Abstract
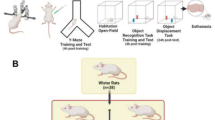
This study aimed to analyze whether taurine has a nootropic effect on short-term and long-term memory in a model of sporadic dementia of the Alzheimer’s type (SDAT). Moreover, we evaluated the immunoreactivity and insulin receptor (IR) distribution and markers for neurons and glial cells in the hippocampus of rats with SDAT and treated with taurine. For this, Male Wistar rats received STZ (ICV, 3 mg/kg, bilateral, 5ul per site, aCFS vehicle) and were treated with taurine (100 mg/kg orally, 1 time per day, saline vehicle) for 25 days. The animals were divided into 4 groups: vehicle (VE), taurine (TAU), ICV-STZ (STZ) and ICV-STZ plus taurine (STZ + TAU). At the end of taurine treatment, short- and long-term memory were assessed by performance on object recognition and Y-maze tasks. Insulin receptor (IR) was evaluated by immunoperoxidase while mature neurons (NeuN), astrocytes (GFAP, S100B, SOX9), and microglia (Iba-1) were evaluated by immunofluorescence. STZ induced worse spatial and recognition memory (INDEX) in YM and ORT tasks. Taurine protected against STZ-induced memory impairment. SDAT reduced the population of mature neurons as well as increased astrocytic and microglial reactivity, and taurine protected against these STZ-induced effects, mainly in the CA1 region of the hippocampus. Taurine increases IR expression in the hippocampus, and protects against the reduction in the density of this receptor in CA1 induced by STZ. In conclusion, these findings demonstrate that taurine is able to enhance memory, up-regulates IR in the hippocampus, protects the neuron population, and reduces the astrogliosis found in SDAT.








Similar content being viewed by others
References
Kim C, Cha YN (2014) Taurine chloramine produced from taurine under inflammation provides anti-inflammatory and cytoprotective effects. Amino Acids 46:89–100. https://doi.org/10.1007/s00726-013-1545-6
Wang GH, Jiang ZL, Fan XJ et al (2007) Neuroprotective effect of taurine against focal cerebral ischemia in rats possibly mediated by activation of both GABAA and glycine receptors. Neuropharmacology 52:1199–1209. https://doi.org/10.1016/j.neuropharm.2006.10.022
Sun M, Gu Y, Zhao Y, Xu C (2011) Protective functions of taurine against experimental stroke through depressing mitochondria-mediated cell death in rats. Amino Acids 40:1419–1429. https://doi.org/10.1007/s00726-010-0751-8
Sun M, Zhao Y, Gu Y, Xu C (2012) Anti-inflammatory mechanism of taurine against ischemic stroke is related to down-regulation of PARP and NF-jB. Amino Acids 42:1735–1747. https://doi.org/10.1007/s00726-011-0885-3
Silva SP, Zago AM, Carvalho FB et al (2021) Neuroprotective Effect of Taurine against Cell Death, Glial Changes, and Neuronal Loss in the Cerebellum of Rats Exposed to Chronic-Recurrent Neuroinflammation Induced by LPS. J Immunol Res 2021:. https://doi.org/10.1155/2021/7497185
Rahmeier FL, Zavalhia LS, Tortorelli LS et al (2016) The effect of taurine and enriched environment on behaviour, memory and hippocampus of diabetic rats. Neurosci Lett 630:84–92. https://doi.org/10.1016/j.neulet.2016.07.032
Möller H-J, Graeber MB (1998) The case described by Alois Alzheimer in 1911. Eur Arch Psychiatry Clin Neurosci 248:111–122. https://doi.org/10.1007/s004060050027
Benilova I, Karran E, De Strooper B (2012) The toxic Aβ oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci 15:349–357. https://doi.org/10.1038/nn.3028
