Abstract
Terpenoids are synthesized naturally by plants as secondary metabolites, and are diverse and complex in structure with multiple applications in bioenergy, food, cosmetics, and medicine. This makes the production of terpenoids such as isoprene, β-phellandrene, farnesene, amorphadiene, and squalene valuable, owing to which their industrial demand cannot be fulfilled exclusively by plant sources. They are synthesized via the Methylerythritol phosphate pathway (MEP) and the Mevalonate pathway (MVA), both existing in plants. The advent of genetic engineering and the latest accomplishments in synthetic biology and metabolic engineering allow microbial synthesis of terpenoids. Cyanobacteria manifest to be the promising hosts for this, utilizing sunlight and CO2. Cyanobacteria possess MEP pathway to generate precursors for terpenoid synthesis. The terpenoid synthesis can be amplified by overexpressing the MEP pathway and engineering MVA pathway genes. According to the desired terpenoid, terpene synthases unique to the plant kingdom must be incorporated in cyanobacteria. Engineering an organism to be used as a cell factory comes with drawbacks such as hampered cell growth and disturbance in metabolic flux. This review set forth a comparison between MEP and MVA pathways, strategies to overexpress these pathways with their challenges.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Terpenoids, also called isoprenoids or terpenes, are the diverse and largest class of organic compounds known in nature. Plants substantially synthesize them as secondary metabolites with repeating C5 isoprenoid units. Other living organisms such as bacteria (Reddy et al. 2020; Rabe et al. 2016), insects (Leonhardt et al. 2010; Darragh et al. 2021), and fungi (Jakubczyk and Dussart 2020) also produce terpenoids. Over 80,000 of them have been identified and characterized so far (Pemberton et al. 2017). Such multitudinous molecules have an extensive range of applications too, both biologically and commercially. Photosynthetic organisms rely on terpenoids as they play an indispensable role in photosynthesis (chlorophylls, carotenoids, rhodopsin). Apart from photosynthesis, they show multifunctional applications in the electron transport chain (plastoquinone), respiration (ubiquinone), membrane stability (sterols), hormones (brassinosteroid phytohormones, gibberellic acid) (Moses et al. 2013). Plants’ essential oil comprises volatile terpenoids, which give them their characterized fragrances. Volatile terpenoids exude from the plant’s root, stem, leaf, and fruits but chiefly by flowers. Volatile floral terpenoids prompt pollination (entomophily, ornithophily, chiropterophily) (Abbas et al. 2017). Recently, the role of floral scent in Collinsia heterophylla (Common name: Chinese Houses, Order: Lamiales, Family: Plantaginaceae) flowers was identified to entice bees for pollination (Larsson et al. 2021). Furthermore, these volatile terpenoids act as defensive and anti-stress compounds against biotic (insects, herbivores) and abiotic stress (temperature, light) (Block et al. 2019; Zhang et al. 2019; Gongora et al. 2020). Occasionally in tea plants such as Oriental beauty, the distinctive aroma and flavor is due to the terpenoids release against attack by tea green leafhopper (Empoasca (Matsumurasca) onukii Matsuda) (Liu et al. 2021).
Terpenoids have ample commercial applications in therapeutics, cosmetics, flavoring, fragrances, agrochemicals, and disinfectants (Ajikumar et al. 2008). Artemisinin, a valuable antimalarial drug, is synthesized by sweet wormwood (Artemisia annua L.) (Lopes et al. 2020). Long agricultural lag, fluctuations in abiotic conditions lead to variation in cost and availability of artemisinin. Since the artemisinin and artemisinin combined therapy (ACT) drugs are estimated to intumesce with the combined annual growth rate of 8.5% from 2017 to 2025 with a market size of $363.8 million in 2017, biotechnological production is the pressing priority (https://www.grandviewresearch.com/industry-analysis/artemisinin-combination-therapy-act-market). Saccharomyces cerevisiae was engineered to produce amorphadiene, a precursor for artemisinin (Westfall et al. 2012). Aside from being an antimalarial drug, it also aids in renal and respiratory diseases, reviewed by Liu et al. 2018, Xia et al. 2020, and Cheong et al. 2020. Another multibillion-dollar drug produced from Taxus brevifolia (Common name: Pacific yew tree, Class: Pinopsida, Order: Pinales, Family: Taxaceae) is Taxol (Paclitaxel). Taxol is a chemotherapeutic drug approved by Food and Drug Administration which stabilizes microtubule assembly and inhibits cell division (Weaver 2014). To rely on natural sources for taxol extraction is not eco-friendly as the yield from the yew tree’s bark is 0.001–0.005% only, requiring chopping four mature trees for treating a single patient (giving 2 g dose) (El-Sayed et al. 2019). The complexity of the taxol's structure and chemical synthesis makes its synthetic production unfavorable. Therefore, concepts of metabolic engineering help in the semisynthetic production of taxol through microorganisms to cope with these barriers (Abdallah et al. 2019). As individuals are inclining toward nature identical flavors and fragrances, their market share was worth $ 30 million in 2017 (Kutyna and Borneman 2018). Terpenes have characteristic aroma and flavors, such as vanillin (from pods of Vanilla planifolia), widely used prominently as a vanilla flavoring substance in bakeries. Similarly, raspberry ketone (from Rubus idaeus), cinnamaldehyde (from Cinnamomum zeylanicum), limonene (from citrus fruits) are to name some (Luziatelli et al. 2019; Wang et al. 2019a; He et al. 2019; Wu et al. 2019). Tetali enlightened the role of terpenes in pharmaceuticals, fragrance, flavors, and biofuels in his review (Tetali 2019). Terpenes are proven to be a promising substitute (biofuel) for petroleum-based fuels. Pahima et al. (2019), through computational studies (density functional theory and ab initio quantum chemistry methods), showed that terpenes have all the entailing characteristics of a biofuel. They reckoned enthalpy of combustion, enthalpy of vaporization, enthalpy of formation, cetane number, boiling point, and vapor pressure of various terpenes, namely, pinene, carene, limonene, terpinene, bisabolene, farnesene, and sabinene.
The constant increase in the extraction of value-added products from plants and animals negatively impacts the environment. A sustainable host is required to produce these products that do not compete with food production and proliferate rapidly to meet the demand. One such propitious host is cyanobacteria. Cyanobacteria fascinate researchers as they are photoautotrophic, i.e., they require sunlight and CO2 as an energy source. Apart from this, C, P, S, N, K, and Fe source is needed, usually provided by BG-11 media in the laboratory (Pattharaprachayakul et al. 2019). They can also be cultivated in wastewater and help in wastewater management (Rueda et al. 2020). They are about 3.5 billion years old and considered to be living fossils (Schopf and Packer 1987). Since they are the first oxygen-producing organisms, plants originated from them (Rai et al 2021). It is also considered that the chloroplast inside the plant’s cell is the cyanobacterium living inside it, a typical case of endosymbiosis (Ishikawa and Kawai-Yamada 2019). Methylerythritol phosphate pathway (MEP), which occurs in cyanobacteria, is possessed by chloroplast in plants, giving evidence of this endosymbiotic theory. In addition to this, cyanobacteria own plants like the C2 glycolate cycle (Eisenhut et al 2006).
Commercially, value-added products are predominantly synthesized by Escherichia coli and yeast. Being heterotrophic, they require a carbon source (sugar) for their growth. Shoot up in the production cost of the sugar, it is feasible to use cyanobacteria for the direct production of products from CO2 (Cheng et al. 2019). Recent advances in the genetic toolboxes for synthetic biology of cyanobacteria expressing heterologous genes became attainable (Sun et al. 2018; Sengupta et al. 2018). Sundry tools like CRISPR/Cas9, promoters, ribosome binding site (RBS), riboswitches were used in hosts like Synechococcus elongatus PCC 7942, Synechocystis sp. PCC 6803, and Synechococcus elongatus UTEX 2973 (Yadav et al. 2021; Pattharaprachayakul et al. 2020; Werner et al. 2018; Liu and Pakrasi 2018; Chi et al. 2019; Yu et al. 2015). This review aims at apprising photosynthetic terpenoid production in cyanobacteria by engineering native or foreign gene(s). It also delineates pathways for terpenoid production, approaches to optimize them, and challenges faced.
