Abstract
Despite advanced imaging techniques, more information on the extent of joint degeneration and modifications in tendon collagen content is required for selecting the appropriate type of shoulder prosthesis. The goal of this study was to compare the thermal parameters of various tissue samples from patients who had shoulder arthroplasty and to correlate the thermal shifts with the outcomes of radiological and histopathological assessments. Rotator cuff (ROC) tendon, hyaline cartilage, and subchondral bone samples were obtained throughout surgery for a comminuted 4-part proximal humerus fracture or cuff tear arthropathy. Thermal parameters were measured using differential scanning calorimetry and thermogravimetry (DTA/TG). ROC: The effect of advanced collagen damage was visible in denaturation curves of osteoarthritis samples. Only moderately increased thermal enthalpy and melting temperature were found in the tissue of the trauma patients. Cartilage: Thermal parameters revealed that structural change generated by pathologic conditions exceeded the effects of trauma. Bone: Thermal parameters from degenerative samples revealed more structural damage and calcification decrease than thermal parameters from trauma samples. Correlation: A statistically significant relationship between the extent of osteoarthritis and the thermal variables of cartilage and subchondral bone was discovered. Authors suggest that thermal analysis may be useful in the diagnostic process of orthopedic and posttraumatic shoulder diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, shoulder joint prostheses have undergone significant improvements. Different implant designs are available for the treatment of dislocated proximal humerus fractures, primary osteoarthritis or rotator cuff tear arthropathy [1,2,3].
Several reviews have recently been published, debating the risks and benefits of shoulder replacements versus other surgical methods or conservative treatment. For the selection of proper implant type, it is essential to know the condition of cartilage, subchondral bone, and importantly, the rotator cuff tendons [4,5,6].
The rotator cuff is responsible for the movements and stabilization of the glenohumeral joint. It consists of four muscles: supraspinatus, infraspinatus, teres minor and subscapularis. Notably, one of the main components of rotator cuff tendons are collagens. Collagens are the most abundant proteins in mammals, with 28 members in their superfamily [7]. Type I collagen fibrils are responsible for the mechanical durability and strength. Meanwhile, Type II collagen is located in tissues where compressive loads are expected, like hyaline cartilage of joint surfaces [8].
The differential scanning calorimetry (DSC) analysis has recently proven to be a useful method in examining proteins with collagen structural changes [9, 10]. In addition, DSC has been utilized to investigate the hyaline cartilage damage of the fractured human femoral head and to demonstrate the effect of cryopreservation on the morphology of human cartilage samples [11, 12]. Thermal analysis was also used successfully in the examination of degenerative shoulder and spinal diseases [13, 14].
According to the literature, there are only a few publications that look at the mineral content of bone of the humerus articular surface in patients who have had shoulder arthroplasty, especially utilizing thermal analysis. TGA has also been used to evaluate the thermal characteristics of femur head subchondral bone samples collected of patients with arthritic and osteoporotic pathologies [15, 16].
Authors have lately focused on the degenerative and inflammatory changes of different types of tendons, ligaments, cartilages and subchondral bones. In this review, we propose to demonstrate the efficacy of thermal analysis in the investigation of arthritic pathologies and consequences of multifragmentary humeral fractures, leading to prosthesis implantation.
Patients and methods
Sample collection
Rotator cuff tendon: the 5 × 5 × 10 mm-sized ROC samples were obtained during surgery from patients whom underwent total reversed shoulder arthroplasty due to degenerative shoulder diseases/rotator cuff tear arthropathies (D group) or comminuted proximal humerus fractures: Type AO/ASIF 11 C3.2–C3.3 (F group). In case of the trauma patients, the indication for reversed shoulder was the age above 70–75 years and/or existing rotator cuff tear. Hyaline cartilage: the 5 × 5 × 2.5 mm-sized sample of the hyaline cartilage of the removed humerus head from the estimated glenohumeral contact area (GCA) was collected during reversed shoulder replacement. Subchondral bone: the 5 × 150 mm (diameter × height)-sized cylinder-shaped cancellous samples were collected from the same patients as described above. The subchondral bone was harvested from removed humerus head, under ‘estimated glenohumeral contact area’ (GCA). The control samples were taken from a young patient who had a dislocated 4-part proximal humerus fracture but was otherwise healthy. Furthermore, histopathological examinations ruled out the existence of ruptures of the rotator cuff, rheumatoid arthritis or cartilage destruction.
All of the collected samples were considered as waste from the point of surgery.
Intraoperative evaluation
Samples of rotator cuff, hyaline cartilage and subchondral bone were collected during surgery (see details elsewhere) [17,18,19]. Importantly, all of these samples were considered as waste from the point of surgery.
