Abstract
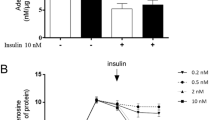
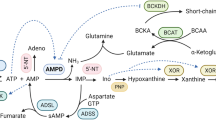
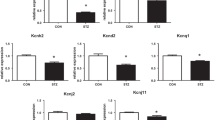
Adenosine is an important physiological regulator of the cardiovascular system. The goal of our study was to assess the expression level of nucleoside transporters (NT) in diabetic rat cardiomyocytes and to examine the activities of adenosine metabolizing enzymes. Isolated rat cardiomyocytes displayed the presence of detectable amounts of mRNA for ENT1, ENT2, CNT1, and CNT2. Overall adenosine (10 µM) transport in cardiomyocytes isolated from normal rat was 36 pmol/mg/min. The expression level of equilibrative transporters (ENT1, ENT2) decreased and of concentrative transporters (CNT1, CNT2) increased in myocytes isolated from diabetic rat. Consequently, overall adenosine transport decreased by 30%, whereas Na+–dependent adenosine uptake increased 2–fold, and equilibrative transport decreased by 60%. The activity ratio of AMP deaminase/5'–nucleotidase in cytosol of normal cardiomyocytes was 11 and increased to 15 in diabetic cells. The activity of ecto–5'–nucleotidase increased 2–fold in diabetic cells resulting in a rise of the activity ratio of ecto–5'–nucleotidase/adenosine deaminase from 28 to 56.
These results indicate that in rat cardiomyocytes diabetes alters activities of adenosine metabolizing enzymes in such a way that conversion of AMP to IMP is favored in the cytosolic compartment, whereas the capability to produce adenosine extracellularly is increased. This is accompanied by an increased unidirectional Na+–dependent uptake of adenosine and significantly reduced bidirectional adenosine transport.
Similar content being viewed by others
References
Nichols GA, Hillier TA, Erbey JR, Brown JB (2001) Congestive heart failure in type- 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care 24:1614–1619
Taegtmeyer H, McNulty P, Young ME (2002) Adaptation and maladaptation of the heart in diabetes: Part I, General concepts. Circulation 105:1727–1733
El-Omar MM, Yang Z-K, Phillips AO, Shah AM (2004) Cardiac dysfunction in the Goto-Kakizaki rat. A model of type II diabetes mellitus. Basic Res Cardiol 99:133–141
Joseph T, Coirault C, Dubourg O, Lecarpentier Y (2005) Changes in crossbridge mechanical properties in diabetic rat cardiomyopathy. Basic Res Cardiol 100:231–239
Mubagwa K, Mullane K, Flameng W (1996) Role of adenosine in the heart and circulation. Cardiovasc Res 32:797–813
Rosales OR, Eades B, Assali AR (2004) Cardiovascular drugs: adenosine role in coronary syndromes and percutaneous coronary interventions. Catheter Cardiovasc Interv 62:358–363
Peart JN, Gross GJ (2005) Cardioprotection following adenosine kinase inhibition in rat hearts. Basic Res Cardiol 100:328–336
Pelleg A, Katchanov G, Xu J (1997) Autonomic neural control of cardiac function: modulation by adenosine and adenosine 5’-triphosphate. Am J Cardiol 79:11–14
Deussen A, Stappert M, Schafer S, Kelm M (1999) Quanti.cation of extracellular and intracellular adenosine production: understanding the transmembranous concentration gradient. Circulation 99:2041–2047
Mattig S, Deussen A (2001) Signi.cance of adenosine metabolism of coronary smooth muscle cells. Am J Physiol Heart Circ Physiol 280:H117–H124
Moser GH, Schrader J, Deussen A (1989) Turnover of adenosine in plasma of human and dog blood. Am J Physiol 256:C799–C806
Sparks HV, Bardenheuer H (1986) Regulation of adenosine formation by the heart. Circ Res 58:193–201
Headric JP, Emerson CS, Berr SS, Berne RM, Matherne GP (1996) Interstitial adenosine and cellular metabolism during β-adrenergic stimulation of the in situ rabbit heart. Cardiovasc Res 31:699–710
Sobrevia L, Jarvis SM, Yudilevich DL (1994) Adenosine transport in cultured human umbilical vein endothelial cells is reduced in diabetes. Am J Physiol Cell Physiol 267:C39–C47
Aguayo C, Flores C, Parodi J, Rojas R, Mann GE, Pearson JD, Sobrevia L (2001) Modulation of adenosine transport by insulin in human umbilical artery smooth muscle cells from normal or gestational diabetic pregnancies. J Physiol 534:243–254