MEP vs. MVA: upstream and downstream module of terpenoid production
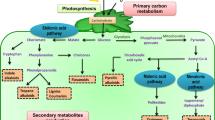
Though terpenoids seem to show diversity in range and their applications, they all are initiated from 5-carbon moiety isopentenyl pyrophosphate (IPP) and its isomeric conformation dimethylallyl pyrophosphate (DMAPP). Isoprene (C5H8) is the most basic terpenoid. In accordance with the “isoprene rule and biogenesis of terpenic compound,” step-by-step addition of DMAPP to IPP units leads to longer chain terpenoid (Ruzicka 1953). This indicates that there is a difference of five-carbon isoprene units in terpenes and are classified based on that: hemiterpenoids (C5), monoterpenoids (C10), sesquiterpenoids (C15), diterpenoids (C20), triterpenoids (C30), tetraterpenoids (C40), and polyterpenes (> C40). The 2-C-methyl-d-erythritol-4-phosphate (MEP) pathway (also called DXP (1-deoxy-d-Xylulose-5-phosphate) pathway) and mevalonate (MVA) pathway account for the generation of IPP and DMAPP, isoprenoid precursors (Fig. 1). Plants possess both pathways, MVA employed in the cytosol, whereas MEP in plastids. Individually MVA pathway is operated in the cytosol of eukaryotes, archaebacteria, some bacteria, and the MEP pathway in prokaryotes (bacteria, algae, cyanobacteria). Both the pathways comprise seven enzyme-catalyzed steps to form IPP and DMAPP; however, both pathways’ initial reactants differ. MEP initiates with glyceraldehyde 3-phosphate (GAP) and pyruvate, and MVA with two acetyl-CoA molecules. The first step of the MEP pathway comprehends an irreversible reaction, condensation of GAP and pyruvate to form DXP. This step is a rate-limiting step catalyzed by a rate-limiting enzyme DXP synthase (DXS). DXP goes through reductive rearrangement to yield MEP, which is then coupled with cytidine triphosphate (CTP) to form 4-(-cytidine 5′-pyrophospho)-2-C-methyl-d-erythritol (CDP-ME). CDP-ME then undergoes phosphorylation, cyclization, and reductive dehydration to give rise to 4-hydroxy-3-methyl-butenyl 1-diphosphate (HMB-PP). Finally, HMB-PP generates either IPP or DMAPP with the help of the enzyme HMB-PP reductase (IspH). Therefore, the enzyme isopentenyl diphosphate isomerase (IDI), which interconverts IPP and DMAPP, is not crucial for the MEP pathway and is required only to balance IPP:DMAPP ratio. In contrast, MVA commences with condensation of two molecules of acetyl-CoA to form acetoacetyl-CoA, which further condensation with the third molecule of acetyl-CoA leads to produce 4-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA). Later, reduction in HMG-CoA forms MVA, whose twofold phosphorylation leads to the formation of mevalonate pyrophosphate. IPP is produced when the third round of phosphorylation and decarboxylation takes place. IDI is the only common enzyme to both MEP and MVA pathways and helps in the interconversion of IPP to DMAPP (Jin et al. 2020).
Schematic representation of MVA and MEP pathways with different upstream and common downstream steps, and different classes of terpenoids with their structure generated from IPP/DMAPP pool. Upstream pathway abbreviations RuBP ribulose-1,5-bisphosphate, 3-PGA 3-phosphoglyceric acid, Ac-CoA acetyl-CoA, AcAc-CoA acetoacetyl-CoA, HMG-CoA 3-hydroxy-3-methylglutaryl-CoA, MVA mevalonate, MVAP mevalonate-5-phosphate, MVAPP mevalonate-5-pyrophosphate, G3P glyceraldehyde 3-phosphate, DXP 1-deoxy-d-xylulose 5-phosphate, MEP methylerythritol-4-phosphate, CDP-ME 4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol, CDP-MEP 2-phospho-4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol, MEcPP 2-C-methyl-d-erythritol 2,4-cyclodiphosphate, HMB-PP 4-hydroxy-3-methylbut-2-enyldiphosphate, AtoB acetoacetyl-CoA thiolase, HMGS HMG-CoA synthase, HMGR HMG-CoA reductase, MK mevalonate kinase, PMK MVAP kinase, PMD MVAPP decarboxylase, DXS DXP synthase, DXR DXP reductoisomerase: IspD: CDP-ME cytidylyltransferase, IspE CDP-ME kinase, IspF MEC synthase, IspG HMBPP synthase, IspH HMBPP reductase. Downstream pathway abbreviations DMAPP dimethylallyl diphosphate, IPP isopentenyl diphosphate, GPP geranyl diphosphate, FPP farnesyl diphosphate, GGPP geranylgeranyl diphosphate, IDI isopentenyl diphosphate isomerase, GPPS GPP synthase, FPPS FPP synthase, GGPPS GGPP synthase, TPS Terpene synthase. *Downstream pathway is common to MVA and MEP pathways and is functional in cytosol and plastid. Besides that, it operates in mitochondria to generate ubiquinone
Generation of IPP and DMAPP from any of the two pathways nevertheless leads to the same downstream processing in any organism. Sequential head-to-tail condensation of IPP and DMAPP takes place to generate various prenyl phosphate of varying length by 5 carbon units like C10 geranyl pyrophosphate (GPP), C15 farnesyl pyrophosphate (FPP), C20 geranylgeranyl pyrophosphate (GGPP). Afterward, these prenyl phosphates, with the help of terpene synthase (TPS), give rise to numerous monoterpenes (from GPP), sesquiterpene (from FPP), diterpene (from GGPP), triterpene (from two molecules of FPP), and tetraterpene (from two molecules of GGPP) (Wang et al. 2019b; Abbas et al. 2021).
To bioengineer cyanobacteria, the MEP pathway is usually targeted for several reasons. First, being the native pathway, it is highly regulated than the MVA pathway (Banerjee and Sharkey 2014). Secondly, as the MVA pathway is non-native to cyanobacteria, it poses difficulties in adding the complete pathway with seven genes, each more than 1.5 kb in length (Betterle and Melis 2019). Moreover, successful expression of the MVA pathway gives the same yield as with MEP pathway engineering (Formighieri and Melis 2016). Thirdly, MEP is scrutinized as an “energy-deficient” pathway converting carbon source more efficiently (83%) for precursor IPP synthesis in comparison to the MVA pathway, which utilizes only 56% carbon (Dugar and Stephanopoulos 2011).
Engineering upstream MEP/MVA pathway to redirect flux toward terpene precursors
Increased production of a terpenoid is directly proportional to the increase in precursor pool, IPP and DMAPP, which can be attained by metabolic engineering of regulatory enzymes in the MEP/MVA pathway. Upregulation of rate-limiting genes (DXS, IspD, IspF, and IDI) and other enzymes of the MEP pathway increases IPP and DMAPP production. Different regulation strategies of the MEP pathway are summarized in a review (Banerjee and Sharkey, 2014). Plethora of research has been conducted in heterotrophic hosts to overexpress these genes to increase the terpenoid yield, which can be taken as a lesson to be applied to phototrophic hosts (cyanobacteria). In one of the studies in E. coli to produce levopimaradiene, bottleneck genes DXS, IspD, IspF, and IDI were incorporated in the chromosome as an operon (Leonard et al. 2010). To increase the copy number of the genes, an operon was inserted in the expression plasmid under the control of the trc promoter. Approximately five auxiliary copies of the genes lead to a more than 600-fold increase in levopimaradiene synthesis. Similarly, a 24-fold increase in squalene production was observed when DXS and IDI genes were overexpressed (Liu et al. 2017). Genes order also plays an influential role in metabolic engineering of the MEP pathway (Lv et al. 2013). DXS expression levels were high irrespective of the order, and high isoprene levels were obtained when DXS, DXR, and IDI were in an order similar to the native pathway. Apart from using wild-type regulatory genes, their sequences can be modified through error-prone PCR, site-directed mutagenesis, protein engineering, and recombineering (Lv et al. 2016; Volke et al. 2019). Site-directed mutagenesis of Poplar DXS reduced its feedback inhibition by IPP, which competes for thiamine diphosphate site on DXS (Banerjee et al. 2016). Recently, exogenous DXS and prenyltransferase (PT) from Vibrio sp. Dhg (a fast-growing microorganism) were incorporated in E. coli (Kim et al. 2019). The group aimed to find enzymes with higher catalytic efficiencies (1.08-fold for DXS and 1.38-fold for PT). Aside from E. coli, Corynebacterium glutamicum was engineered by overexpressing IspD and IspF to produce two terpenoids (Lim et al. 2020).
