Abstract
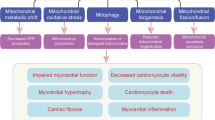
Diabetes mellitus is one of the major causes of ischemic and nonischemic heart failure. While hypertension and coronary artery disease are frequent comorbidities in patients with diabetes, cardiac contractile dysfunction and remodeling occur in diabetic patients even without comorbidities, which is referred to as diabetic cardiomyopathy. Investigations in recent decades have demonstrated that the production of reactive oxygen species (ROS), impaired handling of intracellular Ca2+, and alterations in energy metabolism are involved in the development of diabetic cardiomyopathy. AMP deaminase (AMPD) directly regulates adenine nucleotide metabolism and energy transfer by adenylate kinase and indirectly modulates xanthine oxidoreductase-mediated pathways and AMP-activated protein kinase-mediated signaling. Upregulation of AMPD in diabetic hearts was first reported more than 30 years ago, and subsequent studies showed similar upregulation in the liver and skeletal muscle. Evidence for the roles of AMPD in diabetes-induced fatty liver, sarcopenia, and heart failure has been accumulating. A series of our recent studies showed that AMPD localizes in the mitochondria-associated endoplasmic reticulum membrane as well as the sarcoplasmic reticulum and cytosol and participates in the regulation of mitochondrial Ca2+ and suggested that upregulated AMPD contributes to contractile dysfunction in diabetic cardiomyopathy via increased generation of ROS, adenine nucleotide depletion, and impaired mitochondrial respiration. The detrimental effects of AMPD were manifested at times of increased cardiac workload by pressure loading. In this review, we briefly summarize the expression and functions of AMPD in the heart and discuss the roles of AMPD in diabetic cardiomyopathy, mainly focusing on contractile dysfunction caused by this disorder.


Similar content being viewed by others
Data availability
Not applicable.
References
Hancock CR, Brault JJ, Terjung RL (2006) Protecting the cellular energy state during contractions: role of AMP deaminase. J Physiol Pharmacol 57(Suppl 10):17–29
Zabielska MA, Borkowski T, Slominska EM, Smolenski RT (2015) Inhibition of AMP deaminase as therapeutic target in cardiovascular pathology. Pharmacol Rep 67(4):682–688. https://doi.org/10.1016/j.pharep.2015.04.007
Hong S, Zhou W, Fang B, Lu W, Loro E, Damle M, Ding G, Jager J, Zhang S, Zhang Y, Feng D, Chu Q, Dill BD, Molina H, Khurana TS, Rabinowitz JD, Lazar MA, Sun Z (2017) Dissociation of muscle insulin sensitivity from exercise endurance in mice by HDAC3 depletion. Nat Med 23(2):223–234. https://doi.org/10.1038/nm.4245
Purzycka J (1962) AMP and adenosine aminohydrolases in rat tissues. Acta Biochim Pol 9:83–93
Moriwaki Y, Yamamoto T, Higashino K (1999) Enzymes involved in purine metabolism–a review of histochemical localization and functional implications. Histol Histopathol 14(4):1321–1340. https://doi.org/10.14670/HH-14.1321
Ouyang J, Parakhia RA, Ochs RS (2011) Metformin activates AMP kinase through inhibition of AMP deaminase. J Biol Chem 286(1):1–11. https://doi.org/10.1074/jbc.M110.121806
Lanaspa MA, Cicerchi C, Garcia G, Li N, Roncal-Jimenez CA, Rivard CJ, Hunter B, Andrés-Hernando A, Ishimoto T, Sánchez-Lozada LG, Thomas J, Hodges RS, Mant CT, Johnson RJ (2012) Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver. PLoS ONE 7(11):e48801. https://doi.org/10.1371/journal.pone.0048801
Plaideau C, Liu J, Hartleib-Geschwindner J, Bastin-Coyette L, Bontemps F, Oscarsson J, Hue L, Rider MH (2012) Overexpression of AMP-metabolizing enzymes controls adenine nucleotide levels and AMPK activation in HEK293T cells. FASEB J 26(6):2685–2694. https://doi.org/10.1096/fj.11-198168
Cicerchi C, Li N, Kratzer J, Garcia G, Roncal-Jimenez CA, Tanabe K, Hunter B, Rivard CJ, Sautin YY, Gaucher EA, Johnson RJ, Lanaspa MA (2014) Uric acid-dependent inhibition of AMP kinase induces hepatic glucose production in diabetes and starvation: evolutionary implications of the uricase loss in hominids. FASEB J 28(8):3339–3350. https://doi.org/10.1096/fj.13-243634
Plaideau C, Lai YC, Kviklyte S, Zanou N, Löfgren L, Andersén H, Vertommen D, Gailly P, Hue L, Bohlooly-Y M, Hallén S, Rider MH (2014) Effects of pharmacological AMP deaminase inhibition and Ampd1 deletion on nucleotide levels and AMPK activation in contracting skeletal muscle. Chem Biol 21(11):1497–1510. https://doi.org/10.1016/j.chembiol.2014.09.013
Tandelilin AA, Hirase T, Hudoyo AW, Cheng J, Toyama K, Morisaki H, Morisaki T (2015) AMPD1 regulates mTORC1-p70 S6 kinase axis in the control of insulin sensitivity in skeletal muscle. BMC Endocr Disord 15:11. https://doi.org/10.1186/s12902-015-0010-9
Davis PR, Miller SG, Verhoeven NA, Morgan JS, Tulis DA, Witczak CA, Brault JJ (2020) Increased AMP deaminase activity decreases ATP content and slows protein degradation in cultured skeletal muscle. Metabolism 108:154257. https://doi.org/10.1016/j.metabol.2020.154257
Miller SG, Hafen PS, Law AS, Springer CB, Logsdon DL, O’Connell TM, Witczak CA, Brault JJ (2021) AMP deamination is sufficient to replicate an atrophy-like metabolic phenotype in skeletal muscle. Metabolism 123:154864. https://doi.org/10.1016/j.metabol.2021.154864
Hafen PS, Law AS, Matias C, Miller SG, Brault JJ (2022) Skeletal muscle contraction kinetics and AMPK responses are modulated by the adenine nucleotide degrading enzyme AMPD1. J Appl Physiol 133(5):1055–1066. https://doi.org/10.1152/japplphysiol.00035.2022
Fischer S, Drenckhahn C, Wolf C, Eschrich K, Kellermann S, Froster UG, Schober R (2005) Clinical significance and neuropathology of primary MADD in C34-T and G468-T mutations of the AMPD1 gene. Clin Neuropathol 24(2):77–85
Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL (2004) Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18(1):39–51. https://doi.org/10.1096/fj.03-0610com
Cheng J, Morisaki H, Toyama K, Sugimoto N, Shintani T, Tandelilin A, Hirase T, Holmes EW, Morisaki T (2014) AMPD1: a novel therapeutic target for reversing insulin resistance. BMC Endocr Disord 14:96. https://doi.org/10.1186/1472-6823-14-96
Admyre T, Amrot-Fors L, Andersson M, Bauer M, Bjursell M, Drmota T, Hallen S, Hartleib-Geschwindner J, Lindmark B, Liu J, Löfgren L, Rohman M, Selmi N, Wallenius K (2014) Inhibition of AMP deaminase activity does not improve glucose control in rodent models of insulin resistance or diabetes. Chem Biol 21(11):1486–1496. https://doi.org/10.1016/j.chembiol.2014.09.011
Hudoyo AW, Hirase T, Tandelillin A, Honda M, Shirai M, Cheng J, Morisaki H, Morisaki T (2017) Role of AMPD2 in impaired glucose tolerance induced by high fructose diet. Mol Genet Metab Rep 13:23–29. https://doi.org/10.1016/j.ymgmr.2017.07.006
Dzeja P, Terzic A (2009) Adenylate kinase and AMP signaling networks: metabolic monitoring, signal communication and body energy sensing. Int J Mol Sci 10(4):1729–1772. https://doi.org/10.3390/ijms10041729
Jenkins RL, McDaniel HG, Atkins L (1991) Changes in AMP deaminase activities in the hearts of diabetic rats. Biochim Biophys Acta 1077(3):379–384. https://doi.org/10.1016/0167-4838(91)90554-d
Podgorska M, Kocbuch K, Grden M, Szutowicz A, Pawelczyk T (2006) Prevalence of unidirectional Na+-dependent adenosine transport and altered potential for adenosine generation in diabetic cardiac myocytes. Basic Res Cardiol 101(3):214–222. https://doi.org/10.1007/s00395-005-0578-8
Kouzu H, Miki T, Tanno M, Kuno A, Yano T, Itoh T, Sato T, Sunaga D, Murase H, Tobisawa T, Ogasawara M, Ishikawa S, Miura T (2015) Excessive degradation of adenine nucleotides by up-regulated AMP deaminase underlies afterload-induced diastolic dysfunction in the type 2 diabetic heart. J Mol Cell Cardiol 80:136–145. https://doi.org/10.1016/j.yjmcc.2015.01.004
Castro MC, Villagarcía HG, Schinella G, Massa ML (1868) Francini F (2023) Mechanism of preventive effects of exendin-4 and des-fluoro-sitagliptin in a murine model of fructose-induced prediabetes. Biochim Biophys Acta Mol Cell Biol Lipids 9:159363. https://doi.org/10.1016/j.bbalip.2023.159363
Pawelczyk T, Sakowicz M, Szczepanska-Konkel M, Angielski S (2000) Decreased expression of adenosine kinase in streptozotocin-induced diabetes mellitus rats. Arch Biochem Biophys 375(1):1–6. https://doi.org/10.1006/abbi.1999.1548
Yang H, Wang Q, Xi Y, Yu W, Xie D, Morisaki H, Morisaki T, Cheng J (2023) AMPD2 plays important roles in regulating hepatic glucose and lipid metabolism. Mol Cell Endocrinol 577:112039. https://doi.org/10.1016/j.mce.2023.112039
Andres-Hernando A, Cicerchi C, Garcia GE, Orlicky DJ, Stenvinkel P, Johnson RJ, Lanaspa MA (2023) Phosphate depletion in insulin-insensitive skeletal muscle drives AMPD activation and sarcopenia in chronic kidney disease. Science 26(4):106355. https://doi.org/10.1016/j.isci.2023.106355
Miki T, Yuda S, Kouzu H, Miura T (2013) Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev 18(2):149–166. https://doi.org/10.1007/s10741-012-9313-3
Jia G, Hill MA, Sowers JR (2018) Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res 122(4):624–638. https://doi.org/10.1161/CIRCRESAHA.117.311586
Maack C, Lehrke M, Backs J, Heinzel FR, Hulot JS, Marx N, Paulus WJ, Rossignol P, Taegtmeyer H, Bauersachs J, Bayes-Genis A, Brutsaert D, Bugger H, Clarke K, Cosentino F, De Keulenaer G, Dei Cas A, González A, Huelsmann M, Iaccarino G, Lunde IG, Lyon AR, Pollesello P, Rena G, Riksen NP, Rosano G, Staels B, van Laake LW, Wanner C, Farmakis D, Filippatos G, Ruschitzka F, Seferovic P, de Boer RA, Heymans S (2018) Heart failure and diabetes: metabolic alterations and therapeutic interventions: a state-of-the-art review from the Translational Research Committee of the Heart Failure Association-European Society of Cardiology. Eur Heart J 39(48):4243–4254. https://doi.org/10.1093/eurheartj/ehy596
Ritchie RH, Abel ED (2020) Basic mechanisms of diabetic heart disease. Circ Res 126(11):1501–1525. https://doi.org/10.1161/CIRCRESAHA.120.315913
Rupee S, Rupee K, Singh RB, Hanoman C, Ismail AMA, Smail M, Singh J (2023) Diabetes-induced chronic heart failure is due to defects in calcium transporting and regulatory contractile proteins: cellular and molecular evidence. Heart Fail Rev 28(3):627–644. https://doi.org/10.1007/s10741-022-10271-5
Cusi K, Sanyal AJ, Zhang S, Hartman ML, Bue-Valleskey JM, Hoogwerf BJ, Haupt A (2017) Non-alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes Metab 19(11):1630–1634. https://doi.org/10.1111/dom.12973
Memaj P, Jornayvaz FR (2022) Non-alcoholic fatty liver disease in type 1 diabetes: prevalence and pathophysiology. Front Endocrinol (Lausanne) 13:1031633. https://doi.org/10.3389/fendo.2022.1031633
Tack W, De Cock AM, Dirinck EL, Bastijns S, Ariën F, Perkisas S (2023) Pathophysiological interactions between sarcopenia and type 2 diabetes: a two-way street influencing diagnosis and therapeutic options. Diabetes Obes Metab. https://doi.org/10.1111/dom.15321
Tatekoshi Y, Tanno M, Kouzu H, Abe K, Miki T, Kuno A, Yano T, Ishikawa S, Ohwada W, Sato T, Niinuma T, Suzuki H, Miura T (2018) Translational regulation by miR-301b upregulates AMP deaminase in diabetic hearts. J Mol Cell Cardiol 119:138–146. https://doi.org/10.1016/j.yjmcc.2018.05.003
Igaki Y, Tanno M, Sato T, Kouzu H, Ogawa T, Osanami A, Yano T, Kuno A, Miki T, Nakamura T, Miura T (2021) Xanthine oxidoreductase-mediated injury is amplified by upregulated AMP deaminase in type 2 diabetic rat hearts under the condition of pressure overload. J Mol Cell Cardiol 154:21–31. https://doi.org/10.1016/j.yjmcc.2021.01.002
