Abstract
In the current study, a decahedron-like silver nanostructure (D-AgNs) was successfully created in an aqueous solution in the presence of Polyvinylpyrrolidone (PVP) as a capping agent using the gamma-radiolysis approach without the use of a reducing agent. The synthesized D-AgNs were characterized using various analytical tools such as UV–Vis. spectroscopy, SEM, XRD, HRTEM, EDX and FTIR. UV–Vis. absorption spectra showed considerable surface Plasmon resonance (SPR) bands at 350–600 nm, indicating that colloidal D-AgNs had been successfully synthesized. HRTEM image demonstrates well-dispersed uniformly decahedral shapes that are well separated from each other. The produced nanoparticles were effectively stabilized by PVP through interactions, confirmed by the FTIR anlaysis. The synthesis of D-AgNs using gamma radiation was accomplished, in addition its antimicrobial potential, antibiofilm activity, and the effect of UV rays were assessed. In addition, protein leakage assays and SEM imaging were employed to analyze the antimicrobial reaction’s mechanism. A wide variety of bacteria, including S. aureus, P. aeruginosa, and C. albicans, were deactivated by D-AgNs. In the antibiofilm assay, D-AgNs inhibited the biofilm formation of S. aureus (89.58%), E. coli (80.35%), and P. aureginosa (78.45%). After investigating the effect of D-AgNs on the growth curve of S. aureus, we concluded that D-AgNs affect the growth curve of S. aureus, and the curve was reduced to be 0.125. The formation of holes in the S. aureus cell membrane is explained by the fact that the amount of cellular protein released from the bacteria is directly proportional to the concentration of D-AgNs, which was determined to be 259.25 µg/ml at concentration equal to 1.0 mg/mL.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nanoparticles have been of enormous scientific significance because they filled the gap between bulk materials and atomic and molecular structures [1]. Silver nanostructures (AgNs) are the most attractive due to their largest surface area to volume ratio. The surface of the particles needs to be controlled since variations in surface size may cause changes in the physical and chemical properties of the nanoparticles [2]. But what factors affect these nanoparticles’ properties? What could we influence so that their characteristics change? The electrical, chemical, and physical characteristics of the particles change when they reach a size between 1 and 100 nm, making it evident that the properties are directly correlated with the particle size. As a result, different attributes, including temperature, redox potential, color, conductivity, chemical stability, optics, etc., can be changed by varying the particle's size and shape [3, 4].
Based on the literature review regarding the superior activity of metallic, and metal oxide nanostructure, it was found that the synthesized metallic and metal oxide nanoparticles demonstrated enhanced stability and penetration [5], anti-cancer efficacy [6], interaction with microbiome for clinical management of extended spectrum beta lactamases (ESBL) positive Pseudomonas aeruginosa [7], and the selective binding with A-T bases in minor groove of DNA [8].
One of the most intriguing applications for AgNs is theirpractical use as a promising antibacterial disinfectant agent[9].The scientific community has recently given AgNs much thought due to their catalytic potential [10], antibacterial activity [11], optical [12], thermal [13], and electronic properties [14].
AgNs have been widely used in various fields, including environmental and medicinal applications, microelectronics, optoelectronic devices, the health sector, food storage, textile coating, and catalysts. Besides that, they feature distinct qualities and a high surface area-to-volume ratio. According to research, the shape and size of Ag NPs have a significant impact on their electromagnetic, optical, and catalytic capabilities. AgNs with various morphologies, including discs, rods, wires, prisms, and spheres, have been produced in numerous research. Tetrahedrons, cubes, decahedrons, and pentagons are a few examples of specific shapes successfully synthesized [15].