Cyanobacteria being photosynthetic, acts as an excellent source for terpenoids production. Table 1 summarizes the recent metabolic engineering of cyanobacteria for terpenoids production. Common cyanobacterial species used as cell factories include Synechocystis sp. PCC 6803, PCC 7002, Synechococcus elongatus PCC 7942, and recently discovered fast-growing Synechococcus elongatus UTEX 2973, PCC 11801, and PCC 11802 (Ungener et al. 2018; Jaiswal et al. 2018, 2020). Exploiting the native MEP pathway in cyanobacteria is a common strategy, as stated above in the case of heterotrophic hosts. Terpene synthase and prenyltransferase expression (discussed in detail in the next section) do not increase bisabolene production in Synechocystis sp. PCC 6803 (PCC 6803 hereafter) (Rodrigues and Lindberg 2021). However, overexpression of DXS and IDI doubled the titers in combination with carotenoids. Sporadically, as IPP and DMAPP pool accumulates with no further conversion to desired products, or pigments, it hinders the cell growth. A cumbersome approach was made by engineering the entire MEP pathway by expressing native MEP genes and non-native MEP genes from E. coli in PCC 6803 for comparative analysis to evaluate every step (Englund et al. 2018). In the case of exogenous genes, only DXS and IDI showed increased isoprene production by 14.5 and 3.4-fold, respectively. While in addition to DXS and IDI, IspG also showed an increase in isoprene synthesis in the case of endogenous genes (Gao et al. 2016; Englund et al. 2018). The rate of mRNA and protein synthesis of overexpressed DXS gene and protein differ by 4 and 1.5-fold than the wild-type strain, respectively (Kudoh et al. 2017). This suggests that a high transcription rate interrupts post-translational protein folding leading to a high soluble to insoluble protein ratio. IPP pool inhibits isoprene synthesis by affecting isoprene synthase (IspS) and DXS activity (by feedback inhibition). This shows the importance of the IDI enzyme to convert IPP to DMAPP, leading to an increase in DMAPP/IPP concentration and mitigating the repression on DXS and IspS (Gao et al. 2016). Similar results were acquired by Chaves and Melis (2018) by expressing IDI from Streptococcus pneumoniae. DXS, IDI from E. coli with farnesene synthase and FPP synthase, increase FPP pool and hence farnesene in Synechococcus elongatus PCC 7942 (PCC 7942 hereafter) (Lee et al. 2017; Pattharaprachayakul et al. 2019). The same set of genes with different sources (DXS from Plectranthus barbatus, IDI from E. coli and squalene synthase from Botryococcus braunii) were expressed in PCC 6803 for higher squalene synthesis in comparison to the strain only expressing native squalene synthase (Pattanaik et al. 2020). Analogous to squalene, another triterpenoid that can be used as a fuel precursor is botryococcene, which utilizes the same intermediate presqualene diphosphate (PSPP) as squalene. Botryococcene synthase was first characterized in slow growing microalgae B. braunii Race B, similar to squalene synthase (Okada et al. 2004; Bell et al. 2014). Therefore, botryococcene production is not economically feasible using B. braunii (Melis 2012). The production of botryococcene from cyanobacterial cell factories has not been reported yet and can be a potential prospect for the future.
For Synechococcus elongatus UTEX 2973 (UTEX 2973 hereafter), the upregulated DXS gene is toxic (Lin et al. 2021). However, DXS and IDI combined expression under lacUV5 promoter optimized by IPTG induction increased limonene synthesis. IDI exists in type I and type II forms based on their requirement of divalent metals and reduced flavin mononucleotide and divalent cations, respectively (Thibodeaux and Liu 2017). Gao et al. (2016) cloned type I IDI from Haematococcus pluialis and Saccharomyces cerevisiae and type II IDI from Synechococcus elongatus and Bacillus subtilis in PCC 7942. The strain expressing IDI from S. cerevisiae showed the highest isoprene synthase activity and consequently high isoprene synthesis (without DXS overexpression).
As seen in MEP vs. MVA section, the MVA pathway presents some limitations over the MEP pathway to express in a heterologous host. Nonetheless, the expression of MVA pathway enzymes in heterotrophic and up to some extent in photosynthetic organisms has been explored. Much experimentation has been done by engineering E. coli for the non-native MVA pathway, which can be taken as an epitome for engineering cyanobacteria as cell factories for terpenoid production (Navale et al. 2021). MVA pathway genes can be expressed alone as the MEP pathway is already present in the host (E. coli and cyanobacteria), or MVA and MEP pathway genes can be overexpressed concomitantly. As MVA is a foreign pathway in prokaryotes, all the six genes have to be expressed in the host, namely AtoB (acetoacetyl-CoA thiolase), HMGS (HMG-CoA synthase), HMGR (HMG-CoA reductase), MK (mevalonate kinase), PMK (mevalonate 5-phosphate kinase), and PMD (mevalonate 5-pyrophosphate decarboxylase). These genes can be expressed under the influence of different promoters like trc and T7 and in plasmids with varying copy numbers (Nybo et al. 2017; Liu et al. 2019a, b). One of the commercially available vectors, ready to use, consisting of all the six genes, was deposited by Peralta-Yahya et al. (2011) in the addgene plasmid repository (Addgene plasmid # 35151). MVA pathway genes can be taken from several heterologous sources, for instance, AtoB, HMGS, and HMGR genes from Enterococcus faecalis, MK from Methanosarcina mazei, PMK from Streptococcus pneumoniae, and PMD from Clostridium acetobutylicum (Liu et al. 2019a). A high concentration of IPP/DMAPP proves to be fatal to the cells, and therefore the expression of MVA pathway genes needs to be regulated (Liu et al. 2019b). One way to reduce gene expression is to express the upper half of the pathway genes in a single polycistron and the other half in another polycistron, in a low copy number plasmid. The highest terpenoid producing strain expresses AtoB, HMGS, HMGR, and IDI under T7 promoter; MK, PMK, PMD promoted by trc promoter; and terpene synthase expressing individually. Li et al. (2019) showed that amalgamation of MEP and MVA pathways increased cis-abienol titers by 7 and 31-fold, respectively. Another way to regulate the pathway is to use integrative vectors and strictly analyze the number of copies of the genes needed for balanced expression, as Hussain et al. (2021) showed for lycopene production. The entire MVA pathway for the first time was introduced in cyanobacteria by Bentley et al. (2014) for isoprene production by integrating the pathway genes. The introduction of the MVA pathway instigates a 2.5-fold boost in isoprene yield. Native MEP pathway accompanied by MVA pathway increased flux toward DMAPP and IPP pool, which further enhanced β-phellandrene yield (Formighieri and Melis 2016; Betterle and Melis 2019).
Engineering downstream pathway assisting precursor pool to terpenoid synthesis
It is important to abet IPP and DMAPP pool toward the sink, i.e., desired terpene, to avoid feedback inhibition of upstream pathway enzymes. Downstream processing is the connecting link between precursor (IPP and DMAPP) and final terpene product. It involves two categories of enzyme prenyltransferase and terpene synthase. Prenyltransferase converts DMAPP to GPP, FPP, and GGPP. Prenyltransferase that aids in this conversion are GPP synthase, FPP synthase, and GGPP synthase, respectively, common to MEP and MVA pathway irrespective of the terpenoid to be produced. GPP, FPP, and GGPP act as an immediate substrate for terpene synthesis, and therefore prenyltransferase engineering is vital. Further TPS comes into play, converting GPP, FPP, and GGPP into monoterpenes, sesquiterpene, and diterpene.
