Abstract
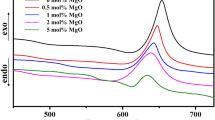
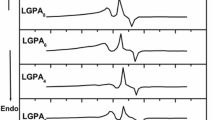
Lithium aluminum germanium phosphate glass–ceramics with NASICON structure find potential application in the field of energy storage device/solid-state battery. Two different glasses with nominal compositions (a) Li1.5Al0.5Ge1.5P2.9Si0.1O12 (LAGP1) and (b) Li1.5Al0.5Ge1.5P2.5Si0.5O12 (LAGP2) were prepared by standard melt-quench technique, and crystallization kinetics phenomenon in these systems was studied using differential thermal analysis technique (DTA). In addition to different conventional methods for crystallization kinetics analysis, model-free kinetics was also applied. The values obtained for activation energy of crystallization (E a) are compared and used for determination of crystallization index, n and m. The E a value obtained for LAGP1 is 375 ± 17 kJ mol−1 which is higher as compared to the E a value of LAGP2, 199 ± 22 kJ mol−1. LAGP2 with higher amount of Si(0.5) causes significant structural modification in the phosphate network and an early phase separation in the silico-phosphate glass. Thus, LAGP2 shows lower activation energy value as compared to LAGP1. The kinetic parameter, n, related to crystal nucleation, was evaluated from crystallized volume fraction (x) at a fixed temperature using predetermined E a value, and other parameter ‘m’ related to the crystal growth was determined using the modified Kissinger equation. The model-free kinetics was used to evaluate the variation of E a, m and n with temperature and suggests a dynamic nucleation and crystallization process with progressive change in kinetic parameters. In LAGP1 sample, an unusual increase in E a value was observed at x value > 0.2 and can be correlated with the existing ‘self-feeding’ process, which is observed in DTA plots. In both of these systems, the values of n and m are found to be equal and more than 3 which suggests three-dimensional growths of Li1.5Ge1.5Al0.5(PO4) crystals on a constant number of already grown nuclei. XRD and micro-Raman spectroscopy were used to identify the crystalline phase formed and various structural units present in the glass and glass–ceramics samples. Minor amount of LiAlPO4 was confirmed from XRD and Raman spectroscopy along with major Li1.5Ge1.5Al0.5(PO4) phase. In addition, using advanced kinetics and technology solution software non-isothermal data were simulated and the isothermal conversion data were extracted for various temperatures which are found to be very close to experimental isothermal data.


















Similar content being viewed by others
References
Wang Y, Richards WD, Ong SP, Mara LJ, Kim JC, Mo Y, Ceder G. Desgin principles for solid-state lithium superionic conductors. Nat Mater. 2015;. https://doi.org/10.1038/NMAT4369.
Bates JB, Dudney NJ, Neudecker B, Ueda A, Evans CD. Thin-film lithium and lithium-ion batteries. Solid State Ion. 2000;135:33–45.
Matusita K, Komatsu T, Yokota Y. Kinetics of non-isothermal crystallization process and activation energy for crystal growth in amorphous materials. J Mater Sci. 1984;19:291–6.
Avrami M. Granulation, phase change, and microstructure kinetics of phase change. III. J Chem Phys. 1941;9:177.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal. 1970;2:301–24.
Kasprzyk M, Sroda M, Szumera M. Influence of Gd2O3 on thermal stability of oxyfluoride glasses. J Therm Anal Calorim. 2017;130:207–20.
Davim EJC, Senos AMR, Fernandes MHV. Non-isothermal crystallization kinetics of a Si–Ca–P–Mg bioactive glass. J Therm Anal Calorim. 2014;117:643–51.
Sinouh H, Bih L, Manoun B, Lazor P. Thermal analysis and crystallization of the glasses inside the BaO–SrO–TiO2–NaPO3 system. J Therm Anal Calorim. 2017;128:883–90.
Basaran C, Canikoglu N, Toplan HO, Toplan N. The crystallization kinetics of the MgO–Al2O3–SiO2–TiO2 glass ceramics system produced from industrial waste. J Therm Anal Calorim. 2016;125:695–701.
Johnson WA, Mehl RF. Reaction kinetics in processes of nucleation and growth. Trans AIME. 1939;135:396–415.
Avrami M. Kinetics of phase change. I general theory. J Chem Phys. 1939;7:1103–12.
Avrami M. Kinetics of phase change. II transformation-time relations for random distribution. J Chem Phys. 1940;8:812–24.
Matusita K, Sakka S. Kinetic study of the crystallization of glass by differential scanning calorimetry. Phys Chem Glasses. 1979;20:81–4.
Golz J. Crystallization of glassy 2PbO·SiO2 studied by DTA. Phys Chem Glasses. 1977;18:32–5.
Marseglia EA. Kinetic theory of crystallization of amorphous material. J Non Cryst Solids. 1980;41:31–6.