The macroscopical observations about the degree of visible rotator cuff tendon tear, bone and cartilage damage were done immediately after collection, following the scoring system published previously [20].
Radiological evaluation
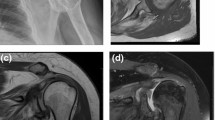
Based on the preoperative imaging, rotator cuff tendon damage was assessed using the Hamada classification, while Walch and Outerbridge classifications were used to analyze primary arthropathies and cartilage damage [21,22,23]. Since these classifications were primarily developed for degenerative (RCA) shoulders, their application for fractured shoulders is very limited. Therefore, we decided to score the level of osteoarthritis (scored from 0 to 7), by calculating the number of signs of arthritis found on conventional two-dimensional radiographs: acromial acetabularization, femoralization of the humeral head, asymmetric wear of the upper glenoid, presence of osteophytes, osteopenia, subchondral sclerosis and anterosuperior escape. Conventional X-rays, CT (Siemens Somatom Perspective Dual 64/128) and MRI (Siemens Magnetom Essenza 1.5 Tesla) examinations were done and analyzes performed with Siemens Syngo.via software (Siemens Healthineers Global).
Histological evaluation
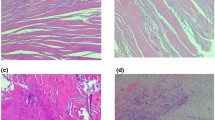
The ROC, cartilage and subchondral bone samples were processed by using standard histological protocols (including decalcification in case of the bone), followed by hematoxylin–eosin staining.
Differential scanning calorimetry (DSC)
Thermal analyses were conducted with the SETARAM Micro DSC-II calorimeter between 0 and 100 °C at a heating rate of 0.3 K min−1 after proper storage. The thermal denaturation was carried out in conventional closed Hastelloy batch vessels (V = 1 mL). As a control, normal saline was used. SETARAM peak integration calorimetric enthalpy was calculated from the area under the heat absorption curve using a two-point setting, and then, the other thermal parameters were calculated (denaturation or melting temperature (Tm), range of denaturation (∆T), half width of transition (T1/2 temperature range, where the maximum of heat flow decreases to its half value) and calorimetric enthalpy (∆Hcal) data of samples were compared. Because of the relatively small mass of cartilage specimens, the scans were smoothed and baseline correction was done by Origin 6.0.
Thermogravimetric analysis (TGA)
The TGA measurements were performed by using an SSC/5200 TG/DTA equipment (SII Seiko Instruments, Japan). The sample holders were open aluminum crucibles with a diameter of 5.2 mm and a depth of 5 mm. The examined temperature range was from ambient up to 550 °C with a heating rate between 10 and 40 K min−1 (in 10 K increments). Evaluations were done under inert nitrogen gas with a flow rate of 100 mL min−1. The measurements were run, and data were collected in DTA (heat flow), TG (mass loss in %) and DTG (rate of mass loss, derivative plot of TGA) modes.
All practices were executed in compliance with the responsible Regional and Institutional Research Ethics Committee’s ethical standards, as well as the Helsinki Declaration of 1975, as revised in 2008.
Results
Preoperative and intraoperative clinical and radiological evaluation of patients underwent total shoulder replacement
Patient demographics
Patients undergoing reversed shoulder arthroplasty with degenerative (orthopedic) or trauma (comminuted fracture) indications were included in the study; the mean age of patients was 73 ± 5 years (mean ± SEM) with a male-to-female ratio: 2/3. In the fracture group, all patients suffered dislocated proximal humerus fractures. Notably, all trauma patients were above the age of 68 years and all of the traumas were due to low energy injuries, mostly domestic falls with osteoporosis in the patient history. All patients with orthopedic indications had developed rotator cuff tear arthropathy with degenerative or autoimmune diseases in medical history.
Radiological evaluation
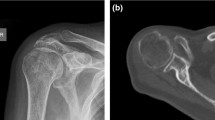
Table 1 demonstrates the results of evaluation of degenerative and trauma shoulders (4-part or Neer Type VI dislocated proximal humerus fractures). The Walch classification, which uses 2-dimensional (2D) axial computed tomography (CT) scans, assesses the glenoid and the position of the humeral head in relation to the glenoid in various degrees of osteoarthritis (Figs. 1, 2). Grade D in sample D2 indicates very severe arthritis with subluxation of the anterior humeral head, grade A1 in sample F2 indicates a well-centered humeral head with minor erosions.
The Hamada classification evaluates the radiological findings after a rotator cuff tear and shows the course of the rotator cuff tear arthropathies (from 1 to 5). Of the trauma specimens, F2 was rated IV/b and orthopedic specimens D1 and D2 were rated IV/a and V, respectively, indicating a poorer prognosis of arthropathy for the orthopedic patients.