TPS is usually not present in cyanobacteria and has to be endowed with heterologous expression. The key impediment in using TPS is its low turnover number (Kcat), which spans in the range of 3–4 s−1 (Betterle and Melis 2019). This barrier can be conquered by escalating TPS concentration by overexpressing the TPS gene or using protein fusion constructs (Fig. 2). This fusion can be between TPS and any other gene like β-subunit of phycocyanin (CpcB) (Chaves et al. 2017; Betterle and Melis 2019). Phycocyanin, a copious protein in cyanobacteria, indicates that the gene is under strong expression (promoter, RBS) control (Zhou et al. 2014). Fusing any gene with CpcB will result in its overexpression. Melis's group exploited this approach by creating several fusion constructs with CpcB, for instance, β-phellandrene synthase (PHLS), isoprene synthase (IspS), geranyllinalool synthase (GLS) (Formighieri and Melis 2016, 2017; Chaves et al. 2017; Betterle and Melis 2019). CpcB fusion with PHLS ensued overexpression of PHLS and increased its concentration in the cell to 20%, thereby improving PHL yield to 100-fold (Formighieri and Melis 2015). In the consecutive years CpcB|PHLS construct was used in conjunction with co-expression of MVA pathway genes and GPPS (Formighieri and Melis 2016; Betterle and Melis 2018, 2019). In continuation to the previous study using CpcB|PHLS fusion construct with entire heterologous MVA pathway gene and GPPS amplified the yield (Formighieri and Melis 2016). Apart from using native genes for fusion construct, other heterologous genes also show high expression in cyanobacteria (Betterle and Melis 2018). These heterologous genes include kanamycin (nptI) and chloramphenicol (cmr) resistance genes. The full-length version of nptI and curtailed version of nptI and cmr were fused with GPPS. CpcB|PHLS with nptI|GPPS gave the highest yield. While the partial cmr sequence from 5′ end to 87 nucleotides, i.e., 29 amino acids called cmr29 fused with GPPS, produced about 2.42 mg PHL per gm of dcw. Similarly, nptI|GPPS followed by half of the MVA pathway enzymes resulted in 24 mg PHL per gm of dcw (with CpcB|PHLS and other half MVA pathway enzymes) (Betterle and Melis 2019). All these strategies resulted in the accumulation of GPPS and PHLS inside the cell factory and increased PHL yield. Other terpenoids such as isoprene and geranyllinalool yield were also escalated by fusion strategy. Chaves et al. (2017) used linker amino acid sequences of varying lengths between CpcB and isoprene synthase (IspS) as there is a decrease in specific activity of IspS by leader sequences of CpcB. Four different linker lengths were used, L7, L10, L16, and L65. CpcB|L7|IspS showed a 27-fold increase in isoprene synthesis than CpcB|IspS, even though only 10% of IspS’s activity was retained. Diterpene geranyllinalool (GL) is synthesized as both extracellular and intracellular products with the help of geranyllinalool synthase (GLS) (Formighieri and Melis 2017). 30–40% GL exude out of the cells and remaining manifests inhibitory effect on PCC 6803 like longer doubling time. CpcB|GLS construct produced up to 390 µg of GL per gm of dcw even with a prolonged doubling time. Cpc operon consists of five genes CpcA, CpcB, CpcC1, CpcC2, and CpcD. α and β subunits of phycocyanin are encoded by CpcA and B, respectively. In contrast, linker polypeptides are encoded by CpcC1, CpcC2, and CpcD, all of which combine to form a phycobilisome, light-harvesting complex. The fusion constructs with CpcB, such as CpcB|PHLS, upregulate when other operon genes are expressed simultaneously (Valsami et al. 2020). Choi et al. (2017) show that PT and TPS can also be fused by fusing FPPS and squalene synthase in PCC 7942. The engineered strain was further upscaled to be grown in a 6 L photobioreactor.
Fusion protein constructs made over the years yielding high terpenoids titer (mg g−1 biomass). CpcA α-subunit of phycocyanin, CpcB β-subunit of phycocyanin, fni IPP from Streptococcus pneumoniae, L7 7-amino acid linker PMPWRVI, PHLS β-phellandrene synthase, nptI Kanamycin resistance gene, GPPS geranyl diphosphate synthase, SF short flexible linker of 4-amino acids, GGGS, NaGLS geranyllinalool synthase from Nicotiana attenuate. References—1Formighieri and Melis (2015); 2Formighieri and Melis (2016); 3Choi et al. (2017); 4Chaves et al. (2017); 5Formighieri and Melis (2017); 6Chaves and Melis (2018); 7Betterle and Melis (2018); 8Betterle and Melis (2019); 9Valsami et al. (2020). The graph is only for representation and not for the scale
Terpene synthase and prenyltransferase engineering does not limit to CpcB promoter only but also to other promoter sequences, altered ribosome binding sites, codon optimization, and mutation of the genes. The expression of amorphadiene synthase and squalene synthase under the influence of trc promoter was analyzed in PCC 7942 (Choi et al. 2016). The recombinant strain expressing TPS alone showed less yield until the FPPS was not overexpressed. FPPS engineered strains rendered a 12-fold and 50,000-fold increase in amorphadiene and squalene production, respectively (with MEP pathway genes, DXS, DXR, and IDI). A nearly similar strain with some further modifications was cultivated in a photobioreactor utilizing industrial flue gas as a carbon source for the generation of squalene (Choi et al. 2020). Evolutionary engineering of PCC 7942 by optimizing RBS (with the help of RBS calculator) of farnesene synthase increased farnesene synthesis by two-fold (Pattharaprachayakul et al. 2019). RBS affects translation initiation rate (TIR) and hence the protein synthesis. Strain having low TIR shows high farnesene production (about 5.66 mg/L). Similar work in PCC 6803 was done to synthesize bisabolene (7.8 mg/L in five days) by codon-optimized bisabolene synthase and different RBS sequences (Sebesta and Peebles 2020). Single nucleotide polymorphism mutation in GPPS in UTEX 2973 (having the shortest doubling time of 1.9 h) produced limonene at a rate of 8.2 mg/L/day (Lin et al. 2021). Mutation in GPPS combined with a mutation in outer membrane protein B, different RBS sequences for GPPS, and overexpression of MEP pathway genes led to this increased rate of limonene production. Not being a native enzyme in cyanobacteria, TPS has to be taken from a heterologous source such as plants. The source from which TPS is excised determines its specific activity, kcat, and expression. Matsudaira et al. (2020) showed valencene synthesis by employing valencene synthase (VLS) gene from three different sources (Citrus sinensis, Vitis vinifera, and Callitropsis nootkatensis). PCC 6803 strains having VLS from C. sinensis and V. vinifera barely showed any signs of valencene production, while VLS from C. nootkatensis produced 9.62 mg/L of valencene.
Lately, an outbreak of novel coronavirus leading to global pandemic questions about biosafety and environmental risks of recombinant strains. In line with this concern, a biocontainment strain was developed by deleting genes encoding for β-carboxysome and carbon concentrating mechanism in farnesene synthesizing PCC 7942 (Lee et al. 2021). The strain produced about 5 mg/L of farnesene and will not grow and produce farnesene at 100% air bubbling.
Challenges
Terpenoids being complex in structure, pose difficulty in their chemical synthesis. Therefore, the biological synthesis of terpenoids is preferred. Cyanobacteria, which are considered green E. coli because they require sunlight and CO2 as energy and carbon source, are used as cell factories for terpenoid production. A photosynthetic organism has a carbon partitioning issue, in which most of the carbon competes for sugar biosynthesis, biomass cumulation, terpenoid synthesis, and other biosynthetic pathways (Melis 2013). Lindberg and co-workers demonstrated flux analysis in PCC 6803, showing only 5% of carbon is allotted to terpene synthesis, in contrast to sugar biosynthesis, 80% (Lindberg et al. 2010). Eliminating competing pathways is one of the strategies to increase terpenoids production. Squalene, a triterpenoid, acts as a precursor for sterols and hopanoids in eukaryotes and prokaryotes, respectively. Accumulation of squalene requires inactivation of the gene(s), which further converts it. One such gene is squalene hopene cyclase (shc) which converts squalene to hopanoids. Inactivation of shc in PCC 6803 leads to accumulation of 5.1 mg/L of squalene (with co-expression of DXS, IDI, and FPPS from different sources) (Pattanaik et al. 2020). The MEP pathway is energy-consuming and requires ATP and NADPH generated by photosynthesis. Photorespiration contends for this ATP and NADPH and effects terpene production. Impeding photorespiration by deleting glycolate dehydrogenase encoding genes (glcD1 and glcD2) has been shown to increase isoprene yield (Zhou et al. 2021). One constraint of impairing photorespiration in the strain is the generation of high carbon dioxide-requiring (HCR) strain. Surprisingly, isoprene-producing strain with impaired photorespiration does not show HCR phenotype.