De Strooper B, Karran E (2016) The Cellular phase of Alzheimer’s Disease. Cell 164:603–615. https://doi.org/10.1016/j.cell.2015.12.056
Allen NJ (2014) Astrocyte regulation of synaptic behavior. Annu Rev Cell Dev Biol 30:439–463. https://doi.org/10.1146/annurev-cellbio-100913-013053
Sofroniew MV, Vinters HV (2010) Astrocytes: Biology and pathology. Acta Neuropathol 119:7–35. https://doi.org/10.1007/s00401-009-0619-8
Craft JM, Watterson DM, Marks A, Van Eldik LJ (2005) Enhanced susceptibility of S-100B transgenic mice to neuroinflammation and neuronal dysfunction induced by intracerebroventricular infusion of human β-amyloid. Glia 51:209–216. https://doi.org/10.1002/glia.20194
Sun W, Cornwell A, Li J et al (2017) SOX9 is an astrocyte-specific nuclear marker in the adult brain outside the neurogenic regions. J Neurosci 37:4493–4507. https://doi.org/10.1523/JNEUROSCI.3199-16.2017
Liu Y, Walter S, Stagi M et al (2005) LPS receptor (CD14): a receptor for phagocytosis of Alzheimer’s amyloid peptide. Brain 128:1778–1789. https://doi.org/10.1093/brain/awh531
Heneka MT, Carson MJ, Khoury J, El et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14:388–405. https://doi.org/10.1016/S1474-4422(15)70016-5
Furman JL, Sama DM, Gant JC et al (2012) Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. J Neurosci 32:16129–16140. https://doi.org/10.1523/JNEUROSCI.2323-12.2012
Parkhurst CN, Yang G, Ninan I et al (2014) Microglia promote learning-dependent synapse formation through BDNF. 155:1596–1609. https://doi.org/10.1016/j.cell.2013.11.030.Microglia
Chen Y, Liang Z, Tian Z et al (2014) Intracerebroventricular streptozotocin exacerbates alzheimer-like changes of 3xTg-AD mice. Mol Neurobiol 49:547–562. https://doi.org/10.1007/s12035-013-8539-y
Grünblatt E, Salkovic-Petrisic M, Osmanovic J et al (2007) Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem 101:757–770. https://doi.org/10.1111/j.1471-4159.2006.04368.x
Kamat PK, Kalani A, Rai S et al (2016) Streptozotocin Intracerebroventricular-Induced neurotoxicity and brain insulin resistance: a therapeutic intervention for treatment of sporadic Alzheimer’s Disease (sAD)-Like Pathology. Mol Neurobiol 53:4548–4562. https://doi.org/10.1007/s12035-015-9384-y
Teixeira FC, Soares MSP, Blödorn EB et al (2022) Investigating the Effect of Inosine on Brain Purinergic receptors and neurotrophic and neuroinflammatory parameters in an experimental model of Alzheimer’s Disease. Mol Neurobiol 59:841–855. https://doi.org/10.1007/s12035-021-02627-z
Teixeira FC, Gutierres JM, Soares MSP et al (2020) Inosine protects against impairment of memory induced by experimental model of Alzheimer disease: a nucleoside with multitarget brain actions. Psychopharmacology 237:811–823. https://doi.org/10.1007/s00213-019-05419-5
Gutierres JM, Carvalho FB, Schetinger MRC et al (2014) Anthocyanins restore behavioral and biochemical changes caused by streptozotocin-induced sporadic dementia of Alzheimer’s type. Life Sci 96:7–17. https://doi.org/10.1016/j.lfs.2013.11.014
Pacheco SM, Soares MSP, Gutierres JM et al (2018) Anthocyanins as a potential pharmacological agent to manage memory deficit, oxidative stress and alterations in ion pump activity induced by experimental sporadic dementia of Alzheimer’s type. J Nutr Biochem 56:193–204. https://doi.org/10.1016/j.jnutbio.2018.02.014
Dodart JC, Mathis C, Ungerer A (1997) Scopolamine-induced deficits in a two-trial object recognition task in mice. NeuroReport 8:1173–1178. https://doi.org/10.1097/00001756-199703240-00023