The Outerbridge classification is originally based on arthroscopic findings, but in our study, we used MRI scans as an advanced imaging tool; Outerbridge 2 in the case of sample D1 indicates blistering swelling/fraying of the articular cartilage extending to the surface, a moderately damaged cartilage.
Meanwhile, the osteoarthritis score clearly demonstrated the presence of advanced osteoarthritis among the orthopedic indication specimens. In case of control samples, healthy tissue structure was confirmed by histological examination (Fig. 3).
Thermal analysis of rotator cuff samples collected from patients during total shoulder arthroplasty
The form of the curves has clearly demonstrated the effect of a moderate damage on tendon structure in case sample D1 and the developed tendon degeneration in case of sample D2, compared to the control. The denaturation curves are of degenerative (orthopedic) samples are widened and flattened, in contrast to the shape of the control (healthy) sample (Fig. 4).
The thermal parameters also supported this notion (Table 2). The calorimetric enthalpy was markedly decreased (∆Hcal: D1 = 3.14, D2 = 1.83 vs. control = 5.01 Jg−1), showing the pathologic alterations in the collagen structure of the degenerative tendons collected from orthopedic patients. The temperature range of denaturation was narrower in the degenerative samples, compared to the control (∆T: D1 = 11.71, D2 = 15.49 vs. control = 18.60 °C).
The change in baseline after denaturation may be indicative of an increase in heat capacity, demonstrating a more compact (dense) structure as a result of less tendon elasticity.
The moderately degenerated tendons (F1) showed a significant difference from the control in the shape of the scans and also in the thermal parameters (Fig. 5). The shape of the denaturation curve was flattened and widened in case of fracture sample F1, comparing to the control and other fracture samples (F2, F3). Meanwhile, the slope of the control sample’s curve is steeper, compared to the fracture samples.
In case of the less damaged F2 and F3 samples, the thermal enthalpy was moderately increased (5.33 and 5.67 Jg−1, respectively), while the F1 fracture sample with degenerative macroscopic signs showed a pronounced decreased (2.69 Jg−1) versus the intact control (5.01 Jg−1). The temperature range of denaturation (∆T) was decreased in all fracture samples (F1 = 13.23, F2 = 12.56, F3 = 12.15 vs. control = 18.60 °C). The half width at the maximal heat flow (T1/2) was increased (2.76 °C), while the peak temperature of the denaturation (Tm) was decreased (61.60 °C) in case of F1 sample, compared to the control (T1/2 = 2.02 °C, Tm = 63.55 °C). The changes in these parameters indicated an opposite pattern in F2 and F3: T1/2 showed a decrease and Tm exhibited an increase in comparison to the control.
Thermal analysis of cartilage samples collected from patients during total shoulder arthroplasty
In terms of sample origin and disease outcome, our main finding is that the denaturation temperature range and half width of the heat flow curves are substantially wider in the degenerative samples (D) than in the control (C) and fracture (F) samples (Figs. 6, 7).
In case of thermal denaturation curves of degenerative samples, an extra peak can be observed only in the ascending part of the curves. Additionally, less pronounced and narrower extra peaks can be seen during both the ascending and the descending phases of the denaturation curves of fracture samples. Since we currently lack the biochemical means to separate the various cartilage compounds, the precise explanation of those extra peaks is beyond the scope of our current study.
The thermal enthalpy (∆Hcal) of both degenerative samples (D1 = 1.84, D2 = 1.04 vs. control = 0.62 Jg−1) showed remarkable differences to the control (Table 3). Interestingly, the thermal enthalpy resulted either in only a slight increase in fracture samples F2 and F3 (1.07 and 1.09 Jg−1), or in a marked decrease in case of sample F1 (0.41 Jg−1). Meanwhile, the melting temperature (Tm) indicated a decrease in the D2 sample with the most severe degenerative changes, comparing to the control (50.0 vs. 56.5 °C). In the fracture group, all samples demonstrated a higher Tm than the orthopedic or control samples. Of the orthopedic cartilages, all samples showed an increase in the denaturation temperature range (∆T), compared to the control (D1 = 27.1, D2 = 20.0 vs. control = 19.0 °C). Contrary, all of the trauma samples exhibited a decrease in the ∆T (F1 = 17.1, F2 = 15.0 and F3 = 14.6 vs. control = 19.0 °C).
The significant decrease in the melting temperatures (Tm) of D2 showed that the structural change caused by degenerative diseases is greater than that caused by traumatic exposure. These findings demonstrate the thermal effects of damaged cartilage’s decreased cooperativity between structural domains.
Thermal analysis of subchondral bone samples collected from patients during total shoulder arthroplasty
In case of the control sample, the DTA result showed four clearly distinguishable thermal transitions (75-103-335 and 407 °C) and a total mass loss of about 40% in the examined temperature range (Figs. 8, 9).
