Abstract
Prior to shoulder replacement, the quality of cancellous bone stock of humerus head should be evaluated. Thermogravimetric analysis (TGA) has already been utilized to assess thermal stability of cancellous bone mineral, collected from the femoral head of patients with osteoarthritis and osteoporosis. Our workgroup has recently examined the thermal parameters of rotator cuff of patients undergoing reversed shoulder replacement. We hypothesized that TGA of humerus head would indicate difference in the bone quality of orthopedic and trauma patients. We also hypothesized that the calorimetric data could correlate with the grade of degenerative changes. Cylindrical subchondral humeral head samples were collected from patients subjected to reversed shoulder replacement due to orthopedic or trauma indications. Then, calorimetric parameters were measured using DTA/TG analysis. Radiological evaluation was also performed to classify the grade of osteoarthritis. In case of orthopedic samples, the calorimetric parameters indicated a moderate to severe degree of bone damage and loss of mineralization, because of the progressed osteoarthritis. Meanwhile, the trauma samples exhibited only moderate or minimal subchondral bone degeneration. DTA curves showed different patterns and indicated shifts in transition temperatures, comparing control and pathologic samples. In addition, correlation was found between the degree of osteoarthritis and calorimetric enthalpy. DTA/TG analysis of humerus head samples indicated marked differences in bone quality of orthopedic and trauma patients. Further investigation is needed to differentiate the calorimetric parameters of different layers of subchondral bone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The number of shoulder arthroplasties has been markedly increased over the last decades [1]. The indication of these surgeries mainly includes degenerative shoulder diseases and comminuted proximal humerus fractures [2, 3]. Prior to operation, quality of cancellous bone stock of humerus head cortical-like bone of the glenoid should be evaluated. The existence of severe osteoarthritis and AVN (avascular necrosis) of the head require different planning and may exclude the use of certain type of prosthesis, such as stemless implants.
While imaging methods, CT-scan and MRI provide useful information for the operational planning, there is still a need for better understanding the biochemical background of bone loos resulting primarily from degenerative diseases [4]. According to the literature, there is only a limited number of publications available investigating the bone density of humerus head and glenoid cavity in patients with shoulder arthroplasty, especially by using thermal analysis.
Thermogravimetric analysis (TGA) has been applied to investigate the effect of distraction forces on the callus formation of metacarpal bones of sheep [5]. The method has also been used to examine pig bone specimens of different postmortem age, concluding that TGA potential to estimate postmortem age of forensic bone specimens [6]. Moreover, TGA was utilized to assess thermal stability of cancellous bone mineral, collected from the femoral head of patients with osteoarthritis and osteoporosis [7].
Our workgroup has recently examined the condition of rotator cuff of patients undergoing reversed shoulder replacement with different indications and we have found marked differences in the thermal parameters of orthopedic and trauma samples [8, 9].
Therefore, we hypothesized that thermogravimetric analysis of human humerus head samples would indicate difference in the bone quality of orthopedic and trauma patients. We expected poorer bone stock and less mineralization in orthopedic samples with existing osteoarthritis or cuff tear arthropathy (CTA), compared to trauma samples. We also hypothesized that the bone loss in orthopedic patients would correlate with the grade of degenerative changes.
Materials and methods
Sample collection
The 5 × 150 mm (diameter × height) sized cylinder-shaped bone samples were harvested from the removed humerus head, under the cartilage layer called the ‘estimated glenohumeral contact area’ (GCA). Then, samples were properly stored for further measurements, as described in details elsewhere [10]. The cancellous bone samples were obtained patients whom underwent reversed shoulder replacement: (1) due to acute fractures, AO-OTA Type 11 C3.1–C3.3 (Trauma samples: T1–T3); (2) due to rotator cuff tear arthropathy (Orthopedic samples: O1, O2). The control sample was collected from a young patient with dislocated four-part proximal humerus fracture and was considered macroscopically as a healthy cancellous bone. This finding was confirmed by histological examination. All of these samples were considered as waste from the point of surgery. Samples from orthopedic surgery ranged in mass from 14 to 110 mg, while traumatic test samples ranged in mass from 12 to 49 mg.
All procedures followed were in accordance with the ethical standards of the responsible Regional and Institutional Research Ethics Committee and with the Helsinki Declaration of 1975, as revised in 2008.
Clinical and radiological evaluation of degenerative changes
Based on patient history and results of the physical examination in case of RCA patients, an individual score system was applied to assess the potential level of rotator cuff damage. Samples of RCA or trauma origin were also macroscopically assessed, looking for signs of tissue damage.