Isoprene and other terpenoids can be toxic to cyanobacterial cells. However, the volatile nature of most of them and extraction with the help of overlay of non-polar solvents (dodecane, hexadecane, tetradecane) lets easy escape from the cells (Choi et al. 2016; Gao et al. 2016; Pattharaprachayakul et al. 2019; Matsudaira et al. 2020). Prenyl phosphate, FPP, too, is found to be noxious for the growth of cyanobacteria. This can be overcome by increasing the activity or number of copies of TPS (Choi et al. 2016, 2017). In addition to this, FPP synthase expression under the influence of strong inducible promoters like tic (having two lac operators) keeps low expression levels until induced by IPTG (Sebesta and Peebles 2020). Recently, it was perceived that some tools from genetic toolboxes like CRISPR/Cas system are lethal to cyanobacteria (Pattharaprachayakul et al. 2020). Adopting Cas12 instead of Cas9 proves to be less toxic.
Terpene synthases are slow enzymes; hence selecting a terpene synthase with high Kcat and low Km is pivotal in terpenoids biosynthesis. Same TPS within different hosts shows a conspicuously distinct activity level (Loeschcke et al. 2017). One possible reason for this could be the codon biasing, for which reason the codon-optimized version of TPS is used (Chaves and Melis 2018; Betterle and Melis 2019; Matsudaira et al. 2020; Lin et al. 2021). Moreover, this limitation can also be conquered by fusing TPS with genes under strong promoter influence (explained in the previous section).
To be genetically modified, comprehension of an organism's complete genome sequence is requisite. Cyanobacteria are advantageous in this case as genome information of the common hosts like Anabaena sp. PCC 7120, Synechocystis sp. PCC 6803, Synechococcus elongatus PCC 7942, Synechococcus sp. PCC 7002 is available at uniport.org. In comparison to E. coli, cyanobacteria have a high doubling time. A newly discovered cyanobacterial strain Synechococcus elongatus UTEX 2973 has the shortest doubling time of 1.9 h, which can be further reduced to 1.5 h under bright light and is the fastest-growing cyanobacteria (Yu et al. 2015; Ungerer et al. 2018). Being polyploidy in nature, cyanobacteria are arduous to create homozygous strains. CRISPR/Cas comes to the rescue for this challenge and is efficiently used to produce homozygous mutants (Choi and Woo 2020).
Concluding remarks: toward green production of terpenoids
Despite the challenges faced in engineering cyanobacteria, carbon partitioning, low yield, and toxicity to the cells, the photoautotrophic production of terpenoids is gaining prominence. Recent advances in genetic toolboxes of cyanobacteria by Sengupta et al. (2018) and Sun et al. (2018) make it easier to manipulate genetic pathways toward the compound of interest. Over-expression of bottleneck enzymes of MEP pathway increases DMAPP/IPP pool which further increases terpenoid production. This also reduces carbon competition toward other necessary pathways like pigment formation, sugar synthesis (Melis 2013). Besides the MEP pathway, introducing a heterologous MVA pathway can also escalate the precursor pool. Expressing the entire MVA pathway with six heterologous genes is laborious, and much work has to be done in this area. The end step in terpenoid synthesis requires terpene synthase, which converts precursors into a different class of terpenoids. Terpene synthases are non-native to cyanobacteria and thereby have to be engineered. Selection of source for terpene synthases has to be chosen wisely with high kcat and codon optimization according to the host is to be done. At times, implementing all these strategies does not give good yield of terpenoids which could be due to the slow growth rate of cyanobacteria. The Discovery of UTEX 2973, PCC 11801, and PCC 11802, having the highest photoautotrophic growth rate, deciphered the issue (Yu et al. 2015; Ungerer et al. 2018; Jaiswal et al. 2018, 2020).
References
Abbas F, Ke Y, Yu R, Yue Y, Amanullah S, Jahangir MM, Fan Y (2017) Volatile terpenoids: multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta 246(5):803–816. https://doi.org/10.1007/s00425-017-2749-x
Abbas F, Ke Y, Zhou Y et al (2021) Genome-wide analysis of ARF transcription factors reveals HcARF5 expression profile associated with the biosynthesis of β-ocimene synthase in Hedychium coronarium. Plant Cell Rep 40(7):1269–1284. https://doi.org/10.1007/s00299-021-02709-1
Abdallah II, Pramastya H, Van Merkerk R, Quax WJ (2019) Metabolic engineering of Bacillus subtilis toward taxadiene biosynthesis as the first committed step for taxol production. Front Microbiol 10:218. https://doi.org/10.3389/fmicb.2019.00218
Ajikumar PK, Tyo K, Carlsen S, Mucha O, Phon TH, Stephanopoulos G (2008) Terpenoids: opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol Pharm 5(2):167–190. https://doi.org/10.1021/mp700151b
Banerjee A, Sharkey TD (2014) Methylerythritol 4-phosphate (MEP) pathway metabolic regulation. Nat Product Rep 31(8):1043–1055. https://doi.org/10.1039/c3np70124g
Banerjee A, Preiser AL, Sharkey TD (2016) Engineering of recombinant poplar deoxy-D-xylulose-5-phosphate synthase (Pt DXS) by site-directed mutagenesis improves its activity. PLoS ONE 11(8):e0161534. https://doi.org/10.1371/journal.pone.0161534
Bell SA, Niehaus TD, Nybo SE, Chappell J (2014) Structure–function mapping of key determinants for hydrocarbon biosynthesis by squalene and squalene synthase-like enzymes from the green alga Botryococcus braunii race B. Biochemistry 53(48):7570–7581. https://doi.org/10.1021/bi501264s
Bentley FK, Zurbriggen A, Melis A (2014) Heterologous expression of the mevalonic acid pathway in cyanobacteria enhances endogenous carbon partitioning to isoprene. Mol Plant 7(1):71–86. https://doi.org/10.1093/mp/sst134
Betterle N, Melis A (2018) Heterologous leader sequences in fusion constructs enhance expression of geranyl diphosphate synthase and yield of β-phellandrene production in cyanobacteria (Synechocystis). ACS Synth Biol 7(3):912–921. https://doi.org/10.1021/acssynbio.7b00431
Betterle N, Melis A (2019) Photosynthetic generation of heterologous terpenoids in cyanobacteria. Biotechnol Bioeng 116(8):2041–2051. https://doi.org/10.1002/bit.26988
Block AK, Vaughan MM, Schmelz EA, Christensen SA (2019) Biosynthesis and function of terpenoid defense compounds in maize (Zea mays). Planta 249(1):21–30. https://doi.org/10.1007/s00425-018-2999-2
Chaves JE, Melis A (2018) Biotechnology of cyanobacterial isoprene production. Appl Microbiol Biotechnol 102(15):6451–6458. https://doi.org/10.1007/s00253-018-9093-3
Chaves JE, Rueda-Romero P, Kirst H, Melis A (2017) Engineering isoprene synthase expression and activity in cyanobacteria. ACS Synthetic Biol 6(12):2281–2292. https://doi.org/10.1021/acssynbio.7b00214
Cheng MH, Huang H, Dien BS, Singh V (2019) The costs of sugar production from different feedstocks and processing technologies. Biofuels, Bioprod Biorefin 13(3):723–739. https://doi.org/10.1002/bbb.1976
Cheong DH, Tan DW, Wong FW, Tran T (2020) Antimalarial drug, artemisinin and its derivatives for the treatment of respiratory diseases. Pharmacol Res 158:104901. https://doi.org/10.1016/j.phrs.2020.104901