Haider S, Sajid I, Batool Z, Madiha S, Sadir S, Kamil N et al (2020) Supplementation of Taurine insulates against oxidative stress, confers Neuroprotection and attenuates memory impairment in noise stress exposed male Wistar rats. Neurochem Res 45:2762–2774. https://doi.org/10.1007/s11064-020-03127-7
Javed H, Khan A, Vaibhav K et al (2013) Taurine ameliorates neurobehavioral, neurochemical and immunohistochemical changes in sporadic dementia of Alzheimer’s type (SDAT) caused by intracerebroventricular streptozotocin in rats. Neurol Sci 34:2181–2192. https://doi.org/10.1007/s10072-013-1444-3
Reeta KH, Singh D, Gupta YK (2017) Chronic treatment with taurine after intracerebroventricular streptozotocin injection improves cognitive dysfunction in rats by modulating oxidative stress, cholinergic functions and neuroinflammation. Neurochem Int 108:146–156. https://doi.org/10.1016/j.neuint.2017.03.006
Dou JT, Chen M, Dufour F et al (2005) Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn Mem 12:646–655. https://doi.org/10.1101/lm.88005
Ronaghi A, Zibaii MI, Pandamooz S et al (2019) Entorhinal cortex stimulation induces dentate gyrus neurogenesis through insulin receptor signaling. Brain Res Bull 144:75–84. https://doi.org/10.1016/j.brainresbull.2018.11.011
Palmano K, Rowan A, Guillermo R et al (2015) The role of gangliosides in neurodevelopment. Nutrients 7:3891–3913. https://doi.org/10.3390/nu7053891
El Idrissi A (2019) Taurine regulation of neuroendocrine function. Adv Exp Med Biol 1155:977–985. https://doi.org/10.1007/978-981-13-8023-5_81
Wang K, Shi Y, Liu W et al (2021) Taurine improves neuron injuries and cognitive impairment in a mouse Parkinson’s disease model through inhibition of microglial activation. Neurotoxicology 83:129–136. https://doi.org/10.1016/j.neuro.2021.01.002
Kowall NW, Beal MF (1991) Glutamate-, glutaminase‐, and taurine‐immunoreactive neurons develop neurofibrillary tangles in Alzheimer’s disease. Ann Neurol 29:162–167. https://doi.org/10.1002/ana.410290208
Du J, Lü W, Wu S et al (2015) Glycine receptor mechanism elucidated by electron cryo-microscopy. Nature 526:224–229. https://doi.org/10.1038/nature14853
Yu J, Zhu H, Lape R et al (2021) Mechanism of gating and partial agonist action in the glycine receptor. Cell 184:957–968e21. https://doi.org/10.1016/j.cell.2021.01.026
Oh SJ, Lee HJ, Jeong YJ et al (2020) Evaluation of the neuroprotective effect of taurine in Alzheimer’s disease using functional molecular imaging. Sci Rep 10:1–9. https://doi.org/10.1038/s41598-020-72755-4
Toma V, Al, Farcas AD, Parvu M et al (2017) CA3 hippocampal field: Cellular changes and its relation with blood nitro-oxidative stress reveal a balancing function of CA3 area in rats exposed to repetead restraint stress. Brain Res Bull 130:10–17. https://doi.org/10.1016/j.brainresbull.2016.12.012
Azevedo H, Khaled NA, Santos P et al (2018) Temporal analysis of hippocampal CA3 gene coexpression networks in a rat model of febrile seizures. DMM Dis Model Mech 11. https://doi.org/10.1242/dmm.029074
Jha MK, Jo M, Kim JH, Suk K (2019) Microglia-Astrocyte Crosstalk: an intimate Molecular Conversation. Neuroscientist 25:227–240. https://doi.org/10.1177/1073858418783959
Farina C, Aloisi F, Meinl E (2007) Astrocytes are active players in cerebral innate immunity. Trends Immunol 28:138–145. https://doi.org/10.1016/j.it.2007.01.005