Several methods, such as electrochemical deposition, irradiation reduction, pulse sono-electrochemical procedures, ultrasonic-assisted solution reduction, and displacement techniques, have been used to produce distinctive metal dendrites [16]. Water-soluble polymers were used in several of these techniques as protective agents, such as polyvinyl alcohol (PVA) and PVP as a capping and stabilising element to boost their stability and avoid precipitation [17]. Due to their unique features, inorganic-polymer nanocomposites have recently experienced tremendous development. Numerous potential uses for these nanocomposite materials have been discovered in optics, electrics, mechanics, and photo-conductors [18]. PVP is one of the most commonly used vinyl polymers and has several intriguing properties, including excellent chemical and heat resistance, biodegradability, and low cytotoxicity [19]. They also have a strong affinity for complicated hydrophilic and hydrophobic compounds because they dissolve well in water and various organic solvents (such as amines, amides, alcohols, acids, etc.) [20]. This polymer is used in various applications, with the pharmaceutical and biomedical industries being the most popular ones [19]. The US Food and Drug Administration (USFDA) has approved this polymer as a safe polymer for biological studies, and it is used in such fields [19, 20].
The strategy of γ-irradiation is the best method for creating metal nanoparticles because no external reductant is added, making it one of the many methods [21]. Thus, the impact of the additional reducing agent's final products can be diminished. The synthesis may also be performed in normal environmental conditions. The reactants are uniformly reduced due to γ-irradiation as well. Several studies have examined the effects of γ-irradiation on the reduction of silver ions in the presence of PVP [22]. Additionally, the quantity and molecular weight of PVP in the irradiation solution affect the size of the produced nanoparticles [23].
The choice of radiation strategy for the synthesis of nanostructured materials has several advantages over the traditional methods due to its simplicity, homogeneous reduction and nucleation of the nanoparticles with absence of undesired oxidation byproduct impurities and cost-effective [24]. The simplicity of this technique being occurs in a single step at ambient temperature and pressure with excellent reproducibility i.e., the radiation technology is an environmentally benign and scalable strategy. More importantly, radiation technique enables the facile and effective tunning of both the particle size and the morphology of the nanomaterials through the simple optimization of the applied irradiation dose or dose rate, nature, and concentration of the precursors, pH, and nature of solvents. Moreover, the radiation strategy has also a sterilization function. On the other hand, the chemical processes in the synthesis of nanomaterials suffer from the broad particle size distributions and contamination of nanoparticles with toxic reducing agents and needing for additional purification steps [25,26,27,28].
One of the most harmful and prevalent illnesses in humans, urinary tract infections (UTIs) affect almost 150 million individuals globally [29]. As a result of the spread of infectious epidemics, the cost of treating those with UTIs is around $ 6 billion per year [30]. The most frequent cause of UTI is Gram-negative E. coli, which can be acquired through hospitals and the community from infected individuals. Large volumes of pus cells, which are created as a result of the presence of microbial pathogens (fungi and bacteria) capable of infiltrating the tissues in the urinary tract cells, serve as a telltale sign of a UTI [31, 32]. Other major organisms that cause UTI include E. faecalis, S. aureus, P. aeruginosa, Proteus mirabilis, K. pneumonia, and Candida sp.[33]. Due to improper use during therapy and the growth and aggressiveness of the microbial pathogen cells, UTIs have become more resistant to many medicines [34]. Moreover, pathogenic bacterial cells can produce biofilms that shield them from curative drugs [35, 36]. Thus, it is now vital for people interested in scientific study and scientists to develop cutting-edge methods for getting rid of the pathogens that cause UTIs [37].
This study attempts to talk about one of the techniques, characterise them, and pay close attention to their effectiveness as an antibacterial and antibiofilm agents. Many physical, chemical, and biological methods have been developed for producing D-AgNs as a result of the enormous expansion of nanotechnology. In this study, D-AgNs will be produced by gamma radiolysis in the presence of PVP, and their capacity to inhibit pathogenic bacteria and unicellular fungi will be assessed. The created D-AgNs was examined using absorption spectroscopy. In addition, studies into the impacts of UV light and the mechanisms behind antimicrobial reaction mechanisms, as well as protein leakage assays and SEM imaging were carried out.
2 Materials and Methods
2.1 Materials
Silver nitrate and Polyvinylpyrrolidone (PVP) (M.wt = 40,000 g/mol) were purschased from Sigma Aldrich. Merck Chemicals Ltd. provided the isopropyl alcohol. Other chemicals and media used in the microbiological methods were of pure grade and used as received without any further purification. Throughout the experiments, deionized water was used.