Pawelczyk T, Podgorska M, Sakowicz M (2003) The effect of insulin on expression level of nucleoside transporters in diabetic rats. Mol Pharmacol 63:81–88
Mackiewicz U, Emanuel K, Lewartowski B (2000) Dihydropyridine receptors functioning as voltage sensors in cardiac myocytes. J Physiol Pharmacol 51:777–798
Pawelczyk T, Bizon D, Angielski S (1992) The distribution of enzymes involved in purine metabolism in rat kidney. Biochim Biophys Acta 1116:309–314
Stockand JD, Al-Baldawi NF, Al-Khalili OK, Worrell RT, Eaton DC (1999) Sadenosyl- L-homocysteine hydrolase regulates aldosterone-induced Na+ transport. J Biol Chem 274:3842–3850
Pitarys CJ, Virmani R, Vildibill HD, Jackson EK, Forman M (1991) Reduction of myocardial reperfusion injury by intravenous adenosine administered during the early reperfusion period. Circulation 83:237–247
Downey JM, Liu GS, Thornton JD (1993) Adenosine and the anti-infarct effects of preconditioning. Cardiovasc Res 27:3–8
Headrick JP, Hack B, Ashton KJ (2003) Acute adenosinergic cardioprotection in ischemic-reperfused hearts. Am J Physiol Heart Circ Physiol 285:H1797–H1818
Belardinelli L (1993) Adenosine system in the heart. Drug Dev Res 28:263–267
Meghji P, Middleton KM, Newby AC (1988) Absolute rates of adenosine formation during ischaemia in rat and pigeon hearts. Biochem J 249:695–703
Kochan Z, Smolenski RT, Yacoub MH, Seymour AML (1994) Nucleotide and adenosine metabolism in different cell types of human and rat heart. J Mol Cell Cardiol 26:1497–1503
Pawelczyk T, Sakowicz M, Szczepanska-Konkel M, Angielski S (2000) Decreased expression of adenosine kinase in streptozotocin- induced diabetes mellitus rats. Arch Biochem Biophys 375:1–6
Sakowicz M, Pawelczyk T (2002) Insulin restores expression of adenosine kinase in streptozotocin-induced diabetes mellitus rats. Mol Cell Biochem 236:163–171
Pawelczyk T, Sakowicz M, Podgorska M, Szczepanska-Konkel M (2003) Insulin induces expression of adenosine kinase gene in rat lymphocytes by signaling through the mitogen-activated protein kinase pathway. Exp Cell Res 286:152–163
Loh E, Rebbeck T, Mahoney P, DeNofrio D, Swain J, Holmes E (1999) Common variant in AMPD1 gene predicts improved clinical outcome in patients with heart failure. Circulation 99: 1422–1425
Yacoub MH, Yuen AH, Kalsi KK, Birks EJ, Taegtmeyer A, Barton PJ, Johnson PH, Suzuki K, Smolenski RT (2004) C34 AMP deaminase 1 gene mutation protects cardiac function in donors. Transplantation 77:1621–1623
Craven PA, DeRubertis FR (1989) Protein kinase C is activated in glomeruli from streptozotocin diabetic rats. Possible mediation by glucose. J Clin Invest 83:1667–1675
Malhotra A, Reich D, Reich D, Nakouzi A, Sanghi V, Geenen DL, Buttrick PM (1997) Experimental diabetes is associated with functional activation of protein kinase CE and phosphorylation of troponin I in the heart, which are prevented by angiotensin II receptor blockade. Circ Res 81:1027–1033
Koya D, King GL (1998) Protein kinase C activation and the development of diabetic complications. Diabetes 47:859–866
Malhotra A, Kang BPS, Cheung S, Opawumi D, Meggs L (2001) Angiotensin II promotes glucose-induced activation of cardiac protein kinase C isozymes and phosphorylation of troponin I. Diabetes 50:1918–1926
Kitakaze M, Hori M, Morioka T, Minamino T, Takashima S, Okazaki Y, Node K, Komamura K, Iwakura K, Itoh T, Inoue M, Kamada T (1995) α1-Adrenoceptor activation increases ecto-5’-nucleotidase activity and adenosine release in rat cardiomyocytes by activating protein kinase C. Circulation 91:2226–2234
Node K, Kitakaze M, Minamino T, Tada M, Inoue M, Hori M, Kamada T (1997) Activation of ecto-5’-nucleotidase by protein kinase C and its role in ischaemic tolerance in the canine heart. Br J Pharmacol 120:273–281
Sato T, Obata T, Yamanaka Y, Arita M (1997) Stimulation of alpha 1-adrenoceptors and protein kinase C-mediated activation of ecto-5’-nucleotidase in rat hearts in vivo. J Physiol 503:119–127