Ogawa T, Kouzu H, Osanami A, Tatekoshi Y, Sato T, Kuno A, Fujita Y, Ino S, Shimizu M, Toda Y, Ohwada W, Yano T, Tanno M, Miki T, Miura T (2023) Downregulation of extramitochondrial BCKDH and its uncoupling from AMP deaminase in type 2 diabetic OLETF rat hearts. Physiol Rep 11(4):e15608. https://doi.org/10.14814/phy2.15608
Osanami A, Sato T, Toda Y, Shimizu M, Kuno A, Kouzu H, Yano T, Ohwada W, Ogawa T, Miura T, Tanno M (2023) Adenosine monophosphate deaminase in the endoplasmic reticulum-mitochondria interface promotes mitochondrial Ca2+ overload in type 2 diabetes rat hearts. J Diabetes Investig 14(4):560–569. https://doi.org/10.1111/jdi.13982
Ogasawara N, Goto H, Yamada Y, Watanabe T (1978) Distribution of AMP-deaminase isozymes in rat tissues. Eur J Biochem 87(2):297–304. https://doi.org/10.1111/j.1432-1033.1978.tb12378.x
Ogasawara N, Goto H, Yamada Y, Watanabe T, Asano T (1982) AMP deaminase isozymes in human tissues. Biochim Biophys Acta 714(2):298–306. https://doi.org/10.1016/0304-4165(82)90337-3
Sabina RL, Mahnke-Zizelman DK (2000) Towards an understanding of the functional significance of N-terminal domain divergence in human AMP deaminase isoforms. Pharmacol Ther 87(2–3):279–283. https://doi.org/10.1016/s0163-7258(00)00040-1
Thakkar JK, Janero DR, Yarwood C, Sharif H, Hreniuk D (1993) Isolation and characterization of AMP deaminase from mammalian (rabbit) myocardium. Biochem J 290(2):335–341. https://doi.org/10.1042/bj2900335
Mahnke-Zizelman DK, Tullson PC, Sabina RL (1998) Novel aspects of tetramer assembly and N-terminal domain structure and function are revealed by recombinant expression of human AMP deaminase isoforms. J Biol Chem 273(52):35118–35125. https://doi.org/10.1074/jbc.273.52.35118
Mahnke-Zizelman DK, Sabina RL (2001) Localization of N-terminal sequences in human AMP deaminase isoforms that influence contractile protein binding. Biochem Biophys Res Commun 285(2):489–495. https://doi.org/10.1006/bbrc.2001.5180
Mahnke-Zizelman DK, Sabina RL (2002) N-terminal sequence and distal histidine residues are responsible for pH-regulated cytoplasmic membrane binding of human AMP deaminase isoform E. J Biol Chem 277(45):42654–42662. https://doi.org/10.1074/jbc.M203473200
Sims B, Mahnke-Zizelman DK, Profit AA, Prestwich GD, Sabina RL, Theibert AB (1999) Regulation of AMP deaminase by phosphoinositides. J Biol Chem 274(36):25701–25707. https://doi.org/10.1074/jbc.274.36.25701
Mahnke-Zizelman DK, D’cunha J, Wojnar JM, Brogley MA, Sabina RL (1997) Regulation of rat AMP deaminase 3 (isoform C) by development and skeletal muscle fibre type. Biochem J 326(2):521–529. https://doi.org/10.1042/bj3260521
Barshop BA, Frieden C (1984) Analysis of the interaction of rabbit skeletal muscle adenylate deaminase with myosin subfragments. A kinetically regulated system. J Biol Chem 259(1):60–66
Marquetant R, Sabina RL, Holmes EW (1989) Identification of a noncatalytic domain in AMP deaminase that influences binding to myosin. Biochemistry 28(22):8744–8749. https://doi.org/10.1021/bi00448a010
Chilson OP, Kelly-Chilson AE, Siegel NR (1997) AMP-deaminases from chicken and rabbit muscle: partial primary sequences of homologous 17-kDa CNBr fragments: autorecognition by rabbit anti-[chicken AMPD]. Comp Biochem Physiol B Biochem Mol Biol 116(3):371–377. https://doi.org/10.1016/s0305-0491(96)00270-2
Ronca F, Raggi A (2023) Role of the interaction between troponin T and AMP deaminase by zinc bridge in modulating muscle contraction and ammonia production. Mol Cell Biochem. https://doi.org/10.1007/s11010-023-04763-7
Wu J, Bond C, Chen P, Chen M, Li Y, Shohet RV, Wright G (2015) HIF-1α in the heart: remodeling nucleotide metabolism. J Mol Cell Cardiol 82:194–200. https://doi.org/10.1016/j.yjmcc.2015.01.014
Milan G, Romanello V, Pescatore F, Armani A, Paik JH, Frasson L, Seydel A, Zhao J, Abraham R, Goldberg AL, Blaauw B, DePinho RA, Sandri M (2015) Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun 6:6670. https://doi.org/10.1038/ncomms7670
Brocca L, Toniolo L, Reggiani C, Bottinelli R, Sandri M, Pellegrino MA (2017) FoxO-dependent atrogenes vary among catabolic conditions and play a key role in muscle atrophy induced by hindlimb suspension. J Physiol 595(4):1143–1158. https://doi.org/10.1113/JP273097
Mi Z, Song Y, Cao X, Lu Y, Liu Z, Zhu X, Geng M, Sun Y, Lan B, He C, Xiong H, Zhang L, Chen Y (2020) Super-enhancer-driven metabolic reprogramming promotes cystogenesis in autosomal dominant polycystic kidney disease. Nat Metab 2(8):717–731. https://doi.org/10.1038/s42255-020-0227-4
Barsacchi R, Ranieri-Raggi M, Bergamini C, Raggi A (1979) Adenylate metabolism in the heart. Regulatory properties of rabbit cardiac adenylate deaminase. Biochem J 182(2):361–366. https://doi.org/10.1042/bj1820361
Spychala J, Marszalek J (1991) Regulatory properties of AMP deaminases from rat tissues. Int J Biochem 23(10):1155–1159. https://doi.org/10.1016/0020-711x(91)90158-j
Thakkar JK, Janero DR, Yarwood C, Sharif HM (1993) Modulation of mammalian cardiac AMP deaminase by protein kinase C-mediated phosphorylation. Biochem J 291(2):523–527. https://doi.org/10.1042/bj2910523
Thakkar JK, Janero DR, Sharif HM, Hreniuk D, Yarwood C (1994) Cardiac adenylate deaminase: molecular, kinetic and regulatory properties under phosphate-free conditions. Biochem J 300(2):359–363. https://doi.org/10.1042/bj3000359
Hu B, Altschuld RA, Hohl CM (1993) Adenosine stimulation of AMP deaminase activity in adult rat cardiac myocytes. Am J Physiol 264(1 Pt 1):C48-53. https://doi.org/10.1152/ajpcell.1993.264.1.C48
Hohl AM, Hohl CM (1999) Isolation and regulation of piglet cardiac AMP deaminase. Mol Cell Biochem 201(1–2):151–158. https://doi.org/10.1023/a:1007083000564
