Abstract
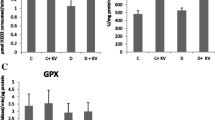
We hypothesized that oxygen free radicals (OFRs) may be involved in pathogenesis of diabetic complications. We therefore investigated the levels of lipid peroxidation by measuring thiobarbituric acid reactive substances (TBARS) and activity of antioxidant enzymes [superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT)] in tissues and blood of streptozotocin (STZ)-induced diabetic rats. The animals were divided into two groups: control and diabetic. After 10 weeks (wks) of diabetes the animals were sacrificed and liver, heart, pancreas, kidney and blood were collected for measurement of various biochemical parameters. Diabetes was associated with a significant increase in TBARS in pancreas, heart and blood. The activity of CAT increased in liver, heart and blood but decreased in kidney. GSH-Px activity increased in pancreas and kidney while SOD activity increased in liver, heart and pancreas. Our findings suggest that oxidative stress occurs in diabetic state and that oxidative damage to tissues may be a contributory factor in complications associated with diabetes.
Similar content being viewed by others
References
Oberley LW: Free radicals and diabetes. Free Rad Biol Med 5: 113–124, 1988
Baynes JW: Perspectives in diabetes, Role of oxidative stress in development of complications in Diabetes. Diabetes 40: 405–412, 1991
Asplund K, Grankvist K, Marklund S, Taljedal IB: Partial protection against streptozotocin induced hyperglycemia by superoxide dismutase linked to polyethylene glycol. Acta Endocrinol 107: 390–394, 1984
Asayama K, English D, Slonim AE, Buer IM: Chemiluminescence as an index of drug induced free radical production in pancreatic islets. Diabetes 33: 160–163, 1984
Hunt JV, Smith CCT, Wolff SP: Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes 39: 1420–1424, 1990
Wolff SP, Dean RT: Glucose autoxidation and protein modification the potential role of autoxidative glycosylation in diabetes. Biochem J 245: 243–250, 1987
Halliwell B, Gutteridge JMC: Lipid peroxidation, oxygen radicals, cell damage and antioxidant therapy. Lancet 1: 1396–1397, 1984
Meerson FZ, Kagan VE, Kozlov YP, Belkina LM, Arkhipenko YV: The role of lipid peroxidation in pathogenesis of ischemic damage and antioxidant protection of the heart. Basic Res Cardiol 77: 465–468, 1982
Salin ML, McCord JM: Superoxide dismutases in polymorphonuclear leukocytes. J Clin Invest 54: 1005–1009, 1974
Freeman BA, Crapo JD: Biology of disease: Free radicals and tissue injury. Lab Invest 47: 412–426, 1982
McCord JM: Oxygen derived free radicals in post-ischemic tissue injury. N Eng J Med 312: 159–163, 1985
Mantha SV, Prasad M, Kalra J, Prasad K: Antioxidant enzymes in hypercholesterolemia and effects of Vitamin E in rabbits. Atherosclerosis 101: 135–144, 1993
Dodson PM: Dyslipidemia and diabetes: an introduction. In: P.M. Dodson and A.H. Barnett (eds.). Lipids, diabetes and vascular disease, Science press Limited, London, 1992, pp. 1–15
Morel DW, Hessler JR, Chisolm GM: Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. Lipid Res 24: 1070–1076, 1983
Matkovics B, Varga SI, Szabo L, Witas H: The effect of diabetes on the activities of the peroxide metabolizing enzymes. Horm Metab Res 14: 77–79, 1983
Like AA, Rossini A: Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science 193: 415–417, 1976
Prasad K, Lee P, Mantha SV, Kalra J, Prasad M, Gupta JB: Detection of ischemia-reperfusion cardiac injury by cardiac muscle chemiluminescence. Mol Cell Biochem 115: 49–58, 1992
Kadish AH, Little RL, Sternberg JC: A new and rapid method for the determination of glucose by measurement of rate of oxygen consumption. Clin Chem 14: 116–131, 1968