The TG/DTA curves of cancellous bone samples of degenerative origin. Samples were run in technical triplicates and the heat flow curves show the averages of three measurements. Data were normalized to dry sample mass. Fig. published here with permission of Journal of Thermal Analysis and Calorimetry
The DTG curves (data are not shown) demonstrated similar pattern to the DTA’s shapes during the ascending phase; there are two recognizable peak temperatures at 1.5 mg min−1 at 2.1 mg min−1. In contrast, only the DTG indicated differentiable peaks at 402 °C at 2.7 mg min−1. In addition, the DTA curve of D1 was significantly narrower, comparing to the run of D2 and control.
Importantly, the D1 degenerative sample had twice the thermal effect of the control specimen. The lower temperature peaks transitioned to 50 and 107 °C, respectively, and the endotherm impact was four times greater than in the control case. Meanwhile, the higher transitions stayed at about the same temperatures (333 and 404 °C). The three graphs revealed a twice greater final mass loss (DTG values: 4.6 mg min−1 at 107 °C and 1.3 mg min−1 at 390 °C) (Table 4).
Differing of the D1 degenerative sample, the D2 sample behaved similarly to the control in terms of characteristics and heat denaturation. For instance, in the lower range, the two denaturation peaks separated more clearly (38 and 118 °C), resulting in the same calorimetric enthalpy. In the higher denaturation range, three peaks were observed (333, 388 and 485 °C) vs. only two peaks of control sample. With the enthalpy contribution of denaturation at 333 °C being larger than that of the control at 335 °C and much larger than the second at 388 °C. The total mass loss was approximately 40%.
When compared to the degenerative samples, a very distinct difference in denaturation process is apparent in the fracture group (Fig. 9). Thereby, it indicates a clear difference in the cancellous bone structure as well. The F1 demonstrated transitions between 50 (inflection) and 120 °C, accompanied with twice of the calorimetric enthalpy of the control.
The denaturation in the higher temperature range shifted to 348 and 423 °C with nearly identical enthalpies. The DTG showed increased value (4 mg min−1) at 100.4 °C, comparing to the control (DTG = 1.5–2.1 mg min−1, Tlow = 60.1—98.9 °C). In the higher range, DTG values were similar to the control (T1 DTG 360–414.3 mg min−1 at Thigh = 360–414.3 °C versus control DTG = 2.7 mg min at Thigh = 402.3 °C).
The F2 sample differed the most from the control or any degenerative or fracture case. Following a distinct pattern, a single transition at around 50 °C, a well-separable double denaturation near 150 °C, as well as the others at 340; 420 and 500 °C were detected. It is quite remarkable that the total calorimetric enthalpy in 20–220 °C was considerably lower than in the control case. The DTG values obtained in the lower range were: ~ 48 °C, 0.23 mg min−1, 137 °C, 0.25 mg min−1 and ~ 150 °C, 0.18 mg min−1. These values were 0.31 mg min−1 at 338 °C and 1.2 mg min−1 at 410 °C in the higher range. The total mass loss was approximately 45%.
Thermal shifts at 90, 110, and 150 °C can be distinguished in the F3 sample. The enthalpy contribution of these three peaks is within the control range of 20–210 °C. At higher temperatures, the enthalpies are also comparable to the control. The DTG readings were 85 °C, 1.12 mg min−1 and 408 °C, 3.3 mg min−1. The total mass loss was 50%.
Relationship between the level of tissue damage, degeneration and thermal parameters
Regression analyses have been performed to find a relationship between the thermal parameters and other indicators of degenerative processes, such as macroscopic scores or radiological evaluations.
Of the cartilage samples, ‘moderately strong’ relationship was observed between osteoarthritis score and denaturation temperature range (R2 = 0.74, p = 0.06, trend to statistical significance) (Fig. 10). Furthermore, plotting the osteoarthritis score as predictor and half width at the highest heat flow as a dependent variable also demonstrated similar pattern (R2 = 0.76, p = 0.05, trend to statistical significance).
Regression analyzes between A hyaline cartilage: osteoarthritis score and denaturation range, B hyaline cartilage: osteoarthritis score and half width of transition and C subchondral bone: osteoarthritis score plotted against the calorimetric enthalpy detected at the lower temperature peaks. Linear regression analysis, p < 0.1; R2 (coefficient of determination) and regression equation are indicated on each graph
Comparing the outcome of the radiological evaluation and the calorimetric measurements, causal relationship between the degree of arthritis (osteoarthritis score) and the calorimetric enthalpy was found at the lower temperature peaks (R2 = 0.61, p = 0.067, trend toward significance). It must be noted that by removing one outliner (sample F2), the correlation would be much stronger (R2 = 0.92). Authors suggest that with a larger sample size, the data could show a statistically significant, strong correlation between radiological and thermal data.