Tovmasian EK, Hairapetian RL, Bykova EV, Severin SE Jr, Haroutunian AV (1990) Phosphorylation of the skeletal muscle AMP-deaminase by protein kinase C. FEBS Lett 259(2):321–323. https://doi.org/10.1016/0014-5793(90)80037-j
Mahnke DK, Sabina RL (2005) Calcium activates erythrocyte AMP deaminase [isoform E (AMPD3)] through a protein-protein interaction between calmodulin and the N-terminal domain of the AMPD3 polypeptide. Biochemistry 44(14):5551–5559. https://doi.org/10.1021/bi048121p
Entman ML, Goldstein MA (1976) Schwartz A (1976) The cardiac sarcoplasmic reticulum—glycogenolytic complex, an internal beta-adrenergic receptor. Life Sci 19(11):1623–1630. https://doi.org/10.1016/0024-3205(76)90066-7
Entman ML, Kanike K, Goldstein MA, Nelson TE, Bornet EP, Futch TW, Schwartz A (1976) Association of gylcogenolysis with cardiac sarcoplasmic reticulum. J Biol Chem 251(10):3140–3146
Entman ML, Bornet EP, Van Winkle WB, Goldstein MA, Schwartz A (1977) Association of glycogenolysis with cardiac sarcoplasmic reticulum: II. Effect of glycogen depletion, deoxycholate solubilization and cardiac ischemia: evidence for a phorphorylase kinase membrane complex. J Mol Cell Cardiol 9(7):515–528. https://doi.org/10.1016/s0022-2828(77)80367-2
Entman ML, Bornet EP, Barber AJ, Schwartz A, Levey GS, Lehotay DC, Bricker LA (1977) The cardiac sarcoplasmic reticulum-glycogenolytic complex. A possible effector site for cyclic AMP. Biochim Biophys Acta 499(2):228–237. https://doi.org/10.1016/0304-4165(77)90005-8
Goldstein MA, Murphy DL, van Winkle WB, Entman ML (1985) Cytochemical studies of a glycogen-sarcoplasmic reticulum complex. J Muscle Res Cell Motil 6(2):177–187. https://doi.org/10.1007/BF00713059
Ashby B, Frieden C, Bischoff R (1979) Immunofluorescent and histochemical localization of AMP deaminase in skeletal muscle. J Cell Biol 81(2):361–373. https://doi.org/10.1083/jcb.81.2.361
Mattii L, Bianchi F, Falleni A, Frascarelli S, Masini M, Alì G, Chiellini G, Sabbatini ARM (2020) Ultrastructural localization of histidine-rich glycoprotein in skeletal muscle fibers: colocalization with AMP deaminase. J Histochem Cytochem 68(2):139–148. https://doi.org/10.1369/0022155419897573
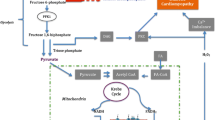
Aragón JJ, Lowenstein JM (1980) The purine-nucleotide cycle. Comparison of the levels of citric acid cycle intermediates with the operation of the purine nucleotide cycle in rat skeletal muscle during exercise and recovery from exercise. Eur J Biochem 110(2):371–377. https://doi.org/10.1111/j.1432-1033.1980.tb04877.x
Graham TE, MacLean DA (1992) Ammonia and amino acid metabolism in human skeletal muscle during exercise. Can J Physiol Pharmacol 70(1):132–141. https://doi.org/10.1139/y92-020
Taegtmeyer H (1985) On the role of the purine nucleotide cycle in the isolated working rat heart. J Mol Cell Cardiol 17(10):1013–1018. https://doi.org/10.1016/s0022-2828(85)80082-1
Davey CL (1961) The amination of inosine monophosphate in skeletal muscle. Arch Biochem Biophys 95:296–304. https://doi.org/10.1016/0003-9861(61)90149-7
Dow JW, Bowditch J, Nigdikar SV, Brown AK (1987) Salvage mechanisms for regeneration of adenosine triphosphate in rat cardiac myocytes. Cardiovasc Res 21(3):188–196. https://doi.org/10.1093/cvr/21.3.188
Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, Jeyaraj D, Youn JY, Ren S, Liu Y, Rau CD, Shah S, Ilkayeva O, Gui WJ, William NS, Wynn RM, Newgard CB, Cai H, Xiao X, Chuang DT, Schulze PC, Lynch C, Jain MK, Wang Y (2016) Catabolic defect of branched-chain amino acids promotes heart failure. Circulation 133(21):2038–2049. https://doi.org/10.1161/CIRCULATIONAHA.115.020226
Li T, Zhang Z, Kolwicz SC Jr, Abell L, Roe ND, Kim M, Zhou B, Cao Y, Ritterhoff J, Gu H, Raftery D, Sun H, Tian R (2017) Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia-reperfusion injury. Cell Metab 25(2):374–385. https://doi.org/10.1016/j.cmet.2016.11.005
Yu JY, Cao N, Rau CD, Lee RP, Yang J, Flach RJR, Petersen L, Zhu C, Pak YL, Miller RA, Liu Y, Wang Y, Li Z, Sun H, Gao C (2023) Cell-autonomous effect of cardiomyocyte branched-chain amino acid catabolism in heart failure in mice. Acta Pharmacol Sin 44(7):1380–1390. https://doi.org/10.1038/s41401-023-01076-9
Mizuno M, Kuno A, Yano T, Miki T, Oshima H, Sato T, Nakata K, Kimura Y, Tanno M, Miura T (2018) Empagliflozin normalizes the size and number of mitochondria and prevents reduction in mitochondrial size after myocardial infarction in diabetic hearts. Physiol Rep 6(12):e13741. https://doi.org/10.14814/phy2.13741
Lin SC, Hardie DG (2018) AMPK: sensing glucose as well as cellular energy status. Cell Metab 27(2):299–313. https://doi.org/10.1016/j.cmet.2017.10.009
Rybakowska I, Slominska EM, Romaszko P, Lipiński M, Zukowska P, Smolenski RT (2014) Activity of AMP-regulated protein kinase and AMP-deaminase in the heart of mice fed high-fat diet. Nucleosides Nucleotides Nucleic Acids 33(4–6):347–352. https://doi.org/10.1080/15257770.2014.880480
Rybakowska IM, Slominska EM, Romaszko P, Olkowicz M, Kaletha K, Smolenski RT (2015) AMP-regulated protein kinase activity in the hearts of mice treated with low- or high-fat diet measured using novel LC-MS method. Mol Cell Biochem 404(1–2):5–10. https://doi.org/10.1007/s11010-015-2360-z
Murase H, Kuno A, Miki T, Tanno M, Yano T, Kouzu H, Ishikawa S, Tobisawa T, Ogasawara M, Nishizawa K, Miura T (2015) Inhibition of DPP-4 reduces acute mortality after myocardial infarction with restoration of autophagic response in type 2 diabetic rats. Cardiovasc Diabetol 14:103. https://doi.org/10.1186/s12933-015-0264-6
Xu KY, Zweier JL, Becker LC (1995) Functional coupling between glycolysis and sarcoplasmic reticulum Ca2+ transport. Circ Res 77(1):88–97. https://doi.org/10.1161/01.res.77.1.88
Goodwin GW, Ahmad F, Taegtmeyer H (1996) Preferential oxidation of glycogen in isolated working rat heart. J Clin Invest 97(6):1409–1416. https://doi.org/10.1172/JCI118561