Heppner FL, Ransohoff RM, Becher B (2015) Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 16:358–372. https://doi.org/10.1038/nrn3880
Gupte R, Christian S, Keselman P et al (2019) Evaluation of taurine neuroprotection in aged rats with traumatic brain injury. Brain Imaging Behav 13:461–471. https://doi.org/10.1007/s11682-018-9865-5
Vargas-Castro V, Gomez-Diaz R, Blanco-Alvarez VM et al (2021) Long-term taurine administration improves motor skills in a tubulinopathy rat model by decreasing oxidative stress and promoting myelination. Mol Cell Neurosci 115. https://doi.org/10.1016/j.mcn.2021.103643
Wang K, Zhang B, Tian T et al (2022) Taurine protects dopaminergic neurons in paraquat-induced Parkinson’s disease mouse model through PI3K/Akt signaling pathways. Amino Acids 54:1–11. https://doi.org/10.1007/s00726-021-03104-6
Liu K, Zhu R, Jiang H et al (2022) Taurine inhibits KDM3a production and microglia activation in lipopolysaccharide-treated mice and BV-2 cells. Mol Cell Neurosci 122:103759. https://doi.org/10.1016/j.mcn.2022.103759
Quintas C, Vale N, Gonçalves J, Queiroz G (2018) Microglia P2Y13 receptors prevent astrocyte proliferation mediated by P2Y1 receptors. Front Pharmacol 9:1–12. https://doi.org/10.3389/fphar.2018.00418
Park JS, Kam TI, Lee S et al (2021) Blocking microglial activation of reactive astrocytes is neuroprotective in models of Alzheimer’s disease. Acta Neuropathol Commun 9:1–15. https://doi.org/10.1186/s40478-021-01180-z
Villarreal A, Vidos C, Monteverde Busso M et al (2021) Pathological Neuroinflammatory Conversion of reactive astrocytes is Induced by Microglia and involves chromatin remodeling. Front Pharmacol 12:1–15. https://doi.org/10.3389/fphar.2021.689346
Zhang H, Wang D, Gong P et al (2019) Formyl peptide receptor 2 Deficiency improves cognition and attenuates tau hyperphosphorylation and astrogliosis in a mouse model of Alzheimer’s Disease. J Alzheimer’s Dis 67:169–179. https://doi.org/10.3233/JAD-180823
Su Y, Fan W, Ma Z et al (2014) Taurine improves functional and histological outcomes and reduces inflammation in traumatic brain injury. Neuroscience 266:56–65. https://doi.org/10.1016/j.neuroscience.2014.02.006
Donato R, Sorci G, Riuzzi F et al (2009) S100B’s double life: intracellular regulator and extracellular signal. Biochim Biophys Acta - Mol Cell Res 1793:1008–1022. https://doi.org/10.1016/j.bbamcr.2008.11.009
Wartchow KM, Rodrigues L, Swierzy I et al (2021) Amyloid-β processing in aged s100b transgenic mice is sex dependent. Int J Mol Sci 22. https://doi.org/10.3390/ijms221910823
Huttunen HJ, Kuja-Panula J, Sorci G et al (2000) Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem 275:40096–40105. https://doi.org/10.1074/jbc.M006993200
Ma Rhong, Zhang Y, Hong X, yue et al (2017) Role of microtubule-associated protein tau phosphorylation in Alzheimer’s disease. J Huazhong Univ Sci Technol - Med Sci 37:307–312. https://doi.org/10.1007/s11596-017-1732-x
Langeh U, Singh S (2020) Targeting S100B protein as a surrogate biomarker and its role in various neurological Disorders. Curr Neuropharmacol 19:265–277. https://doi.org/10.2174/1570159x18666200729100427
Michetti F, D’Ambrosi N, Toesca A et al (2019) The S100B story: from biomarker to active factor in neural injury. J Neurochem 148:168–187. https://doi.org/10.1111/jnc.14574
Shimamoto S, Tsuchiya M, Yamaguchi F et al (2014) Ca2+/S100 proteins inhibit the interaction of FKBP38 with Bcl-2 and Hsp90. Biochem J 458:141–152. https://doi.org/10.1042/BJ20130924