Discussion
Despite recent improvements in treatment protocols, research to explore the biochemical background of rotator cuff and hyaline cartilage and subchondral bone damages are still required [24,25,26]. These studies could provide the surgeon with more detail about the estimated grade of arthritis, particularly where if MRI scan is not readily accessible.
Thermal analyzes of rotator cuff tendon
We found that modification in the thermal properties of ROC, cartilage and subchondral bone samples was able to predict the magnitude of degenerative changes. We also discovered significant changes when comparing denaturation curves of injured and orthopedic patients.
Type I collagen is known to be the major structural element of the extracellular matrix (ECM) in tendons, and covalent cross-linking of collagen molecules in connective tissues is essential for normal function. Collagen cross-linking and the total amount of collagen have been observed to increase with age, which leads to decreases in both versatility and capacity to recover from trauma [27].
Several recent studies have examined the pathologic mechanisms of rotator cuff damages but only a few have used differential scanning calorimetry (DSC) to assess thermodynamic parameters. In an animal study of rabbit rotator cuff injury, Szabó et al. [28] discovered distinct differences between healthy and ruptured tendons. Using DSC, they discovered a loss of thermal cooperation of the components, lower melting temperatures, and an increase in thermal enthalpy of pathologic biceps tendons. These findings were explained by the increased collagen levels and secondary bonds of fibers [20]. The Tm was also found to be significantly higher in samples taken from acutely damaged lower extremity tendons, owing to a lower concentration of bound water in the injured fibers [29].
Cuff tear arthropathies have all been linked to aging, overuse and repetitive microtraumas [30]. Using human cadaver tendons, Bognár et al. discovered significant alterations to the long biceps head’s thermal denaturation properties, parallel with aging. In their study, the age-dependent thermal enthalpy showed a widespread with a peak at 51 years [31]. It should be noted that in those studies, healthy human tendons were collected from cadavers while we were using in vivo collected samples throughout our experiments.
In our current study, a relatively young, otherwise healthy patient’s samples were used as a control. However, for future experiments, we are considering the comparation with a sample collected from an older patient with minimal evidence of osteopenia and/or osteoporosis to account for age-related changes in collagen.
Thermal analyzes of hyaline cartilage
We have shown that the structural changes in human hyaline cartilage induced by degenerative diseases are greater than the changes observed in trauma patients based on the DSC waveforms and thermodynamic properties of samples from different origins. The injury discovered in trauma patients could have been caused by a degenerative disease or by the trauma itself. Based on examination of the histopathologic changes of the humeral head in CTA versus OA, the cartilage layer was found thicker in CTA [32].
The human hyaline cartilage is divided into three zones (superficial, transitional, and deep), each with its own function and consistency [33]. Remarkably, we discovered that the denaturation curves of specific cartilages showed ‘transitions,’ which could be caused by an interaction of one of the contents of certain layers. To differentiate the cartilage layers exceeded the scope of our current study.
It is known that the pathomechanism of cartilage damage observed in the later stage avascular necrosis of the femoral head is different from the osteoarthritis. After the ischemic bony lesions’ collapse, the cartilage will go through a degenerative process described with decreased stiffness and proteoglycan loss. In another set of experiments, the effect of time elapsed since femoral neck fracture on thermal properties was examined. They detected changes in Tm and ΔHcal, supporting the notion that human hyaline cartilage undergoes significant structural changes when blood supply is inadequate [11]. Additionally, it was shown that the duration of cryopreservation in a bone bank could affect the morphology of human cartilage samples as well [12].
Thermal analyzes of subchondral bone
Osteoarthritis is a degenerative and inflammatory process, described with hyaline cartilage destruction and a decrease in the mineral content of cancellous bone. The number of trabeculae and bone quantity increase with osteoarthritis, but the bone is hypomineralized and of poor quality in contrast to normal subchondral tissue [34, 35]. When comparing the more affected orthopedic sample (D1) to the control, we consistently found a relatively increased mass loss. The fracture samples, on the other hand, showed comparable mass loss to the normal structure.
The subchondral structure is separated anatomically into two parts: the subchondral bone plate, which is directly beneath the cartilage, and the deeper trabecular layer [36, 37]. Our thermal analyses revealed that the control sample had four distinct thermal shifts. The lower temperature shift peaks in the D1 degenerative sample were transitioned, resulting in a fourfold increase in endotherm effect. Meanwhile, higher peaks stayed within the same temperature zone. The thermal properties of the other orthopedic samples (D2) were rather similar to those of the control.