Goodwin GW, Ahmad F, Doenst T, Taegtmeyer H (1998) Energy provision from glycogen, glucose, and fatty acids on adrenergic stimulation of isolated working rat hearts. Am J Physiol 274(4):H1239–H1247. https://doi.org/10.1152/ajpheart.1998.274.4.H1239
Katz A (2022) A century of exercise physiology: key concepts in regulation of glycogen metabolism in skeletal muscle. Eur J Appl Physiol 122(8):1751–1772. https://doi.org/10.1007/s00421-022-04935-1
Panayiotou C, Solaroli N, Karlsson A (2014) The many isoforms of human adenylate kinases. Int J Biochem Cell Biol 49:75–83. https://doi.org/10.1016/j.biocel.2014.01.014
Dzeja PP, Vitkevicius KT, Redfield MM, Burnett JC, Terzic A (1999) Adenylate kinase-catalyzed phosphotransfer in the myocardium: increased contribution in heart failure. Circ Res 84(10):1137–1143. https://doi.org/10.1161/01.res.84.10.1137
Aksentijević D, Lygate CA, Makinen K, Zervou S, Sebag-Montefiore L, Medway D, Barnes H, Schneider JE, Neubauer S (2010) High-energy phosphotransfer in the failing mouse heart: role of adenylate kinase and glycolytic enzymes. Eur J Heart Fail 12(12):1282–1289. https://doi.org/10.1093/eurjhf/hfq174
Prandi FR, Evangelista I, Sergi D, Palazzuoli A, Romeo F (2023) Mechanisms of cardiac dysfunction in diabetic cardiomyopathy: molecular abnormalities and phenotypical variants. Heart Fail Rev 28(3):597–606. https://doi.org/10.1007/s10741-021-10200-y
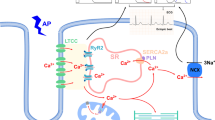
Jaquenod De Giusti C, Palomeque J, Mattiazzi A (2022) Ca2+ mishandling and mitochondrial dysfunction: a converging road to prediabetic and diabetic cardiomyopathy. Pflugers Arch 474(1):33–61. https://doi.org/10.1007/s00424-021-02650-y
Jenkins MJ, Pearson JT, Schwenke DO, Edgley AJ, Sonobe T, Fujii Y, Ishibashi-Ueda H, Kelly DJ, Yagi N, Shirai M (2013) Myosin heads are displaced from actin filaments in the in situ beating rat heart in early diabetes. Biophys J 104(5):1065–1072. https://doi.org/10.1016/j.bpj.2013.01.037
Waddingham MT, Edgley AJ, Astolfo A, Inagaki T, Fujii Y, Du CK, Zhan DY, Tsuchimochi H, Yagi N, Kelly DJ, Shirai M, Pearson JT (2015) Chronic Rho-kinase inhibition improves left ventricular contractile dysfunction in early type-1 diabetes by increasing myosin cross-bridge extension. Cardiovasc Diabetol 14:92. https://doi.org/10.1186/s12933-015-0256-6
Funakoshi A, Miyasaka K, Shinozaki H, Masuda M, Kawanami T, Takata Y, Kono A (1995) An animal model of congenital defect of gene expression of cholecystokinin (CCK)-A receptor. Biochem Biophys Res Commun 210(3):787–796. https://doi.org/10.1006/bbrc.1995.1728
Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T (1992) Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes 41(11):1422–1428. https://doi.org/10.2337/diab.41.11.1422
Bi S, Moran TH (2016) Obesity in the Otsuka Long Evans Tokushima fatty rat: mechanisms and discoveries. Front Nutr 27(3):21. https://doi.org/10.3389/fnut.2016.00021
Yagi K, Kim S, Wanibuchi H, Yamashita T, Yamamura Y, Iwao H (1997) Characteristics of diabetes, blood pressure, and cardiac and renal complications in Otsuka Long-Evans Tokushima Fatty rats. Hypertension 29(3):728–735. https://doi.org/10.1161/01.hyp.29.3.728
Mizushige K, Yao L, Noma T, Kiyomoto H, Yu Y, Hosomi N, Ohmori K, Matsuo H (2000) Alteration in left ventricular diastolic filling and accumulation of myocardial collagen at insulin-resistant prediabetic stage of a type II diabetic rat model. Circulation 101(8):899–907. https://doi.org/10.1161/01.cir.101.8.899
Yu Y, Ohmori K, Kondo I, Yao L, Noma T, Tsuji T, Mizushige K, Kohno M (2002) Correlation of functional and structural alterations of the coronary arterioles during development of type II diabetes mellitus in rats. Cardiovasc Res 56(2):303–311. https://doi.org/10.1016/s0008-6363(02)00513-8
Hosomi N, Noma T, Ohyama H, Takahashi T, Kohno M (2002) Vascular proliferation and transforming growth factor-beta expression in pre- and early stage of diabetes mellitus in Otsuka Long-Evans Tokushima fatty rats. Atherosclerosis 162(1):69–76. https://doi.org/10.1016/s0021-9150(01)00683-9
Saito F, Kawaguchi M, Izumida J, Asakura T, Maehara K, Maruyama Y (2003) Alteration in haemodynamics and pathological changes in the cardiovascular system during the development of Type 2 diabetes mellitus in OLETF rats. Diabetologia 46(8):1161–1169. https://doi.org/10.1007/s00125-003-1156-y
Sakata S, Lebeche D, Sakata Y, Sakata N, Chemaly ER, Liang LF, Padmanabhan P, Konishi N, Takaki M, del Monte F, Hajjar RJ (2006) Mechanical and metabolic rescue in a type II diabetes model of cardiomyopathy by targeted gene transfer. Mol Ther 13(5):987–996. https://doi.org/10.1016/j.ymthe.2006.01.002
Sakata S, Lebeche D, Sakata N, Sakata Y, Chemaly ER, Liang LF, Tsuji T, Takewa Y, del Monte F, Peluso R, Zsebo K, Jeong D, Park WJ, Kawase Y, Hajjar RJ (2007) Restoration of mechanical and energetic function in failing aortic-banded rat hearts by gene transfer of calcium cycling proteins. J Mol Cell Cardiol 42(4):852–861. https://doi.org/10.1016/j.yjmcc.2007.01.003
Takada A, Miki T, Kuno A, Kouzu H, Sunaga D, Itoh T, Tanno M, Yano T, Sato T, Ishikawa S, Miura T (2012) Role of ER stress in ventricular contractile dysfunction in type 2 diabetes. PLoS ONE 7(6):e39893. https://doi.org/10.1371/journal.pone.0039893
She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ (2007) Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab 293(6):E1552–E1563. https://doi.org/10.1152/ajpendo.00134.2007
Herman MA, She P, Peroni OD, Lynch CJ, Kahn BB (2010) Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J Biol Chem 285(15):11348–11356. https://doi.org/10.1074/jbc.M109.075184
