu. Plant Physiol 137(2):700–712. https://doi.org/10.1104/pp.104.054445
Sharkey TD, Wiberley AE, Donohue AR (2007) Isoprene emission from plants: why and how. Ann Bot 101:5–18. https://doi.org/10.1093/aob/mcm240
Surzycki R, Greenham K, Kitayama K, Dibal F, Wagner R, Rochaix JD, Ajam T, Surzycki S (2009) Factors effecting expression of vaccines in microalgae. Biologicals 37:133–138. https://doi.org/10.1016/j.biologicals.2009.02.005
Tran M, Zhou B, Pettersson PL, Gonzalez MJ, Mayfield SP (2009) Synthesis and assembly of a full-length human monoclonal antibody in algal chloroplasts. Biotechnol Bioeng 104:663–673. https://doi.org/10.1002/bit.22446
Ungerer J, Tao L, Davis M, Ghirardi ML, Maness P-C, Yu J (2012) Sustained photosynthetic conversion of CO2 to ethylene in recombinant cyanobacterium Synechocystis 6803. Energy Environ Sci 5:8998–9006. https://doi.org/10.1039/C2EE22555G
Velikova V, Loreto F (2005) On the relationship between isoprene emission and thermotolerance in Phragmites australis leaves exposed to high temperatures and during the recovery from a heat stress. Plant, Cell Environ 28:318–327. https://doi.org/10.1111/j.1365-3040.2004.01314.x
Wang F, Yuanyuan Gao Y, Yang G (2020) Recent advances in synthetic biology of cyanobacteria for improved chemicals production. Bioengineered 11:1208–1220. https://doi.org/10.1080/21655979.2020.1837458
Wendt KE, Walker P, Sengupta A, Ungerer J, Pakrasi HB (2022) Engineering natural competence into the fast-growing cyanobacterium Synechococcus elongatus strain UTEX 2973. Appl Environ Microbiol 88(1):e0188221. https://doi.org/10.1128/AEM.01882-21
Whited GM, Feher FJ, Benko DA, Cervin MA, Chotani GK, McAuliffe JC, LaDuca RJ, Ben-Shoshan EA, Sanford KJ (2010) Technology update: development of a gas-phase bioprocess for isoprene-monomer production using metabolic pathway engineering. Ind Biotechnol 6:152–163. https://doi.org/10.1089/ind.2010.6.152
Włodarczyk A, Selão TT, Norling B, Nixon PJ (2020) Newly discovered Synechococcus sp. PCC 11901 is a robust cyanobacterial strain for high biomass production. Commun Biol 3:215. https://doi.org/10.1038/s42003-020-0910-8
Woo HM (2017) Solar-to-chemical and solar-to-fuel production from CO2 by metabolically engineered microorganisms. Curr Opin Biotechnol 45:1–7. https://doi.org/10.1016/j.copbio.2016.11.017
Xie M, Wang W, Zhang W, Chen L, Lu X (2017) Versatility of hydrocarbon production in cyanobacteria. Appl Microbiol Biotechnol 101:905–919. https://doi.org/10.1007/s00253-016-8064-9
Yadav I, Rautela A, Gangwar A, Kesari V, Padhi AK, Kumar S (2023) Geranyl diphosphate dynthase (CrtE) inhibition using alendronate enhances isoprene production in recombinant Synechococcus elongatus UTEX 2973: A Step towards Isoprene Biorefinery. Fermentation 9(3):217. https://doi.org/10.3390/fermentation9030217
Yahya RZ, Wellman GB, Overmans S, Lauersen KJ (2023) Engineered production of isoprene from the model green microalga Chlamydomonas reinhardtii. Metab Eng Comm 16:e00221. https://doi.org/10.1016/j.mec.2023.e00221
Yao L, Cengic I, Anfelt J, Hudson EP (2016) Multiple gene repression in cyanobacteria using CRISPRi. ACS Synth Biol 5(3):207–212. https://doi.org/10.1021/acssynbio.5b00264
Yu J, Liberton M, Cliften PF, Head RD, Jacobs JM, Smith RD, Koppenaal DW, Brand JJ, Pakrasi HB (2015) Synechococcus elongatus UTEX 2973, a fast-growing cyanobacterial chassis for biosynthesis using light and CO2. Sci Rep 5:8132. https://doi.org/10.1038/srep08132
Zhang XN, Betterle N, Hidalgo Martinez D, Melis A (2021) Recombinant protein stability in cyanobacteria. ACS Synth Biol 10(4):810–825. https://doi.org/10.1021/acssynbio.0c00610
Zhou J, Yang F, Zhang F, Meng H, Zhang Y, Li Y (2021) Impairing photorespiration increases photosynthetic conversion of CO2 to isoprene in engineered cyanobacteria. Bioresour Bioprocess 8(1):1–13. https://doi.org/10.1186/s40643-021-00398-y
Author information
Authors and Affiliations
Contributions
AM, DAHM and NB conducted work reported in this review. AM wrote the manuscript, edited by DAHM, and NB.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Melis, A., Hidalgo Martinez, D.A. & Betterle, N. Perspectives of cyanobacterial cell factories. Photosynth Res (2023). https://doi.org/10.1007/s11120-023-01056-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11120-023-01056-4