Chi X, Zhang S, Sun H, Duan Y, Qiao C, Luan G, Lu X (2019) Adopting a theophylline-responsive riboswitch for flexible regulation and understanding of glycogen metabolism in Synechococcus elongatus PCC7942. Frontiers in Microbiol 10:551. https://doi.org/10.3389/fmicb.2019.00551
Choi SY, Woo HM (2020) CRISPRi-dCas12a: a dCas12a-mediated CRISPR interference for repression of multiple genes and metabolic engineering in cyanobacteria. ACS Synth Biol 9(9):2351–2361. https://doi.org/10.1021/acssynbio.0c00091
Choi SY, Lee HJ, Choi J et al (2016) Photosynthetic conversion of CO2 to farnesyl diphosphate-derived phytochemicals (amorpha-4, 11-diene and squalene) by engineered cyanobacteria. Biotechnol Biofuels 9(1):1–12. https://doi.org/10.1186/s13068-016-0617-8
Choi SY, Wang JY, Kwak HS et al (2017) Improvement of squalene production from CO2 in Synechococcus elongatus PCC 7942 by metabolic engineering and scalable production in a photobioreactor. ACS Synth Biol 6(7):1289–1295. https://doi.org/10.1021/acssynbio.7b00083
Choi SY, Sim SJ, Ko SC et al (2020) Scalable cultivation of engineered cyanobacteria for squalene production from industrial flue gas in a closed photobioreactor. J Agric Food Chem 68(37):10050–10055. https://doi.org/10.1021/acs.jafc.0c03133
Darragh K, Orteu A, Black D et al (2021) A novel terpene synthase controls differences in anti-aphrodisiac pheromone production between closely related Heliconius butterflies. PLoS Bio 19(1):e3001022. https://doi.org/10.1371/journal.pbio.3001022
Dienst D, Wichmann J, Mantovani O, Rodrigues JS, Lindberg P (2020) High density cultivation for efficient sesquiterpenoid biosynthesis in Synechocystis sp. PCC 6803. Sci Rep 10(1):1–16. https://doi.org/10.1038/s41598-020-62681-w
Dugar D, Stephanopoulos G (2011) Relative potential of biosynthetic pathways for biofuels and bio-based products. Nat Biotechnol 29(12):1074–1078. https://doi.org/10.1038/nbt.2055
Eisenhut M, Kahlon S, Hasse D, Ewald R, Lieman-Hurwitz J, Ogawa T et al (2006) The plant-like C2 glycolate cycle and the bacterial-like glycerate pathway cooperate in phosphoglycolate metabolism in cyanobacteria. Plant Physiol 142(1):333–342. https://doi.org/10.1104/pp.106.082982
El-Sayed AS, Ali DM, Yassin MA, Zayed RA, Ali GS (2019) Sterol inhibitor “Fluconazole” enhance the Taxol yield and molecular expression of its encoding genes cluster from Aspergillus flavipes. Process Biochem 76:55–67. https://doi.org/10.1016/j.procbio.2018.10.008
Englund E, Shabestary K, Hudson EP, Lindberg P (2018) Systematic overexpression study to find target enzymes enhancing production of terpenes in Synechocystis PCC 6803, using isoprene as a model compound. Metab Eng 49:164–177. https://doi.org/10.1016/j.ymben.2018.07.004
Formighieri C, Melis A (2015) A phycocyanin· phellandrene synthase fusion enhances recombinant protein expression and β-phellandrene (monoterpene) hydrocarbons production in Synechocystis (cyanobacteria). Metabolic Eng 32:116–124. https://doi.org/10.1016/j.ymben.2015.09.010
Formighieri C, Melis A (2016) Sustainable heterologous production of terpene hydrocarbons in cyanobacteria. Photosynth Res 130(1):123–135. https://doi.org/10.1007/s11120-016-0233-2
Formighieri C, Melis A (2017) Heterologous synthesis of geranyllinalool, a diterpenol plant product, in the cyanobacterium Synechocystis. Appl Microbiol Biotechnol 101(7):2791–2800. https://doi.org/10.1007/s00253-016-8081-8
Gao X, Gao F, Liu D, Zhang H, Nie X, Yang C (2016) Engineering the methylerythritol phosphate pathway in cyanobacteria for photosynthetic isoprene production from CO2. Energy Environ Sci 9(4):1400–1411. https://doi.org/10.1039/C5EE03102H
Góngora CE, Tapias J, Jaramillo J, Medina R, Gonzalez S, Casanova H, Ortiz A, Benavides P (2020) Evaluation of terpene-volatile compounds repellent to the coffee berry borer, Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae). J Chem Ecol 46(9):881–890. https://doi.org/10.1007/s10886-020-01202-5
Hasunuma T, Takaki A, Matsuda M, Kato Y, Vavricka CJ, Kondo A (2019) Single-stage astaxanthin production enhances the nonmevalonate pathway and photosynthetic central metabolism in Synechococcus sp. PCC 7002. ACS Synth Biol 8(12):2701–2709. https://doi.org/10.1021/acssynbio.9b00280
He Z, Huang Z, Jiang W, Zhou W (2019) Antimicrobial activity of cinnamaldehyde on Streptococcus mutans biofilms. Front Microbiol 10:2241. https://doi.org/10.3389/fmicb.2019.02241
Hussain MH, Hong Q, Zaman WQ et al (2021) Rationally optimized generation of integrated Escherichia coli with stable and high yield lycopene biosynthesis from heterologous mevalonate (MVA) and lycopene expression pathways. Synth Syst Biotechnol 6(2):85–94. https://doi.org/10.1016/j.synbio.2021.04.001
Ishikawa Y, Kawai-Yamada M (2019) Physiological significance of NAD kinases in cyanobacteria. Front Plant Sci 10:847. https://doi.org/10.3389/fpls.2019.00847
Jaiswal D, Sengupta A, Sohoni S, Sengupta S, Phadnavis AG, Pakrasi HB, Wangikar PP (2018) Genome features and biochemical characteristics of a robust, fast growing and naturally transformable cyanobacterium Synechococcus elongatus PCC 11801 isolated from India. Sci Rep 8(1):1–13. https://doi.org/10.1038/s41598-018-34872-z
Jaiswal D, Sengupta A, Sengupta S, Madhu S, Pakrasi HB, Wangikar PP (2020) A novel cyanobacterium Synechococcus elongatus PCC 11802 has distinct genomic and metabolomic characteristics compared to its neighbor PCC 11801. Sci Rep 10(1):1–15. https://doi.org/10.1038/s41598-019-57051-0
Jakubczyk D, Dussart F (2020) Selected fungal natural products with antimicrobial properties. Molecules 25(4):911. https://doi.org/10.3390/molecules25040911
Jin X, Baysal C, Gao L et al (2020) The subcellular localization of two isopentenyl diphosphate isomerases in rice suggests a role for the endoplasmic reticulum in isoprenoid biosynthesis. Plant Cell Rep 39(1):119–133. https://doi.org/10.1007/s00299-019-02479-x
Kim MJ, Noh MH, Woo S, Lim HG, Jung GY (2019) Enhanced lycopene production in Escherichia coli by expression of two MEP pathway enzymes from Vibrio sp. dhg. Catalysts 9(12):1003. https://doi.org/10.3390/catal9121003
Kudoh K, Hotta S, Sekine M, Fujii R, Uchida A, Kubota G, Kawano Y, Ihara M (2017) Overexpression of endogenous 1-deoxy-d-xylulose 5-phosphate synthase (DXS) in cyanobacterium Synechocystis sp. PCC6803 accelerates protein aggregation. J Biosci Bioeng 123(5):590–596. https://doi.org/10.1016/j.jbiosc.2017.01.001
Kutyna DR, Borneman AR (2018) Heterologous production of flavour and aroma compounds in Saccharomyces cerevisiae. Genes 9(7):326. https://doi.org/10.3390/genes9070326
Larsson MC, Madjidian JA, Lankinen Å (2021) Floral scent and pollinator visitation in relation to floral colour morph in the mixed-mating annual herb Collinsia heterophylla. Nordic J Bot. https://doi.org/10.1111/njb.03025
Lee HJ, Lee J, Lee SM, Um Y, Kim Y, Sim SJ, Choi JI, Woo HM (2017) Direct conversion of CO2 to α-farnesene using metabolically engineered Synechococcus elongatus PCC 7942. J Agric Food Chem 65(48):10424–10428. https://doi.org/10.1021/acs.jafc.7b03625
Lee HJ, Choi JI, Woo HM (2021) Biocontainment of engineered Synechococcus elongatus PCC 7942 for photosynthetic production of α-farnesene from CO2. J Agric Food Chem 69(2):698–703. https://doi.org/10.1021/acs.jafc.0c07020