Wiklund P, Zhang X, Pekkala S, Autio R, Kong L, Yang Y, Keinänen-Kiukaanniemi S, Alen M, Cheng S (2016) Insulin resistance is associated with altered amino acid metabolism and adipose tissue dysfunction in normoglycemic women. Sci Rep 6:24540. https://doi.org/10.1038/srep24540
Kammermeier H (1987) High energy phosphate of the myocardium: concentration versus free energy change. Basic Res Cardiol 82(Suppl 2):31–36. https://doi.org/10.1007/978-3-662-11289-2_3
Hu Y, Suarez J, Fricovsky E, Wang H, Scott BT, Trauger SA, Han W, Hu Y, Oyeleye MO, Dillmann WH (2009) Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose. J Biol Chem 284(1):547–555. https://doi.org/10.1074/jbc.M808518200
Diaz-Juarez J, Suarez J, Cividini F, Scott BT, Diemer T, Dai A, Dillmann WH (2016) Expression of the mitochondrial calcium uniporter in cardiac myocytes improves impaired mitochondrial calcium handling and metabolism in simulated hyperglycemia. Am J Physiol Cell Physiol 311(6):C1005–C1013. https://doi.org/10.1152/ajpcell.00236.2016
Ji L, Liu F, Jing Z, Huang Q, Zhao Y, Cao H, Li J, Yin C, Xing J, Li F (2017) MICU1 alleviates diabetic cardiomyopathy through mitochondrial Ca2+-dependent antioxidant response. Diabetes 66(6):1586–1600. https://doi.org/10.2337/db16-1237
Suarez J, Cividini F, Scott BT, Lehmann K, Diaz-Juarez J, Diemer T, Dai A, Suarez JA, Jain M, Dillmann WH (2018) Restoring mitochondrial calcium uniporter expression in diabetic mouse heart improves mitochondrial calcium handling and cardiac function. J Biol Chem 293(21):8182–8195. https://doi.org/10.1074/jbc.RA118.002066
Dia M, Gomez L, Thibault H, Tessier N, Leon C, Chouabe C, Ducreux S, Gallo-Bona N, Tubbs E, Bendridi N, Chanon S, Leray A, Belmudes L, Couté Y, Kurdi M, Ovize M, Rieusset J, Paillard M (2020) Reduced reticulum-mitochondria Ca2+ transfer is an early and reversible trigger of mitochondrial dysfunctions in diabetic cardiomyopathy. Basic Res Cardiol 115(6):74. https://doi.org/10.1007/s00395-020-00835-7
Velmurugan S, Liu T, Chen KC, Despa F, O’Rourke B, Despa S (2023) Distinct effects of mitochondrial Na+/Ca2+ exchanger inhibition and Ca2+ uniporter activation on Ca2+ sparks and arrhythmogenesis in diabetic rats. J Am Heart Assoc 12(14):e029997. https://doi.org/10.1161/JAHA.123.029997
Yu Z, Quamme GA, McNeill JH (1994) Depressed [Ca2+]i responses to isoproterenol and cAMP in isolated cardiomyocytes from experimental diabetic rats. Am J Physiol 266(6 Pt 2):H2334–H2342. https://doi.org/10.1152/ajpheart.1994.266.6.H2334
Lagadic-Gossmann D, Buckler KJ, Le Prigent K, Feuvray D (1996) Altered Ca2+ handling in ventricular myocytes isolated from diabetic rats. Am J Physiol 270(5 Pt 2):H1529–H1537. https://doi.org/10.1152/ajpheart.1996.270.5.H1529
Belke DD, Swanson EA, Dillmann WH (2004) Decreased sarcoplasmic reticulum activity and contractility in diabetic db/db mouse heart. Diabetes 53(12):3201–3208. https://doi.org/10.2337/diabetes.53.12.3201
Garbincius JF, Elrod JW (2022) Mitochondrial calcium exchange in physiology and disease. Physiol Rev 102(2):893–992. https://doi.org/10.1152/physrev.00041.2020
Kaludercic N, Di Lisa F (2020) Mitochondrial ROS formation in the pathogenesis of diabetic cardiomyopathy. Front Cardiovasc Med 7:12. https://doi.org/10.3389/fcvm.2020.00012
Privratsky JR, Wold LE, Sowers JR, Quinn MT, Ren J (2003) AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes: role of the AT1 receptor and NADPH oxidase. Hypertension 42(2):206–212. https://doi.org/10.1161/01.HYP.0000082814.62655.85
Zhang M, Kho AL, Anilkumar N, Chibber R, Pagano PJ, Shah AM, Cave AC (2006) Glycated proteins stimulate reactive oxygen species production in cardiac myocytes: involvement of Nox2 (gp91phox)-containing NADPH oxidase. Circulation 113(9):1235–1243. https://doi.org/10.1161/CIRCULATIONAHA.105.581397
Roe ND, Thomas DP, Ren J (2011) Inhibition of NADPH oxidase alleviates experimental diabetes-induced myocardial contractile dysfunction. Diabetes Obes Metab 13(5):465–473. https://doi.org/10.1111/j.1463-1326.2011.01369.x
Varga ZV, Kupai K, Szűcs G, Gáspár R, Pálóczi J, Faragó N, Zvara A, Puskás LG, Rázga Z, Tiszlavicz L, Bencsik P, Görbe A, Csonka C, Ferdinandy P, Csont T (2013) MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J Mol Cell Cardiol 62:111–121. https://doi.org/10.1016/j.yjmcc.2013.05.009
Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD (2009) Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol 54(20):1891–1898. https://doi.org/10.1016/j.jacc.2009.07.031
Mariappan N, Elks CM, Sriramula S, Guggilam A, Liu Z, Borkhsenious O, Francis J (2010) NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res 85(3):473–483. https://doi.org/10.1093/cvr/cvp305
Bombicino SS, Iglesias DE, Mikusic IAR, D’Annunzio V, Gelpi RJ, Boveris A, Valdez LB (2016) Diabetes impairs heart mitochondrial function without changes in resting cardiac performance. Int J Biochem Cell Biol 81(Pt B):335–345. https://doi.org/10.1016/j.biocel.2016.09.018
Koncsos G, Varga ZV, Baranyai T, Boengler K, Rohrbach S, Li L, Schlüter KD, Schreckenberg R, Radovits T, Oláh A, Mátyás C, Lux Á, Al-Khrasani M, Komlódi T, Bukosza N, Máthé D, Deres L, Barteková M, Rajtík T, Adameová A, Szigeti K, Hamar P, Helyes Z, Tretter L, Pacher P, Merkely B, Giricz Z, Schulz R, Ferdinandy P (2016) Diastolic dysfunction in prediabetic male rats: Role of mitochondrial oxidative stress. Am J Physiol Heart Circ Physiol 311(4):H927–H943. https://doi.org/10.1152/ajpheart.00049.2016
Jeong EM, Chung J, Liu H, Go Y, Gladstein S, Farzaneh-Far A, Lewandowski ED, Dudley SC Jr (2016) Role of mitochondrial oxidative stress in glucose tolerance, insulin resistance, and cardiac diastolic dysfunction. J Am Heart Assoc 5(5):e003046. https://doi.org/10.1161/JAHA.115.003046
Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87(1):315–424. https://doi.org/10.1152/physrev.00029.2006