One possible explanation is that the different peaks represent various layers of bone. Lower peaks relate to the more responsive transition zone or subchondral plate, while higher, more stable thermodynamic shifts correspond to the less affected deeper layers [38].
Notably, the thermal properties of the F2 trauma sample were significantly different from those of the other specimens. For example, a single transition was found at ~ 50 °C, a well separable double denaturation near 150 °C, and three more at 340 °C; 420 and 500 °C. In each of the three denaturation phases, the enthalpy contribution was greater than in the control. Furthermore, the final mass loss was the greatest, at 60%. The distinct patterns of the scans may have been caused by more intense microstructural damages or necrosis in response to inadequate blood supply due to the acute trauma.
It is characterized by lower-than-normal maximum bone mass and higher-than-normal bone loss. Hormonal effects cause increased absorption in conjunction with a lack of calcium and vitamin D, leading to decreased bone formation [39]. Given our patient demographics, it is likely that our fracture patients with multifragmentary proximal humerus fractures also had osteoporosis. The different thermal consequences of trauma specimens compared to controls could be explained by osteoporosis-related decreased bone density and calcification.
Another possibility is that during the measurements, the structural impact of the trauma was recognized. In case of a multifragmentary proximal humeral fracture affecting the anatomic neck, the inadequate blood supply will lead to avascular necrosis (AVN) [40, 41]. Because of the period that refers to the duration between fracture and surgical intervention, which is typically 5–10 days, the disrupted blood flow may not have led to a substantial loss of mineral deposits, but modified metabolic activity and cellular function. It could have contributed to different thermal parameters, particularly in comparison to the arthritic samples with partially different pathologies.
Relationship between clinical and thermodynamic parameters
We have demonstrated moderately strong relationship between thermal parameters and osteoarthritis scores, using regression analysis in case of hyaline cartilage samples and subchondral cancellous bone samples. Interestingly, in case of the rotator cuff samples, there was no statistically supported correlation found between the radiological scores and outcome of thermal measurements. Possible explanation is that osteoarthritis score is based on radiological morphology of glenohumeral joint, describing the bone consistency, joint space, etc. Neither conventional radiographs, nor CT scans can provide details about the degree of rotator cuff tendon damage. The likely degenerative changes of rotator cuff tendon could only indirectly be concluded from radiological morphology of the joint.
Potential limitations
One potential limitation of the study is that evaluation based on diagnostic imaging was only conducted in a relatively small group of the patients. The rational is that the currently provided classifications were designed to assess RCA shoulders rather than comminuted fractures. Furthermore, because of the existence of alloy implants, panic attacks, and other factors, MRI may not always be applicable.
A further limitation of the study is that, because of the limited sample size, only the average values have been calculated, with no further statistical analysis. Nevertheless, the significant differences in denaturation curves between and pathologic specimens, as well as the excellent reproducibility of the experiments (temperature: ± 0.2 °C, enthalpy < 0.05 J) support our finding that thermodynamic analysis is a reliable tool to assess degenerative shoulder tissue samples with human origin.
In our current study, macroscopically and microscopically healthy tissue samples were used as controls, obtained from a relatively young patient without concomitant diseases. Most of trauma patients exhibited less radiological or macroscopic signs of OA or CTA than the orthopedic patients; therefore, the thermal results of trauma samples were closer to the healthy samples from the point of view of degenerative changes. The injuries were acute, and there was not enough time to develop avascular necrosis or tendon contractures.
During surgery, we collected 5 × 150 mm (diameter × height) cylindrical bone sample involve both layers of subchondral bone. Therefore, thermal analysis was conducted on the total cylindrical sample and data from all layers were obtained in the same experiment. As a result, it cannot be ruled out that a larger sclerotic portion of subchondral cysts affects the measurement.
It is necessary to note that the p value of our data showed only a trend to significance (p < 0.1). However, based on R2 values, authors suggest that in a future study with a larger sample size, strong correlation would be found between radiological and thermal data. Therefore, thermal parameters could be a reliable predictor of the degenerative and traumatic tissue injuries.
Clinical relevance
The differences between sample origins may not always be obvious. Patients suffering from trauma could have pre-existing arthritic signs, and orthopedic patients could develop avascular necrosis as a consequence of prior trauma. In the ‘outliner’ trauma sample, we are likely to have found degenerative condition and impact of acute injury at the same time.
In everyday clinical settings, it is generally more challenging to prepare a trauma patient for surgery since acute fractures require quicker decisions and early surgical treatment. Osteoarthritis and rotator cuff arthropathies, on the other hand, rarely demand immediate surgery, giving the surgeon more opportunity to prepare for the operation.