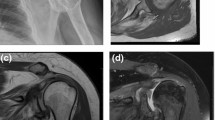
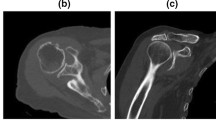
The rotator cuff tendon damage was radiologically scored with Hamada classification [11]. For the CT-based evaluation of primary arthropathies, Walch [12] classification was applied. Osteoarthritis score (0–7) was assessed by calculating the number of arthritis signs found on conventional two-dimensional X-rays: acromial acetabularization, femoralization of humeral head, asymmetric superior glenoid wear, presence of osteophytes, osteopenia, subchondral sclerosis (snowcap-sign) and anterosuperior escape. Radiological analysis of conventional X-rays, CT and MRI images was performed by using Siemens Syngo.via software (Siemens Healthineers Global).
Histological processing
The subchondral bone samples were processed by utilizing standard decalcification and staining protocols. During slide preparation and visual analysis, Leica Biosystems ST 5020 Multistainer, Leica Biosystems CV 5030 and Nikon Eclipse Ci-L microscope were used.
Thermogravimetry
The thermoanalytical investigation of bone samples was performed by an SSC/5200 TG/DTA equipment made by SII Seiko Instruments (Japan). The sample holders were open aluminum crucibles with a diameter of 5.2 mm and a depth of 5 mm. The investigated temperature range was from ambient up to 550 °C (aluminum sample holders!). The applied heating rate was between 10 and 40 K min−1 (in 10 K steps). Measurements were performed under an inert nitrogen gas with a flow rate of 100 mL min−1. The detected signals were DTA (“heat flow”), TG (mass loss in %) and DTG (“speed” of mass loss) curves.
Statistical analysis
Correlation analysis and plot chart were performed by using MS Excel software.
Results
Radiological and histological evaluation
Based on the Hamada and Walch classifications, we have found that samples with orthopedic origin (O1, O2) showed significantly more severe signs of preexisting degenerative conditions, such as osteoarthritis and rotator cuff tear arthropathy, compared to the trauma samples (T1–T3).
According to the fact that preoperative CT-scan was not available in all orthopedic cases, osteoarthritis was additionally graded by analyzing conventional two-plain radiographs. The results, indicated as ‘osteoarthritis-score’ on Table 1, clearly demonstrated the presence of more developed osteoarthritis among the samples with orthopedic indication. These data were confirmed by the macroscopical observations about the degree of visible bone and cartilage damage during surgery and sample collection.
In case of control sample, histopathological analyses excluded the presence of osteoarthritis or degenerative cartilage and bone damage (Fig. 1).
TG/DTA measurements
The TG/DTA scans in case of orthopedic samples can be seen in Fig. 2. The plots contain the average of three measurements in case of 10 K min−1 heating rate.
The DTA result in case of control sample exhibited four well separable thermal transitions (75–103–335 and 407 °C) and about a total 40% mass loss in the scanned temperature range. The DTG curves (they are not plotted) followed the DTA curves in case of the first two peak temperatures with 1.5 and 2.1 mg min−1, but in case of the higher thermal transitions we could detect only one DTG peak around 402 °C with 2.7 mg min−1 value.
The O1 orthopedic sample showed twice a big thermal effect than the control one. The lower temperature peaks shifted to 50 and 107 °C and the endotherm effect was four times bigger than in case of control. The higher transitions remained roughly at the same temperatures (333 and 404 °C). The twice a greater final mass loss jumped out from the three graphs (DTG was at 107 °C ~ 4.6 mg min−1 and 1.3 mg min−1 at 390 °C).
The O2 sample exhibited in the characteristic and denaturation heat similar tendency as it was in case of the control. The two lower denaturation peaks separated more clearly (38 and 118 °C) resulting same calorimetric enthalpy. In the higher temperature range instead of two (see control) three denaturation peaks appeared (333, 388 and 485 °C), where the enthalpy contribution of denaturation at 333 °C was greater than that of control at 335 °C and much greater than the second one at 388 °C (this last one was smaller than that of the similar control at 407 °C). The total mass loss was about 40%, and DTG values for the biggest lower transition were 1.43 mg min−1 and at 380 °C 2.3 mg min−1.
From Table 2. and Fig. 2—despite of the fact that we have no enough sample to perform statistical analysis—the differences among control and O1 as well O2 test materials can be seen by Tms, enthalpies, mass losses and “speed” of mass loss.
The same TG/DTA curves of traumatic bones and control can be seen in Fig. 3.