Soliman H, Gador A, Lu YH, Lin G, Bankar G, MacLeod KM (2012) Diabetes-induced increased oxidative stress in cardiomyocytes is sustained by a positive feedback loop involving Rho kinase and PKCβ2. Am J Physiol Heart Circ Physiol 303(8):H989–H1000. https://doi.org/10.1152/ajpheart.00416.2012
Lin C, Guo Y, Xia Y, Li C, Xu X, Qi T, Zhang F, Fan M, Hu G, Zhao H, Zhao H, Liu R, Gao E, Yan W, Tao L (2021) FNDC5/Irisin attenuates diabetic cardiomyopathy in a type 2 diabetes mouse model by activation of integrin αV/β5-AKT signaling and reduction of oxidative/nitrosative stress. J Mol Cell Cardiol 160:27–41. https://doi.org/10.1016/j.yjmcc.2021.06.013
Rajesh M, Mukhopadhyay P, Bátkai S, Mukhopadhyay B, Patel V, Haskó G, Szabó C, Mabley JG, Liaudet L, Pacher P (2009) Xanthine oxidase inhibitor allopurinol attenuates the development of diabetic cardiomyopathy. J Cell Mol Med 13(8B):2330–2341. https://doi.org/10.1111/j.1582-4934.2008.00564.x
Battelli MG, Bolognesi A (1842) Polito L (2014) Pathophysiology of circulating xanthine oxidoreductase: new emerging roles for a multi-tasking enzyme. Biochim Biophys Acta 9:1502–1517. https://doi.org/10.1016/j.bbadis.2014.05.022
Faria A, Persaud SJ (2017) Cardiac oxidative stress in diabetes: mechanisms and therapeutic potential. Pharmacol Ther 172:50–62. https://doi.org/10.1016/j.pharmthera.2016.11.013
Kakkar R, Kalra J, Mantha SV, Prasad K (1995) Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol Cell Biochem 151(2):113–119. https://doi.org/10.1007/BF01322333
Vincent HK, Powers SK, Dirks AJ, Scarpace PJ (2001) Mechanism for obesity-induced increase in myocardial lipid peroxidation. Int J Obes 25(3):378–388. https://doi.org/10.1038/sj.ijo.0801536
Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED (2007) Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 56(10):2457–2466. https://doi.org/10.2337/db07-0481
Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN (2004) Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes 53(5):1336–1343. https://doi.org/10.2337/diabetes.53.5.1336
Shen X, Zheng S, Thongboonkerd V, Xu M, Pierce WM Jr, Klein JB, Epstein PN (2004) Cardiac mitochondrial damage and biogenesis in a chronic model of type 1 diabetes. Am J Physiol Endocrinol Metab 287(5):E896-905. https://doi.org/10.1152/ajpendo.00047.2004
Herlein JA, Fink BD, O’Malley Y, Sivitz WI (2009) Superoxide and respiratory coupling in mitochondria of insulin-deficient diabetic rats. Endocrinology 150(1):46–55. https://doi.org/10.1210/en.2008-0404
Turko IV, Murad F (2003) Quantitative protein profiling in heart mitochondria from diabetic rats. J Biol Chem 278(37):35844–35849. https://doi.org/10.1074/jbc.M303139200
Umbarkar P, Singh S, Arkat S, Bodhankar SL, Lohidasan S, Sitasawad SL (2015) Monoamine oxidase-A is an important source of oxidative stress and promotes cardiac dysfunction, apoptosis, and fibrosis in diabetic cardiomyopathy. Free Radic Biol Med 87:263–273. https://doi.org/10.1016/j.freeradbiomed.2015.06.025
Sun Y, Tian Z, Liu N, Zhang L, Gao Z, Sun X, Yu M, Wu J, Yang F, Zhao Y, Ren H, Chen H, Zhao D, Wang Y, Dong S, Xu C, Lu F, Zhang W (2018) Exogenous H2S switches cardiac energy substrate metabolism by regulating SIRT3 expression in db/db mice. J Mol Med (Berl) 96(3–4):281–299. https://doi.org/10.1007/s00109-017-1616-3
Sun Y, Teng Z, Sun X, Zhang L, Chen J, Wang B, Lu F, Liu N, Yu M, Peng S, Wang Y, Zhao D, Zhao Y, Ren H, Cheng Z, Dong S, Lu F, Zhang W (2019) Exogenous H2S reduces the acetylation levels of mitochondrial respiratory enzymes via regulating the NAD+-SIRT3 pathway in cardiac tissues of db/db mice. Am J Physiol Endocrinol Metab 317(2):E284–E297. https://doi.org/10.1152/ajpendo.00326.2018
Tan Y, Ichikawa T, Li J, Si Q, Yang H, Chen X, Goldblatt CS, Meyer CJ, Li X, Cai L, Cui T (2011) Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes. https://doi.org/10.2337/db10-1164
Luo J, Yan D, Li S, Liu S, Zeng F, Cheung CW, Liu H, Irwin MG, Huang H, Xia Z (2020) Allopurinol reduces oxidative stress and activates Nrf2/p62 to attenuate diabetic cardiomyopathy in rats. J Cell Mol Med 24(2):1760–1773. https://doi.org/10.1111/jcmm.14870
Oka SI, Byun J, Huang CY, Imai N, Ralda G, Zhai P, Xu X, Kashyap S, Warren JS, Alan Maschek J, Tippetts TS, Tong M, Venkatesh S, Ikeda Y, Mizushima W, Kashihara T, Sadoshima J (2021) Nampt potentiates antioxidant defense in diabetic cardiomyopathy. Circ Res 129(1):114–130. https://doi.org/10.1161/CIRCRESAHA.120.317943
Kim BS, Serebreni L, Fallica J, Hamdan O, Wang L, Johnston L, Kolb T, Damarla M, Damico R, Hassoun PM (2015) Cyclin-dependent kinase five mediates activation of lung xanthine oxidoreductase in response to hypoxia. PLoS ONE 10(4):e0124189. https://doi.org/10.1371/journal.pone.0124189
Ghio AJ, Kennedy TP, Stonehuerner J, Carter JD, Skinner KA, Parks DA, Hoidal JR (2002) Iron regulates xanthine oxidase activity in the lung. Am J Physiol Lung Cell Mol Physiol 283(3):L563–L572. https://doi.org/10.1152/ajplung.00413.2000
Martelin E, Lapatto R, Raivio KO (2002) Regulation of xanthine oxidoreductase by intracellular iron. Am J Physiol Cell Physiol 283(6):C1722–C1728. https://doi.org/10.1152/ajpcell.00280.2002
Petrosillo G, Matera M, Moro N, Ruggiero FM, Paradies G (2009) Mitochondrial complex I dysfunction in rat heart with aging: critical role of reactive oxygen species and cardiolipin. Free Radic Biol Med 46(1):88–94. https://doi.org/10.1016/j.freeradbiomed.2008.09.031
Chen CL, Zhang L, Jin Z, Kasumov T, Chen YR (2022) Mitochondrial redox regulation and myocardial ischemia-reperfusion injury. Am J Physiol Cell Physiol 322(1):C12–C23. https://doi.org/10.1152/ajpcell.00131.2021