Yagi K: Assay for blood plasma or serum. In: L. Packer (ed.). Methods in Enzymology. Academic press, New York, Vol. 105, 1984, pp. 328–331
Aebi H: Catalase. In: Methods of enzymatic analysis. Bergmeyer HV (Ed.), Chemie, Weinheim, F.R.G., pp. 673–684, 1974
Paglia DE, Valentine WN: Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70: 158–169, 1967
Lawrence RA, Burk RF: Glutathione peroxidase activity in selenium deficient rat liver. Biochem Biophys Res Commun 71: 952–958, 1976
Sun Y, Peterson TE, McCormick ML, Oberley LW, Osborne JW: Improved superoxide dismutase assay for clinical use. Clin Chem 35: 1265–1266, 1989
Gornall AG, Bardwill CJ, David MM: Determination of serum proteins by means of biuret reaction. J Biol Chem 177: 751–766, 1949
Eilers RJ: Notification of final adoption of an international method and standard solution for haemoglobinometry. Specifications for preparation of standard solution. Am J Clin Pathol 47: 212–214, 1967
Malaisse WJ: Alloxan toxicity to the pancreatic B-cell. Biochem Pharmacol 31: 3527–3534, 1982
Sato Y, Hotta N, Sakamoto N et al.: Lipid peroxide level in plasma of diabetic patients. Biochem Med 25: 373–378, 1981
Takasu N, Komiya I, Asasa T, Nagasawa Y, Yamada T: Streptozotocin and Alloxan induced H2O2 generation and DNA fragmentation in pancreatic islets. Diabetes 40: 1141–1145, 1991
Tjalve H, Wilander E, Johansson E: Distribution of labelled streptozotocin in mice: uptake and retention in pancreatic islets. J Endocrinol 69: 455–456, 1976
Wohaeib SA, Godin DV: Starvation related alterations in free radical defense mechanisms in rats. Diabetes 36: 169–173, 1987
Horie S, Ishii H, Suga T: Changes in peroxisomal fatty acid oxidation in diabetic rat liver. J Biochem 90: 1691–1696, 1981
Aust SD, Morehouse LA, Thomas CE: Role of metals in oxygen radical reaction. J Free Rad Biol Med 1: 3–25, 1985
Halliwell JMC, Gutteridge JMC: Role of free radicals and catalytic metal ions in human disease: An overview. In: L. Packer and A.N. Glazer (eds). Methods in Enzymolgy, Academic press Inc., San Diego, 1990, vol. 186, pp 1–25
Srivastava P, Saxena AK, Kale RK, Baquer NZ: Insulin like effects of lithium and vanadate on the altered antioxidant status of diabetic rats. Res Commun Chem Pathol Pharmacol 80: 283–293, 1993
Kono Y, Fridovich I: Superoxide radicals inhibit catalase. J Biol Chem 257: 5751–5754, 1982
De duve C, Baudhhuin P: Peroxisomes (Microbodies and related particles). Physiol Rev 46: 323–341, 1966
Doroshow JH, Lockes GY, Myers GE: Enzymatic defences of the mouse heart against reactive oxygen metabolites. J Clin Invest 65: 128–135, 1980
Frank L, Messaro D: Oxygen toxicity. Am J Med 69: 117–126, 1980
Oei HH, Stroo WE, Burton KP, Schaffer SW: A possible role of xanthine oxidase in producing oxidative stress in heart of chronically ethanol treated rats. Res Commun Chem Pathol Pharmacol 38: 453, 1982
Shull S, Heintz NH, Periasamy M, Manohar M, Janssen YM, Marsh JP, Mossman BT: Differential regulation of antioxidant enzymes in response to oxidants. J Biol Chem 266: 24398–24403, 1991
Bray RC, Cockle SA, Martin-Fielder E, Roberts PB, Rotillo G, Calabrase L: Reduction and inactivation of superoxide dismutase by hydrogen peroxide. Biochem J 139: 43–48, 1974
Kono Y, Fridovich I: Inhibition and reactivation of Mn-catalase. J Biol Chem 258: 13646–13648, 1983
Blum J, Fridovich I: Inactivation of glutathione peroxidase by superoxide radicals. Arch Biochem Biophys 240: 500–508, 1985
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kakkar, R., Kalra, J., Mantha, S.V. et al. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol Cell Biochem 151, 113–119 (1995). https://doi.org/10.1007/BF01322333
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01322333