Leonard E, Ajikumar PK, Thayer K, Xiao WH, Mo JD, Tidor B, Stephanopoulos G, Prather KL (2010) Combining metabolic and protein engineering of a terpenoid biosynthetic pathway for overproduction and selectivity control. Proc Natl Acad Sci 107(31):13654–13659. https://doi.org/10.1073/pnas.1006138107
Leonhardt SD, Zeilhofer S, Blüthgen N, Schmitt T (2010) Stingless bees use terpenes as olfactory cues to find resin sources. Chem Senses 35(7):603–611. https://doi.org/10.1093/chemse/bjq058
Li L, Wang X, Li X, Shi H, Wang F, Zhang Y, Li X (2019) Combinatorial engineering of mevalonate pathway and diterpenoid synthases in Escherichia coli for cis-Abienol production. J Agric Food Chem 67(23):6523–6531. https://doi.org/10.1021/acs.jafc.9b02156
Lim H, Park J, Woo HM (2020) Overexpression of the key enzymes in the methylerythritol 4-phosphate pathway in Corynebacterium glutamicum for improving farnesyl diphosphate-derived terpene production. J Agric Food Chem 68(39):10780–10786. https://doi.org/10.1021/acs.jafc.0c04307
Lin PC, Saha R, Zhang F, Pakrasi HB (2017) Metabolic engineering of the pentose phosphate pathway for enhanced limonene production in the cyanobacterium Synechocystis sp. PCC 6803. Sci Rep 7(1):1–10. https://doi.org/10.1038/s41598-017-17831-y
Lin PC, Zhang F, Pakrasi HB (2021) Enhanced limonene production in a fast-growing cyanobacterium through combinatorial metabolic engineering. Metab Eng Commun 12:e00164. https://doi.org/10.1016/j.mec.2021.e00164
Lindberg P, Park S, Melis A (2010) Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab Eng 12(1):70–79. https://doi.org/10.1016/j.ymben.2009.10.001
Liu D, Pakrasi HB (2018) Exploring native genetic elements as plug-in tools for synthetic biology in the cyanobacterium Synechocystis sp. PCC 6803. Microb Cell Factories 17(1):1–8. https://doi.org/10.1186/s12934-018-0897-8
Liu H, Han S, Xie L, Pan J, Zhang W, Gong G, Hu Y (2017) Overexpression of key enzymes of the 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway for improving squalene production in Escherichia coli. Afr J Biotechnol 16(50):2307–2316. https://doi.org/10.5897/AJB2017.16235
Liu Z, Qu M, Yu L, Song P, Chang Y (2018) Artesunate inhibits renal ischemia-reperfusion-mediated remote lung inflammation through attenuating ROS-induced activation of NLRP3 inflammasome. Inflammation 41(4):1546–1556. https://doi.org/10.1007/s10753-018-0801-z
Liu CL, Bi HR, Bai Z, Fan LH, Tan TW (2019a) Engineering and manipulation of a mevalonate pathway in Escherichia coli for isoprene production. Appl Microbiol Biotechnol 103(1):239–250. https://doi.org/10.1007/s00253-018-9472-9
Liu CL, Dong HG, Zhan J, Liu X, Yang Y (2019b) Multi-modular engineering for renewable production of isoprene via mevalonate pathway in Escherichia coli. J Appl Microbiol 126(4):1128–1139. https://doi.org/10.1111/jam.14204
Liu H, Li S, Xiao G, Wang Q (2021) Formation of volatiles in response to tea green leafhopper (Empoasca onukii Matsuda) herbivory in tea plants: a multi-omics study. Plant Cell Rep 40(4):753–766. https://doi.org/10.1007/s00299-021-02674-9
Loeschcke A, Dienst D, Wewer V et al (2017) The photosynthetic bacteria Rhodobacter capsulatus and Synechocystis sp. PCC 6803 as new hosts for cyclic plant triterpene biosynthesis. PLoS ONE 12(12):e0189816. https://doi.org/10.1371/journal.pone.0189816
Lopes EM, Guimarães-Dias F, Gama TDS et al (2020) Artemisia annua L. and photoresponse: from artemisinin accumulation, volatile profile and anatomical modifications to gene expression. Plant Cell Rep 39(1):101–117. https://doi.org/10.1007/s00299-019-02476-0
Luziatelli F, Brunetti L, Ficca AG, Ruzzi M (2019) Maximizing the efficiency of vanillin production by biocatalyst enhancement and process optimization. Front Bioeng Biotechnol 7:279. https://doi.org/10.3389/fbioe.2019.00279
Lv X, Xu H, Yu H (2013) Significantly enhanced production of isoprene by ordered co-expression of genes dxs, dxr, and idi in Escherichia coli. Appl Microbiol Biotechnol 97(6):2357–2365. https://doi.org/10.1007/s00253-012-4485-2
Lv X, Gu J, Wang F, Xie W, Liu M, Ye L, Yu H (2016) Combinatorial pathway optimization in Escherichia coli by directed co-evolution of rate-limiting enzymes and modular pathway engineering. Biotechnol Bioeng 113(12):2661–2669. https://doi.org/10.1002/bit.26034
Matsudaira A, Hoshino Y, Uesaka K, Takatani N, Omata T, Usuda Y (2020) Production of glutamate and stereospecific flavors, (S)-linalool and (+)-valencene, by Synechocystis sp. PCC6803. J Biosci Bioeng 130(5):464–470. https://doi.org/10.1016/j.jbiosc.2020.06.013
Melis A (2012) Photosynthesis-to-fuels: from sunlight to hydrogen, isoprene, and botryococcene production. Energy Environ Sci 5(2):5531–5539. https://doi.org/10.1039/C1EE02514G
Melis A (2013) Carbon partitioning in photosynthesis. Curr Opin Chem Biol 17(3):453–456. https://doi.org/10.1016/j.cbpa.2013.03.010
Moses T, Pollier J, Thevelein JM, Goossens A (2013) Bioengineering of plant (tri) terpenoids: from metabolic engineering of plants to synthetic biology in vivo and in vitro. New Phytol 200(1):27–43. https://doi.org/10.1111/nph.12325
Navale GR, Dharne MS, Shinde SS (2021) Metabolic engineering and synthetic biology for isoprenoid production in Escherichia coli and Saccharomyces cerevisiae. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-020-11040-w
Nybo SE, Saunders J, McCormick SP (2017) Metabolic engineering of Escherichia coli for production of valerenadiene. J Biotechnol 262:60–66. https://doi.org/10.1016/j.jbiotec.2017.10.004
Okada S, Devarenne TP, Murakami M, Abe H, Chappell J (2004) Characterization of botryococcene synthase enzyme activity, a squalene synthase-like activity from the green microalga Botryococcus braunii. Race B Arch Biochem Biophys 422(1):110–118. https://doi.org/10.1016/j.abb.2003.12.004
Pahima E, Hoz S, Ben-Tzion M, Major DT (2019) Computational design of biofuels from terpenes and terpenoids. Sustain Energy Fuels 3(2):457–466. https://doi.org/10.1039/C8SE00390D
Pattanaik B, Englund E, Nolte N, Lindberg P (2020) Introduction of a green algal squalene synthase enhances squalene accumulation in a strain of Synechocystis sp. PCC 6803. Metab Eng Commun 10:e00125. https://doi.org/10.1016/j.mec.2020.e00125
Pattharaprachayakul N, Lee HJ, Incharoensakdi A, Woo HM (2019) Evolutionary engineering of cyanobacteria to enhance the production of α-farnesene from CO2. J Agric Food Chem 67(49):13658–13664. https://doi.org/10.1021/acs.jafc.9b06254
Pattharaprachayakul N, Lee M, Incharoensakdi A, Woo HM (2020) Current understanding of the cyanobacterial CRISPR-Cas systems and development of the synthetic CRISPR-Cas systems for cyanobacteria. Enzyme Microb Technol 140:109619. https://doi.org/10.1016/j.enzmictec.2020.109619
Pemberton TA, Chen M, Harris GG et al (2017) Exploring the influence of domain architecture on the catalytic function of diterpene synthases. Biochemistry 56(14):2010–2023. https://doi.org/10.1021/acs.biochem.7b00137
Peralta-Yahya PP, Ouellet M, Chan R, Mukhopadhyay A, Keasling JD, Lee TS (2011) Identification and microbial production of a terpene-based advanced biofuel. Nat Commun 2(1):1–8. https://doi.org/10.1038/ncomms1494
Rabe P, Schmitz T, Dickschat JS (2016) Mechanistic investigations on six bacterial terpene cyclases. Beilstein J Org Chem 12(1):1839–1850. https://doi.org/10.3762/bjoc.12.173
Rai R, Singh S, Rai KK, Raj A, Sriwastaw S, Rai LC (2021) Regulation of antioxidant defense and glyoxalase systems in cyanobacteria. Plant Physiol Biochem 168:353–372. https://doi.org/10.1016/j.plaphy.2021.09.037