Jang S, Javadov S (2017) Association between ROS production, swelling and the respirasome integrity in cardiac mitochondria. Arch Biochem Biophys 630:1–8. https://doi.org/10.1016/j.abb.2017.07.009
Moe GW, Marín-García J (2016) Role of cell death in the progression of heart failure. Heart Fail Rev 21(2):157–167. https://doi.org/10.1007/s10741-016-9532-0
Del Re DP, Amgalan D, Linkermann A, Liu Q, Kitsis RN (2019) Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol Rev 99(4):1765–1817. https://doi.org/10.1152/physrev.00022.2018
Jia G, DeMarco VG, Sowers JR (2016) Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol 12(3):144–153. https://doi.org/10.1038/nrendo.2015.216
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94(3):909–950. https://doi.org/10.1152/physrev.00026.2013
Bauer TM, Murphy E (2020) Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ Res 126(2):280–293. https://doi.org/10.1161/CIRCRESAHA.119.316306
Bernardi P, Carraro M, Lippe G (2022) The mitochondrial permeability transition: recent progress and open questions. FEBS J 289(22):7051–7074. https://doi.org/10.1111/febs.16254
Miki T, Miura T, Hotta H, Tanno M, Yano T, Sato T, Terashima Y, Takada A, Ishikawa S, Shimamoto K (2009) Endoplasmic reticulum stress in diabetic hearts abolishes erythropoietin-induced myocardial protection by impairment of phospho-glycogen synthase kinase-3beta-mediated suppression of mitochondrial permeability transition. Diabetes 58(12):2863–2872. https://doi.org/10.2337/db09-0158
Hotta H, Miura T, Miki T, Togashi N, Maeda T, Kim SJ, Tanno M, Yano T, Kuno A, Itoh T, Satoh T, Terashima Y, Ishikawa S, Shimamoto K (2010) Angiotensin II type 1 receptor-mediated upregulation of calcineurin activity underlies impairment of cardioprotective signaling in diabetic hearts. Circ Res 106(1):129–132. https://doi.org/10.1161/CIRCRESAHA.109.205385
Miki T, Itoh T, Sunaga D, Miura T (2012) Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovasc Diabetol 11:67. https://doi.org/10.1186/1475-2840-11-67
Itoh T, Kouzu H, Miki T, Tanno M, Kuno A, Sato T, Sunaga D, Murase H, Miura T (2012) Cytoprotective regulation of the mitochondrial permeability transition pore is impaired in type 2 diabetic Goto-Kakizaki rat hearts. J Mol Cell Cardiol 53(6):870–879. https://doi.org/10.1016/j.yjmcc.2012.10.001
Kortüm F, Jamra RA, Alawi M, Berry SA, Borck G, Helbig KL, Tang S, Huhle D, Korenke GC, Hebbar M, Shukla A, Girisha KM, Steinlin M, Waldmeier-Wilhelm S, Montomoli M, Guerrini R, Lemke JR, Kutsche K (2018) Clinical and genetic spectrum of AMPD2-related pontocerebellar hypoplasia type 9. Eur J Hum Genet 26(5):695–708. https://doi.org/10.1038/s41431-018-0098-2
Gilboa T, Elefant N, Meiner V, Hacohen N (2023) Delineating the phenotype and genetic basis of AMPD2-related pontocerebellar hypoplasia. Neurogenetics 24(1):61–66. https://doi.org/10.1007/s10048-022-00706-4
Kalsi KK, Yuen AH, Rybakowska IM, Johnson PH, Slominska E, Birks EJ, Kaletha K, Yacoub MH, Smolenski RT (2003) Decreased cardiac activity of AMP deaminase in subjects with the AMPD1 mutation–a potential mechanism of protection in heart failure. Cardiovasc Res 59(3):678–684. https://doi.org/10.1016/s0008-6363(03)00497-8
Kalsi KK, Yuen AH, Johnson PH, Birks EJ, Yacoub MH, Smolenski RT (2005) AMPD1 C34T mutation selectively affects AMP-deaminase activity in the human heart. Nucleos Nucleot Nucl Acids 24(4):287–288. https://doi.org/10.1081/NCN-59721
Smolenski RT, Rybakowska I, Turyn J, Romaszko P, Zabielska M, Taegtmeyer A, Słomińska EM, Kaletha KK, Barton PJ (2014) AMP deaminase 1 gene polymorphism and heart disease-a genetic association that highlights new treatment. Cardiovasc Drugs Ther 28(2):183–189. https://doi.org/10.1007/s10557-013-6506-5
Marx N, Federici M, Schütt K, Müller-Wieland D, Ajjan RA, Antunes MJ, Christodorescu RM, Crawford C, Di Angelantonio E, Eliasson B, Espinola-Klein C, Fauchier L, Halle M, Herrington WG, Kautzky-Willer A, Lambrinou E, Lesiak M, Lettino M, McGuire DK, Mullens W, Rocca B, Sattar N, ESC Scientific Document Group (2023) 2023 ESC guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J 44(39):4043–4140. https://doi.org/10.1093/eurheartj/ehad192.Erratum.In:EurHeartJ.2023;44(48):5060
Abovich A, Matasic DS, Cardoso R, Ndumele CE, Blumenthal RS, Blankstein R, Gulati M (2023) The AHA/ACC/HFSA 2022 heart failure guidelines: changing the focus to heart failure prevention. Am J Prev Cardiol 15:100527. https://doi.org/10.1016/j.ajpc.2023.100527
Cheng J, Morisaki H, Sugimoto N, Dohi A, Shintani T, Kimura E, Toyama K, Ikawa M, Okabe M, Higuchi I, Matsuo S, Kawai Y, Hisatome I, Sugama T, Holmes EW, Morisaki T (2014) Effect of isolated AMP deaminase deficiency on skeletal muscle function. Mol Genet Metab Rep 1:51–59. https://doi.org/10.1016/j.ymgmr.2013.12.004
Funding
TM is funded by Grant 21K08035 from the Japan Society for the Promotion of Science, Tokyo, Japan and a Grant for Scientific Research from Hokkaido University of Science, Sapporo, Japan. HK is funded by Grant 23K07481 from the Japan Society for the Promotion of Science, Tokyo, Japan. MT is funded by Grant 21K08110 from the Japan Society for the Promotion of Science, Tokyo, Japan. AK is funded by Grant 23K06152 from the Japan Society for the Promotion of Science, Tokyo, Japan.
Author information
Authors and Affiliations
Contributions
TM conceptualized the topic and wrote the first draft of the manuscript. HK, MT, YK, and AK commented on previous versions of the manuscript. All figures were prepared by TM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have not disclosed any competing interests.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miura, T., Kouzu, H., Tanno, M. et al. Role of AMP deaminase in diabetic cardiomyopathy. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-024-04951-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-024-04951-z