At first glance a very definite different denaturation process (this way internal structure) can be seen compared with the orthopedic samples (see Fig. 2). The sample T1 showed transitions around 50 (inflection) and 120 °C with twice a bigger calorimetric enthalpy as the control. The denaturations in higher temperature range shifted to 348 and 423 °C with practically same enthalpies. The mass loss was smaller, about 30%. The DTG exhibited at 100 °C higher, 4 mg min−1 value and at 414 °C 2.7 mg min−1, also higher than for control.
T2 sample showed the biggest difference compared with either control or any orthopedic and traumatic case. A single transition at ~ 50 °C, a well separable double denaturation in the vicinity of 150 °C, as well as the others at 340; 420 and 500 °C. It is very surprising that the total calorimetric enthalpy in 20–220 °C much lower than in case of control. The enthalpy contribution in each three denaturation phases higher compared with the control. The final mass loss is ~ 60%, the DTG values ~ 48 °C 0.23 mg min−1, ~ 137 °C 0.25 mg min−1 and ~ 150 °C 0.18 mg min−1. In the higher range, these values are at 338 °C 0.31 mg min−1, at 410 °C 1.2 mg min−1.
In case of T3 sample, we can distinguish thermal transitions at 90, 110 and 150 °C. The enthalpy contribution of these three peaks is in the range of the control in 20–210 °C interval. The enthalpies at higher temperature range are also similar to the control. The full mass loss 35%, the DTG values are at 85 °C 1.12 mg min−1 and at 408 °C 3.3 mg min−1.
Similar to the orthopedic surgical materials, Fig. 3 and the data of Table 3 well demonstrate the alterations among the different traumatic as well as orthopedic probes.
Correlation analysis
In comparison with the results of radiological evaluation and calorimetric measurements, we have demonstrated a correlation between the grade of arthritis (osteoarthritis score) and calorimetric enthalpy detected at the lower temperature peaks. The correlation analysis showed a ‘moderate’ correlation (R2 = 0.609) with a p = 0.067, indicating a trend to statistical significance (Fig. 4). It is necessary to note that by excluding one ‘outliner’ (sample T2, see explanation in discussion), the correlation would be much stronger (R2 = 0.922). We believe that in a further study with an increased number of n, the data could indicate a statistically significant, strong correlation between radiological scores and calorimetric data.
Correlation between osteoarthritis score and calorimetric enthalpy. The osteoarthritis score (0–7) was plotted against the calorimetric enthalpy (ΔHcal, Jg−1) measured at the lower temperature peaks. Correlation analysis, linear trend line, n = 6, R2 = 0.694, p = 0.067, trend to significance. Empty square indicates control, solid triangles orthopedic and solid rounds trauma samples
Discussion
Thermal stability of human bone tissue by TGA method has been recently studied by Lozano et al. [13]. They performed DSC, infrared spectroscopy (FTIR) measurements and gas chromatography, to extract and scrutinize collagen structures. Authors claimed that there is an interaction between type I collagen and carbonate hydroxyapatite nanocrystals and changes of collagen during (de)mineralization could affect the fiber elasticity and the strength of bone tissue. Although bone samples of our current study were collected from different location and they represent histologically different bone types (humerus head subchondral cancellous bone vs. healthy skull and radius cortical bone), the thermal data (calorimetric enthalpy, mass loss) of our study are comparable with Lozano’s results.
Osteoarthritis is a degenerative joint disease, characterized with chronic inflammation, cartilage damage and hypomineralization of the subchondral bone. During this process, there is a loss of mineral contents and the exposure of subchondral bone will contribute to the pain accompanied with progressed osteoarthritis [14]. The subchondral bone can be divided into two different anatomical parts: subchondral bone plate laying directly under the cartilage and the deeper trabecular bone [15].
According to our thermal analyses, control sample exhibited four, well separable thermal transitions. In case of O1 orthopedic samples, the lower temperature transition peaks were shifted, accompanied with a four times larger endotherm effect. Meanwhile, higher peaks remained at the same temperature range. The other orthopedic sample (O2) showed similar thermal characteristics to the control. A possible explanation is that the different peaks belong to different layers of the subchondral bone. There is a functional unit, called the ‘osteochondral junction’ providing biochemical contact between different layers and alteration of this functional unit will affect the maintenance of the joint [16]. It can be speculated that the lower peaks represent the more sensitive transition zone or subchondral plate, while the thermodynamically more stable higher peaks represent the less effected deeper layers.
There is an increase in the number of trabeculas and bone volume in osteoarthritis; however, the bone is hypomineralized and lower quality, compared to the healthy cancellous bone structure [17, 18]. Consistently, we have found a relatively high mass loss in case the more affected orthopedic sample (O1), compared to the control. Meanwhile, the trauma samples exhibited similar mass loss, compared to the control sample.