As a result, data demonstrating differences in the anticipated grade of the rotator cuff, hyaline cartilage and subchondral bone injury in trauma patients versus orthopedic patients might facilitate the surgical planning as well as optimize therapeutic guidelines.
Conclusions
These findings suggest that, based on the predictive values of the denaturation variables, that thermal analysis may be helpful in subsequent studies in the diagnostic process of shoulder disorders.
References
Lübbeke A, Rees JL, Barea C, Combescure C, Carr AJ, Silman AJ. International variation in shoulder arthroplasty. Acta Orthop. 2017;88:592–9.
Fitschen-Oestern S, Behrendt P, Martens E, Finn J, Schiegnitz J, Borzikowsky C, et al. Reversed shoulder arthroplasty for the treatment of proximal humerus fracture in the elderly. J Orthop. 2020;17:180–6.
Collin P, Hervé A, Walch G, Boileau P, Muniandy M, Chelli M. Mid-term results of reverse shoulder arthroplasty for glenohumeral osteoarthritis with posterior glenoid deficiency and humeral subluxation. J Shoulder Elb Surg U S. 2019;28:2023–30.
Mata-Fink A, Meinke M, Jones C, Kim B, Bell J-E. Reverse shoulder arthroplasty for treatment of proximal humeral fractures in older adults: a systematic review. J Shoulder Elb Surg U S. 2013;22:1737–48.
Stone MA, Namdari S. Surgical considerations in the treatment of osteoporotic proximal humerus fractures. Orthop Clin North Am U S. 2019;50:223–31.
Holschen M, Siemes M-K, Witt K-A, Steinbeck J. Five-year outcome after conversion of a hemiarthroplasty when used for the treatment of a proximal humeral fracture to a reverse total shoulder arthroplasty. Bone Jt J Engl. 2018;100-B:761–6.
Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3:a004978.
Buckley MR, Evans EB, Matuszewski PE, Chen Y-L, Satchel LN, Elliott DM, et al. Distributions of types I, II and III collagen by region in the human supraspinatus tendon. Connect Tissue Res Engl. 2013;54:374–9.
Chaudhury S, Holland C, Porter D, Tirlapur UK, Vollrath F, Carr AJ. Torn human rotator cuff tendons have reduced collagen thermal properties on differential scanning calorimetry. J Orthop Res Off Publ Orthop Res Soc. 2011;29:1938–43.
Torres K, Trębacz H, Bącik-Donica M, Atras A, Torres A, Plewa Z. Does thermodynamic stability of peritoneal collagen change during laparoscopic cholecystectomies? A differential scanning calorimetry (DSC) study. Surg Endosc Germany. 2014;28:2623–6.
Naumov I, Wiegand N, Patczai B, Vámhidy L, Lőrinczy D. Differential scanning calorimetric examination of the human hyaline cartilage of the femoral head after femoral neck fracture. J Therm Anal Calorim. 2012;108:59–65.
Patczai B, Mintál T, Nőt LG, Wiegand N, Lőrinczy D. Effects of deep-freezing and storage time on human femoral cartilage. J Therm Anal Calorim. 2017;127:1177–80.
Csotye J, Aigner Z, Sohár G, Szabó-Révész P, Tóth K. Calorimetric properties of degenerative human shoulder joint hyaline cartilage. J Therm Anal Calorim. 2009;95:805–8.
Mécs L, Aigner Z, Sohár G, Szabó-Révész P, Tóth K. Characterization of human cartilage in degenerated spine disease with differential scanning calorimetry. J Therm Anal Calorim. 2009;95:809–11.
Lénárt G, Pflüger G, Bidló G, Pintér J, Fischerleitner F. Kristallographische untersuchung des verlängerungskallus. Arch Orthop Trauma Surg Germany. 1979;93:303–5.
Mkukuma LD, Imrie CT, Skakle JMS, Hukins DWL, Aspden RM. Thermal stability and structure of cancellous bone mineral from the femoral head of patients with osteoarthritis or osteoporosis. Ann Rheum Dis. 2005;64:222–5.
Nöt LG, Bata A, Szabó H, Cifra J, Lőrinczy D. DSC examination of rotator cuff damage in patients with total shoulder arthroplasty. J Therm Anal Calorim. 2021;146:165–70.
Bata A, Nöt LG, Szabó H, Cifra J, Lőrinczy D. DSC examination of cartilage damage of patients undergoing shoulder replacement. J Therm Anal Calorim. 2022;147:1275–80.
Bata A, Nöt LG, Szabó H, Cifra J, Lőrinczy D. Thermogravimetric analysis of cancellous bone of humerus head in patients undergoing total shoulder arthroplasty. J Therm Anal Calorim. 2022;147:3107–15.