Li D, Li K, Chen G et al (2016) S100B suppresses the differentiation of C3H/10T1/2 murine embryonic mesenchymal cells into osteoblasts. Mol Med Rep 14:3878–3886. https://doi.org/10.3892/mmr.2016.5697
Tsoporis JN, Overgaard CB, Izhar S, Parker TG (2009) S100B modulates the hemodynamic response to norepinephrine stimulation. Am J Hypertens 22:1048–1053. https://doi.org/10.1038/ajh.2009.145
Wen XH, Duda T, Pertzev A et al (2012) S100B serves as a ca 2 + sensor for ROS-GC1 guanylate cyclase in cones but not in rods of the murine retina. Cell Physiol Biochem 29:417–430. https://doi.org/10.1159/000338496
Agam G, Almog O (2015) Calbindin D28k and S100B have a similar interaction site with the lithium-inhibitable enzyme inositol monophosphatase-1: a new drug target site. J Med Chem 58:2042–2044. https://doi.org/10.1021/jm5019324
Gógl G, Alexa A, Kiss B et al (2016) Structural basis of ribosomal S6 kinase 1 (RSK1) inhibition by S100B protein: modulation of the extracellular signal-regulated kinase (ERK) signaling cascade in a calcium-dependent way. J Biol Chem 291:11–27. https://doi.org/10.1074/jbc.M115.684928
Lee SG, Yoo DY, Jung HY et al (2015) Neurons in the hippocampal CA1 region, but not the dentate gyrus, are susceptible to oxidative stress in rats with streptozotocin-induced type 1 diabetes. Neural Regen Res 10:451–456. https://doi.org/10.4103/1673-5374.153695
Zhang Y, Chen K, Sloan SA et al (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34:11929–11947. https://doi.org/10.1523/JNEUROSCI.1860-14.2014
Farmer WT, Abrahamsson T, Chierzi S et al (2016) Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Sci (80-) 351:849–854. https://doi.org/10.1126/science.aab3103
Nagao M, Ogata T, Sawada Y, Gotoh Y (2016) Zbtb20 promotes astrocytogenesis during neocortical development. Nat Commun 7:1–14. https://doi.org/10.1038/ncomms11102
Zhang Y, Sloan SA, Clarke LE et al (2016) Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89:37–53. https://doi.org/10.1016/j.neuron.2015.11.013.Purification
Acknowledgements
The authors are grateful for all the support offered by the technician of the Pathology Research Laboratory, Teresinha Stein.
Author information
Authors and Affiliations
Contributions
F.H, A.M.Z, G.N.S and J.M.G performed stereotactic surgery, drug preparation and animal care; G.N.S and F.H performed behavioral tests; F.H, A.M.Z and G.N.S performed immunofluorescence assays; M.C.F, F.H and L.F.K performed the optical density microscopic analysis; J.M.G, M.C.F and F.H wrote the main text of the manuscript and J.M.G and F.H prepared scheme 1 and Figs. 1, 2, 3, 4, 5, 6 and 7. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Founding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. The authors would like to thank the scholarships that allowed the execution of this project. These scholarships were funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) Finance Code 001. Fernanda Huf, Gabrielle N. da Silva, Luiz Felipe C. Koenig and Adriana M. Zago received PhD scholarships from the CAPES program, and Jessié M. Gutierres was supported by the National Postdoctoral Program (PNPD/CAPES).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huf, F., Gutierres, J.M., da Silva, G.N. et al. Neuroprotection elicited by taurine in sporadic Alzheimer-like disease: benefits on memory and control of neuroinflammation in the hippocampus of rats. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04872-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04872-3