Hohl CM (1999) AMP deaminase in piglet cardiac myocytes: effect on nucleotide metabolism during ischemia. Am J Physiol Heart Circ Physiol 276:H1502–H1510
Hohl AM, Hohl CM (1997) Regulation of piglet cardiac AMP deaminase. J Mol Cell Cardiol 29:A197
Tovmasian EK, Hairapetian RL, Bykova EV, Severin SE, Haroutunian AV (1990) Phosphorylation of the skeletal muscle AMP-deaminase by protein kinase C. FEBS Lett 259:321–323
Thakkar JK, Janero DR, Yarwood C, Sharif HM (1993) Modulation of mammalian cardiac AMP deaminase by protein kinase C-mediated phosphorylation. Biochem J 291:523–527
Meghji P, Pearson JD, Slakey LL (1992) Regulation of extracellular adenosine production by ectonucleotidases of adult rat ventricular myocytes. Am J Physiol Heart Circ Physiol 263:H40–H47
Dutta AK, Sabirov RZ, Uramoto H, Okada Y (2004) Role of ATP-conductive anion chanel in ATP release from neonatal rat cardiomyocytes in ischaemic or hypoxic conditions. J Physiol 559:799–812
Borst MM, Schrader J (1991) Adenine nucleotide release from isolated perfused guinea pig hearts and extracellular formation of adenosine. Cir Res 68:797–806
Headrick JP, Matherne GP, Berne RM (1992) Myocardial adenosine formation during hypoxia: effects of ecto-5’- nucleotidase inhibition. J Mol Cell Cardiol 24:295–303
Obata T (2002) Adenosine production and its interaction with protection of ischemic and reperfusion injury of the myocardium. Life Sci 71:2083–2103
Schutz W, Schrader J, Gerlach E (1981) Different sites of adenosine formation in the heart. Am J Physiol Heart Circ Physiol 240:H963–H970
Bardenheuer H, Whelton B, Sparks HV Jr (1987) Adenosine release by the isolated guinea pig heart in response to isoproterenol, acetylcholine, and acidosis: The minimal role of vascular endothelium. Cir Res 61:594–600
Ghosh S, Standen NB, Galinanes M (2001) Failure to precondition pathological human myocardium. J Am Coll Cardiol 37:711–718
Lee T-M, Chou T-F (2003) Impairment of myocardial protection in type 2 diabetic patients. J Clin Endocrinol Metab 88:531–537
Hayat SA, Patel B, Khattar RS, Malik RA (2004) Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin Sci 107:539–557
Swynghedauw B (1999) Molecular mechanisms of myocardial remodeling. Physiol Rev 79:215–262
Bell DSH (2003) Heart failure. The frequent, forgotten, and often fatal complication of diabetes. Diabetes Care 26:2433–24441
Simpson P (1983) Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an alpha 1 adrenergic response. J Clin Invest 72:732–738
Yamazaki T, Komuro I, Yazaki Y (1998) Signaling pathways for cardiac hypertrophy. Cell Signal 10:693–698
Villarreal F, Zimmermann S, Makhsudova L, Montag AC, Erion MD, Bullough DA, Ito BR (2003) Modulation of cardiac remodeling by adenosine: In vitro and in vivo effects. Mol Cell Biochem 251:17–26
Dubey RK, Gillespie DG, Jackson EK (1998) Adenosine inhibits collagen and protein synthesis in cardiac fibroblasts. Role of A2b receptors. Hypertension 31:943–948
Dubey RK, Gillespie DG, Zacharia LC, Mi Z, Jackson EK (2001) A2B receptors mediate the antimitogenic effects of adenosine in cardiac fibroblasts. Hypertension 37:716–721
Gan XT, Rajapurohitam V, Haist JV, Chidiac P, Cook MA, Karmazyn M (2005) Inhibition of phenylephrine-induced cardiomiocyte hypertrophy by action of multiple adenosine receptor subtypes. J Pharmacol Exp Ther 312:27–34
Liao Y, Takashima S, Asano Y, Asakura M, Ogai A, Shintani Y, Minamino T, Asanuma H, Sanada S, Kim J, Ogita H, Tomoike H, Hori M, Kitakaze M (2003) Activation of adenosine A1 receptor attenuates cardiac hypertrophy and prevents heart failure in murine left ventricular pressure-overload model. Circ Res 93:759–766
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Podgorska, M., Kocbuch, K., Grden, M. et al. Prevalence of unidirectional Na+–dependent adenosine transport and altered potential for adenosine generation in diabetic cardiac myocytes. Basic Res Cardiol 101, 214–222 (2006). https://doi.org/10.1007/s00395-005-0578-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-005-0578-8