Szabó I, Bognár G, Kereskai L, Szász K, Lőrinczy D. Differential scanning calorimetric and histological examinations of the long head of the biceps in cadavers. J Therm Anal Calorim. 2007;88:343–9.
Brolin TJ, Updegrove GF, Horneff JG. Classifications in brief: Hamada classification of massive rotator cuff tears. Clin Orthop Relat Res. 2017;475:2819–23.
Bercik MJ, Kruse K 2nd, Yalizis M, Gauci M-O, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elb Surg U S. 2016;25:1601–6.
Slattery C, Kweon CY. Classifications in brief: Outerbridge classification of chondral lesions. Clin Orthop Relat Res. 2018;476:2101–4.
Smith GCS, Bateman E, Cass B, Damiani M, Harper W, Jones H, et al. Reverse shoulder arthroplasty for the treatment of proximal humeral fractures in the elderly (ReShAPE trial): study protocol for a multicentre combined randomised controlled and observational trial. Trials. 2017;18:91.
Launonen AP, Fjalestad T, Laitinen MK, Lähdeoja T, Ekholm C, Wagle T, et al. Nordic innovative trials to evaluate osteoPorotic fractures (NITEP) collaboration: the nordic DeltaCon trial protocol-non-operative treatment versus reversed total shoulder arthroplasty in patients 65 years of age and older with a displaced proximal hume. BMJ Open. 2019;9:e024916.
Wilcox RB, Arslanian LE, Millett P. Rehabilitation following total shoulder arthroplasty. J Orthop Sports Phys Ther. 2005;35:821–36.
Arnesen SM, Lawson MA. Age-related changes in focal adhesions lead to altered cell behavior in tendon fibroblasts. Mech Ageing Dev Irel. 2006;127:726–32.
Szabó I, Bognár G, Magda I, Garamvölgyi R, Czobel G, Nőth L, et al. DSC examination of the rotator cuff muscles in rabbits. Thermochim Acta. 2006;445:179–84.
Wiegand N, Naumov I, Vámhidy L, Kereskai L, Lőrinczy D, Nöt LG. Comparative calorimetric analysis of 13 different types of human healthy and pathologic collagen tissues. Thermochim Acta. 2013;568:171–4.
Trębacz H, Szczęsna A, Arczewska M. Thermal stability of collagen in naturally ageing and in vitro glycated rabbit tissues. J Therm Anal Calorim. 2018;134:1903–11.
Bognár G, Szabó I, Pintér C, Ligeti E, Lőrinczy D. Changes in thermal denaturation properties of the long head of the biceps during lifetime. J Therm Anal Calorim. 2010;102:65–8.
Toma T, Suenaga N, Taniguchi N, Oizumi N, Yamaguchi H, Tome Y, et al. Humeral head histopathological changes in cuff tecar arthropathy. J Orthop Surg (Hong Kong). 2019;27:2309499018816428.
Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1:461–8.
Henrotin Y, Pesesse L, Sanchez C. Subchondral bone and osteoarthritis: biological and cellular aspects. Osteoporos Int. 2012;23(Suppl 8):S847–51.
Bettica P, Cline G, Hart DJ, Meyer J, Spector TD. Evidence for increased bone resorption in patients with progressive knee osteoarthritis: longitudinal results from the Chingford study. Arthritis Rheum U S. 2002;46:3178–84.
Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15:223.
Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–7.
Suri S, Walsh DA. Osteochondral alterations in osteoarthritis. Bone U S. 2012;51:204–11.
Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD. Low bone mineral density and fracture burden in postmenopausal women. C Can Med Assoc. 2007;177:575–80.
Maier D, Jaeger M, Izadpanah K, Strohm PC, Suedkamp NP. Proximal humeral fracture treatment in adults. J Bone Jt Surg Am. 2014;96:251–61.
Lafforgue P. Pathophysiology and natural history of avascular necrosis of bone. Jt Bone Spine Fr. 2006;73:500–7.
Funding
Open access funding provided by University of Pécs. This research was funded by CO-272 (OTKA) grant (D.L.). Open access funding provided by University of Pécs.
Author information
Authors and Affiliations
Contributions
DL helped in investigation, writing—original draft and funding acquisition; AB performed in formal analysis; ZL helped in visualization; FS, HS and JC helped in investigation; LGN contributed to writing—review and editing, supervision and funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Local Ethics Committee of Balassa János Tolna County Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lőrinczy, D., Bata, A., Lábas, Z. et al. Thermal analysis of tendon, cartilage and bone samples from shoulder joint replacement patients using differential scanning calorimetry (DSC) and thermogravimetry (DTA/TG). J Therm Anal Calorim (2023). https://doi.org/10.1007/s10973-023-12250-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-023-12250-w