Similar to osteoarthritis, a large part of the elderly population is affected by osteoporosis. The osteoporosis can be described by lower-than-normal maximum bone mass and greater-than-normal bone loss. Due to an imbalance between bone resorption and formation, a pathologic, fragile bone tissue will be gradually replacing the healthy tissue. As the result of hormonal effects, the will an increase in resorption combined with deficiency of calcium and vitamin D leading to impaired bone deposition [19]. In case of trauma patients, there were no preoperative screening or DEXA performed to assess the level of osteoporosis. Additionally, CT’s and conventional X-ray’s capacity to estimate the level of osteoporosis is very limited.
However, considering the demographic data of our patients, there is a high possibility that our trauma patients with comminuted four-part humerus fractures had osteoporosis as well. The decreased bone mass and mineralization accompanied with osteoporosis could be an explanation for the different thermal results of trauma samples, compared to the control.
Another possible explanation is that the mechanical consequence of the trauma itself was detected during the thermal analyses. It is known that in case of a dislocated proximal humerus fractures involving the anatomical neck (fracture type AO 11 C1–C3) [20], there is a high chance of loss of blood supply and development of avascular necrosis (AVN) of the humerus head [21]. As the result of the interruption in blood supply, hemopoietic, then bone cells (osteoblasts, osteoclasts, etc.) and bone marrow fat cells will be affected. With the lack of reperfusion, the angiogenesis and reparation phase could not be initiated and therefore, avascular necrosis (AVN) would start to develop [22]. Considering the time elapsed between the trauma and operation, generally 5–10 days, the interrupted blood supply could not have caused significant loss of mineralization but the altered metabolism and cellular function might have resulted in a different thermal characteristic [23], compared to the orthopedic samples with in part different pathologies.
It is important to note that the T2 trauma sample showed markedly different thermal characteristics, compared to other samples. For instance, a single transition was found at ~ 50 °C, a well separable double denaturation in the vicinity of 150 °C, as well as three others at 340; 420 and 500 °C. The enthalpy contribution in each three denaturation phases higher compared with the control. In addition, the final mass loss was the largest, ~ 60%. The different pattern of the curves could be explained by either a more intensive acute microstructural damage caused by the trauma itself or by a more rapid avascular necrosis caused by the ruptured microcirculation during anatomical neck dislocation.
Additionally, the differences between orthopedic and trauma samples cannot be always clearly distinguished. Trauma patients can exhibit preexisting symptoms of osteoarthritis and orthopedic patients may develop signs of avascular necrosis due to previous trauma. It is likely that in case of the ‘outliner’ trauma sample we were able to detect a mixture of degenerative disease and an enhanced effect of acute trauma as well.
Possible limitation of the study is that the collected 5 × 150 mm (diameter × height) sized cylinder-shaped bone samples involves both layers of the subchondral bone. Therefore, the thermoanalysis was performed on whole cylinder and the data were collected of both layers during the same measurement. It is known that the deeper, trabecular layer would show different pattern at the different grades of osteoarthritis: subchondral microdamage, subchondral trabecular sclerosis and the formation of subchondral cysts, parallel to the loss of mineralization [14]. Therefore, one cannot exclude that a larger sized of sclerotic part of subchondral cysts interferes with the measurement.
Another limitation of this study is that only the average values were calculated due to the relatively small number of samples. However, we believe that DTA curves and thermogravimetric analyses indicated marked differences to draw our conclusion.
Conclusions
DTA/TG analysis of human humerus head samples indicated marked differences in bone quality of orthopedic and trauma patients, compared to the control. Further investigation is needed to differentiate the calorimetric parameters of different layers of subchondral bone.
Availability of data and material
There are no additional available data to upload.
References
Lübbeke A, Rees JL, Barea C, Combescure C, Carr AJ, Silman AJ. International variation in shoulder arthroplasty. Acta Orthop. 2017;88:592–9.
Collin P, Hervé A, Walch G, Boileau P, Muniandy M, Chelli M. Mid-term results of reverse shoulder arthroplasty for glenohumeral osteoarthritis with posterior glenoid deficiency and humeral subluxation. J Shoulder Elb Surg. 2019;28:2023–30.
Fitschen-Oestern S, Behrendt P, Martens E, Finn J, Schiegnitz J, Borzikowsky C, et al. Reversed shoulder arthroplasty for the treatment of proximal humerus fracture in the elderly. J Orthop. 2020;17:180–6.