Wang HY, He K, Zu KC, Chen J. Study on crystallization kinetics of Li2O–Al2O3–GeO2–P2O5 glass by non-isothermal technique. Adv Mater Res. 2012;535–537:1629–33.
Wang J, Lio C, Zhang G, Xie J, Han J, Zhao X. Crystallization properties of magnesium aluminosilicate glass–ceramics with and without rare-earth oxides. J Non Cryst Solids. 2015;419:1–5.
Khawam A, Douglas R, Flanagan DR. Role of isoconversional methods in varying activation energies of solid-state kinetics II. Nonisothermal kinetic studies. Thermochim Acta. 2005;436:101–12.
Pacurariu C, Lazau RI, Lazau I, Ianos R, Tita B. Non-isothermal crystallization kinetics of some basaltic glass–ceramics containing CaF2 as nucleation agent. J Therm Anal Calorim. 2009;97:507–13.
Vlase T, Pacurariu C, Lazau RI, Lazau I. Kinetic studies of the crystallization process of glass–ceramics based on basalt. J Therm Anal Calorim. 2007;88:625–9.
Pacurariu C, Lazau I. Non-isothermal crystallization kinetics of some glass–ceramics with pyroxene structure. J Non Cryst Solids. 2012;358:3332–7.
Starink MJ. Activation energy determination for linear heating experiments; deviation due to neglecting the low temperature end of the temperature integral. J Mater Sci. 2007;42:483–9.
Kun H, Yanhang W, Chengkui Z, Huifeng Z, Yonghua L, Jiang C, Bin H, Juanrong M. Influence of Al2O3 additions on crystallization mechanism and conductivity of Li2O–GeO2–P2O5 glass–ceramics. Physica B. 2011;406:3947–50.
Rodrigues AM, Narváez-Semanate JL, Cabral AA, Rodrigues ACM. Deteremination of crystallization kinetics parameters of a Li1.5Al0.5Ge1.5(PO4)3 (LAGP) glass by differential scanning calorimetry. Mater Res. 2013;16:811–6.
Kun H, Yanhang W, Chengkui Z, Yonghua L, Huifeng Z, Bin H, Jiang C. Crystallization kinetics of lithium aluminum germanium phosphate glass by DSC technique. J Wuhan Univ Technol Mater Sci Ed. 2012;27:63–6.
Mohoric I, Krajnc M, Sebenik U. Model-free kinetics analysis of thermal degradation of polysiloxane lubricant. Chem Biochem Eng. 2009;23(4):493–6.
Syam Prasad N, Varma KBR. Crystallization kinetics of the LiBO2–Nb2O5 glass using differential thermal analysis. J Am Ceram Soc. 2005;88:357–61.
Doyle CD. Kinetic analysis of thermogravimetric data. J Appl Polym Sci. 1961;5:285–92.
Sestak J. Thermophysical properties of solids, their measurements and theoretical analysis, vol. 12D. Amsterdam: Elsevier; 1984.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Flyn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Nat Bur Stand. 1966;70A:487–523.
Yang Q, Jiang Z. The study of criterion for glass crystallization kinetics. J Chin Ceram Soc. 1994;22:419–26.
Imran MMA. Crystallization kinetics, glass transition kinetics, and thermal stability of Se70−x Ga30In x (x = 5,10,15 and 20) semiconducting glasses. Physica B. 2011;406(3):482–7.
Speyer RF. Kinetics of phase transformations in amorphous materials by DSC. Part I. Thermochim Acta. 1988;131:211–24.
Augis JA, Bennett JE. Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J Therm Anal. 1978;13:283–92.
Zhang LY, Li H, Hu LL. Statistical structure analysis of GeO2 modified Yb3+: phosphate glasses based on Raman and FTIR study. J Alloys Compd. 2017;698:103–13.
Kumar S, Murugavel S, Rao KJ. Absence of germanate anomaly in ternary lithium germanophosphate glasses: modification behavior of mixed glass system of strong and fragile formers. J Phys Chem B. 2001;105:5862–73.
Shibata N, Horigudhi M, Edahiro T. Raman spectra of binary high-silica glasses and fibers containing GeO2, P2O5 and B2O3. J Non Cryst Solids. 1981;45:115–26.
Francisco BE, Stoldt CR. Energetics ion transport in NASICON-type electrolytes. J Phys Chem C. 2015;119:16432–42.
Plotnichenko VG, Sokolov VO, Koltashev VV, Dianov EM. On the structure of phosphosilicate glasses. J Non Cryst Solids. 2002;306:209–26.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Das, A., Goswami, M. & Krishnan, M. Crystallization kinetics of Li2O–Al2O3–GeO2–P2O5 glass–ceramics system. J Therm Anal Calorim 131, 2421–2431 (2018). https://doi.org/10.1007/s10973-017-6856-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6856-5