Reddy GK, Leferink NG, Umemura M, Ahmed ST, Breitling R, Scrutton NS, Takano E (2020) Exploring novel bacterial terpene synthases. PLoS ONE 15(4):e0232220. https://doi.org/10.1371/journal.pone.0232220
Rodrigues JS, Lindberg P (2021) Metabolic engineering of Synechocystis sp. PCC 6803 for improved bisabolene production. Metabolic Eng Commun 12:e00159. https://doi.org/10.1016/j.mec.2020.e00159
Rueda E, García-Galán MJ, Díez-Montero R, Vila J, Grifoll M, García J (2020) Polyhydroxybutyrate and glycogen production in photobioreactors inoculated with wastewater borne cyanobacteria monocultures. Bioresour Technol 295:122233. https://doi.org/10.1016/j.biortech.2019.122233
Ruzicka L (1953) The isoprene rule and the biogenesis of terpenic compounds. Experientia 9(10):357–367. https://doi.org/10.1007/BF02167631
Schopf JW, Packer BM (1987) Early Archean (3.3-billion to 3.5-billion-year-old) microfossils from Warrawoona Group, Australia. Science 237(4810):70–73. https://doi.org/10.1126/science.11539686
Sebesta J, Peebles CA (2020) Improving heterologous protein expression in Synechocystis sp. PCC 6803 for alpha-bisabolene production. Metabolic Eng Commun 10:e00117. https://doi.org/10.1016/j.mec.2019.e00117
Sengupta A, Pakrasi HB, Wangikar PP (2018) Recent advances in synthetic biology of cyanobacteria. Appl Microbiol Biotechnol 102(13):5457–5471. https://doi.org/10.1007/s00253-018-9046-x
Shimada N, Okuda Y, Maeda K, Umeno D, Takaichi S, Ikeuchi M (2020) Astaxanthin production in a model cyanobacterium Synechocystis sp. PCC 6803. J Gen Appl Microbiol. https://doi.org/10.2323/jgam.2020.01.003
Sun T, Li S, Song X, Diao J, Chen L, Zhang W (2018) Toolboxes for cyanobacteria: recent advances and future direction. Biotechnol Adv 36(4):1293–1307. https://doi.org/10.1016/j.biotechadv.2018.04.007
Taylor GM, Hitchcock A, Heap JT (2021) Combinatorial assembly platform enabling engineering of genetically stable metabolic pathways in cyanobacteria. Nucleic Acids Res 49(21):e123–e123. https://doi.org/10.1093/nar/gkab791
Tetali SD (2019) Terpenes and isoprenoids: a wealth of compounds for global use. Planta 249(1):1–8. https://doi.org/10.1007/s00425-018-3056-x
Thibodeaux CJ, Liu HW (2017) The type II isopentenyl Diphosphate: dimethylallyl diphosphate isomerase (IDI-2): a model for acid/base chemistry in flavoenzyme catalysis. Arch Biochem Biophys 632:47–58. https://doi.org/10.1016/j.abb.2017.05.017
Ungerer J, Lin PC, Chen HY, Pakrasi HB (2018) Adjustments to photosystem stoichiometry and electron transfer proteins are key to the remarkably fast growth of the cyanobacterium Synechococcus elongatus UTEX 2973. Mbio 9(1):e02327-e2417. https://doi.org/10.1128/mbio.02327-17
Valsami EA, Psychogyiou ME, Pateraki A, Chrysoulaki E, Melis A, Ghanotakis DF (2020) Fusion constructs enhance heterologous β-phellandrene production in Synechocystis sp. PCC 6803. J Appl Phycol 32(5):2889–2902. https://doi.org/10.1007/s10811-020-02186-1
Vavitsas K, Rue EØ, Stefánsdóttir LK, Gnanasekaran T, Blennow A, Crocoll C, Gudmundsson S, Jensen PE (2017) Responses of Synechocystis sp. PCC 6803 to heterologous biosynthetic pathways. Microb Cell Factories 16(1):1–11. https://doi.org/10.1186/s12934-017-0757-y
Volke DC, Rohwer J, Fischer R, Jennewein S (2019) Investigation of the methylerythritol 4-phosphate pathway for microbial terpenoid production through metabolic control analysis. Microb Cell Factories 18(1):1–15. https://doi.org/10.1186/s12934-019-1235-5
Wang C, Zheng P, Chen P (2019a) Construction of synthetic pathways for raspberry ketone production in engineered Escherichia coli. Appl Microbiol and Biotechnol 103(9):3715–3725. https://doi.org/10.1007/s00253-019-09748-5
Wang J, Lin HX, Su P, Chen T, Guo J, Gao W, Huang LQ (2019b) Molecular cloning and functional characterization of multiple geranylgeranyl pyrophosphate synthases (ApGGPPS) from Andrographis paniculata. Plant Cell Rep 38(1):117–128. https://doi.org/10.1007/s00299-018-2353-y
Weaver BA (2014) How Taxol/paclitaxel kills cancer cells. Mol Biol Cell 25(18):2677–2681. https://doi.org/10.1091/mbc.E14-04-0916
Werner A, Oliver K, Miller AD, Sebesta J, Peebles CA (2018) Discovery and characterization of Synechocystis sp. PCC 6803 light-entrained promoters in diurnal light: dark cycles. Algal Res 30:121–127. https://doi.org/10.1016/j.algal.2017.12.012
Westfall PJ, Pitera DJ, Lenihan JR et al (2012) Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc Natl Acad Sci 109(3):E111–E118. https://doi.org/10.1073/pnas.1110740109
Wu J, Cheng S, Cao J, Qiao J, Zhao GR (2019) Systematic optimization of limonene production in engineered Escherichia coli. J Agric Food Chem 67(25):7087–7097. https://doi.org/10.1021/acs.jafc.9b01427
Xia M, Liu D, Liu Y, Liu H (2020) The therapeutic effect of artemisinin and its derivatives in kidney disease. Front Pharmacol 11:380. https://doi.org/10.3389/fphar.2020.00380
Yadav I, Rautela A, Kumar S (2021) Approaches in the photosynthetic production of sustainable fuels by cyanobacteria using tools of synthetic biology. World J Microbiol Biotechnol 37:201. https://doi.org/10.1007/s11274-021-03157-5
Yu J, Liberton M, Cliften PF et al (2015) Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO 2. Sci Rep 5(1):1–10. https://doi.org/10.1038/srep08132
Zhang X, Niu M, da Silva JAT et al (2019) Identification and functional characterization of three new terpene synthase genes involved in chemical defense and abiotic stresses in Santalum album. BMC Plant Biol 19(1):1–18. https://doi.org/10.1186/s12870-019-1720-3
Zhou J, Zhang H, Meng H, Zhu Y, Bao G, Zhang Y, Li Y, Ma Y (2014) Discovery of a super-strong promoter enables efficient production of heterologous proteins in cyanobacteria. Sci Rep 4(1):1–6. https://doi.org/10.1038/srep04500
Zhou J, Yang F, Zhang F, Meng H, Zhang Y, Li Y (2021) Impairing photorespiration increases photosynthetic conversion of CO2 to isoprene in engineered cyanobacteria. Bioresour Bioprocess 8(1):1–13. https://doi.org/10.1186/s40643-021-00398-y
Acknowledgements
AR is grateful to the Ministry of Human Resource and Development (MHRD), India, for financial aid.
Funding
Financial assistance for this work was provided by the Ministry of Human Resource and Development (MHRD) India.
Author information
Authors and Affiliations
Contributions
AR was involved in conceptualization, original draft, writing review, and editing. SK was involved in supervision, formal analysis, validation, review, and editing.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Ethical approval
This submitted work has not been published previously. It is not under consideration for publication elsewhere. Its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out. If accepted, it will not be published elsewhere in the same form, in English, or any other language, including electronically, without the written consent of the copyright holder.
Additional information
Communicated by Wusheng Liu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rautela, A., Kumar, S. Engineering plant family TPS into cyanobacterial host for terpenoids production. Plant Cell Rep 41, 1791–1803 (2022). https://doi.org/10.1007/s00299-022-02892-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-022-02892-9