Mahaffy MD, Knowles NK, Berkmortel C, Abdic S, Walch G, Johnson JA, et al. Density distribution of the type E2 glenoid in cuff tear arthropathy. J Shoulder Elb Surg. 2020;29:167–74.
Lénárt G, Pflüger G, Bidló G, Pintér J, Fischerleitner F. Kristallographische untersuchung des verlängerungskallus. Arch Orthop Trauma Surg. 1979;93:303–5.
Raja S, Thomas PS, Stuart BH, Guerbois JP, O’Brien C. The estimation of pig bone age for forensic application using thermogravimetric analysis. J Therm Anal Calorim. 2009;98:173–6.
Mkukuma LD, Imrie CT, Skakle JMS, Hukins DWL, Aspden RM. Thermal stability and structure of cancellous bone mineral from the femoral head of patients with osteoarthritis or osteoporosis. Ann Rheum Dis. 2005;64:222–5.
Nöt LG, Bata A, Szabó H, Cifra J, Lőrinczy D. DSC examination of rotator cuff damage in patients with total shoulder arthroplasty. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-10402-w.
Bata A, Nöt LG, Szabó H, Cifra J, Lőrinczy D. DSC examination of cartilage damage of patients undergoing shoulder replacement. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-10421-7.
Patczai B, Mintál T, Nőt LG, Wiegand N, Lőrinczy D. Effects of deep-freezing and storage time on human femoral cartilage. J Therm Anal Calorim. 2017;127:1177–80.
Brolin TJ, Updegrove GF, Horneff JG. Classifications in brief: Hamada classification of massive rotator cuff tears. Clin Orthop Relat Res. 2017;475:2819–23.
Bercik MJ, Kruse K 2nd, Yalizis M, Gauci M-O, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elb Surg. 2016;25:1601–6.
Lozano LF, Peña-Rico MA, Heredia A, Ocotlán-Flores J, Gómez-Cortés A, Velázquez R, et al. Thermal analysis study of human bone. J Mater Sci. 2003;38:4777–82.
Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15:223.
Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–7.
Suri S, Walsh DA. Osteochondral alterations in osteoarthritis. Bone. 2012;51:204–11.
Henrotin Y, Pesesse L, Sanchez C. Subchondral bone and osteoarthritis: biological and cellular aspects. Osteoporos Int. 2012;23(Suppl 8):S847–51.
Bettica P, Cline G, Hart DJ, Meyer J, Spector TD. Evidence for increased bone resorption in patients with progressive knee osteoarthritis: longitudinal results from the Chingford study. Arthritis Rheum . 2002;46:3178–84.
Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD. Low bone mineral density and fracture burden in postmenopausal women. Can Med Assoc. 2007;177:575–80.
Meinberg EG, Agel J, Roberts CS, Karam MD, Kellam JF. Fracture and dislocation classification compendium—2018. J Orthop Trauma. 2018;32(Suppl 1):S1-10.
Maier D, Jaeger M, Izadpanah K, Strohm PC, Suedkamp NP. Proximal humeral fracture treatment in adults. J Bone Joint Surg Am. 2014;96:251–61.
Lafforgue P. Pathophysiology and natural history of avascular necrosis of bone. J Bone Spine. 2006;73:500–7.
Schnetzke M, Bockmeyer J, Loew M, Studier-Fischer S, Grützner P-A, Guehring T. Rate of avascular necrosis after fracture dislocations of the proximal humerus: timing of surgery. Obere Extrem. 2018;13:273–8.
Acknowledgements
This work was supported by CO-272 (OTKA) grant (D.L.).
Funding
Open access funding provided by University of Pécs.
Author information
Authors and Affiliations
Contributions
Dr. András Bata involved in sample collection and handling, data analysis and manuscript writing. Dr. László G Nöt participated in operations, sample collection and handling, data analysis and manuscript writing. Dr. János Cifra involved in histological examination. Dr. Hajnalka Szabó participated in radiological evaluation. Prof. Dr. Dénes Lőrinczy corresponding author, principle investigator, TG/TGA experiments, data analysis and manuscript writing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
All procedures followed were approved and in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the revised Helsinki Declaration of 1975.
Consent to participate
Consent from patients to participate in the study has been obtained.
Consent for publication
Copyright form has been uploaded with the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bata, A., Nöt, L.G., Szabó, H. et al. Thermogravimetric analysis of cancellous bone of humerus head in patients undergoing total shoulder arthroplasty. J Therm Anal Calorim 147, 3107–3115 (2022). https://doi.org/10.1007/s10973-021-10702-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10702-9