2.2 Synthesis of Decahedron-Like Silver Nanostructure (D-AgNs)
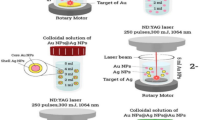
With slight adjustments, the bottom-up approach (radiation synthesis) method proposed by Sheikh et al. [38] was used to create silver nanostructures that resemble decahedrons. To make the polymer completely soluble, 0.15 g of PVP was dissolved in 80 mL of deionized water and stirred using a magnetic stirrer at room temperature for an hour. Afterthat, 20 mL of (0.1 M) Ag NO3 was added to the PVP solution and kept there for about an hour at room temperature with constant stirring in order to guarantee that the solution was homogenous. The aforesaid mixture was then given 10 mL of isopropanol solution to prevent the reverse reaction. Finally, the PVP/Ag+ solution was exposed to gamma-ray dosages ranging from 1 to 100 kGy at the NCRRT, EAEA, Nasr City, Cairo, Egypt, using Co-60-cell-220 sources (manufactured by the Atomic Energy Authority of India at a dosage rate of 1.1 kGy/h).
2.3 Characterization Techniques
The optical properties of the synthesized D-AgNs were assessed using a Unicam Double Beam Double Monochromator UV–Visible spectrophotometer in the wavelength range of 400–900 nm (made in England). D-AgNs surface and morphological properties were examined using SEM (SEM, ZEISS, EVO-MA10, Germany). Also, using EDX spectrum analysis, the elemental analysis, purity, and relationship of each metal were assessed (BRUKER, Nano GmbH, D-12489, 410-M, Germany). The X-ray diffraction (XRD) pattern was examined using an X-ray diffractometer (Shimadzu-XRD-6000, Tokyo, Japan). The particle size and shape of the generated D-AgNs were measured using a high-resolution transmission electron microscope (HRTEM, JEM2100, Jeol, Japan). The function group was identified and a structural study using Fourier transform infrared spectroscopy in attenuated total reflection (ATR) mode was carried out using a Bruker Vertex 70 FTIR spectrophotometer.
2.4 Antimicrobial Potential
The antibacterial, and antifungal potential of the synthesised D-AgNs (10 µg/mL) was assessed using the agar-disc diffusion experiment [39] against several chosen pathogenic unicellular fungi (yeast) and bacteria. Gram-positive bacteria include Bacillus subtilis and Staphylococcus aureus, while Gram-negative bacteria include Proteus vulgaris, Pseudomonas aeruginosa, Escherichia coli, Proteus mirabilis, Salmonella typhi, and Klebsiella pneumoniae.
As unicellular fungus, the pathogenic yeasts Candida tropicalis and Candida albicans were examined. These microbial strains were obtained from the culture collection at the Drug Microbiology Lab, Drug Radiation Research Department, NCRRT, EAEA, Cairo, Egypt (clinical isolates from urine samples). The bacteria under examination were plated on nutrient agar and cultured at 37 °C for one day before being stored to 4 °C [40]. The microbial inoculums of all the tested microorganisms must be fixed to a certain standard known as 0.5 McFarland, which is set at 2 × 108 CFU/mL for the tested bacteria and 3 × 108 CFU/mL for unicellular fungi, prior to completing the microbial surface inoculation and zone of inhibition (ZOI) measuring [7, 41]. To assess the antimicrobial potential of the synthesised samples, the ZOI test must be conducted alongside the addition of amoxicillin (25 µg/mL),a common antibacterial agent, and nystatin (100 µg/mL), a common antifungal agent (positive controls) [42]. All Petri plates required to be incubated for overnight at 37 °C [5], in order to determine the antimicrobial potential as the diameter of ZOI of the investigated samples [41, 43].
2.5 Antibiofilm Activity of the Synthesized D-AgNs
The antibiofilm potential of the synthesised D-AgNs (at concentration 10 µg/mL) was assessed in triplicate after the test tube assay and testing against a few selected harmful microorganisms (tested in the ZOI assay). Finally, the semi-qualitative analysis for the microbial biofilm hindrance was performed using the method published by Christensen et al., [44]. The obtained results were compared with the control non-treated samples.
Before the antibiofilm assay, the inoculums of the tested bacteria and unicellular fungus must be fixed according to 0.5 McFarland and adjusted at 2 × 108 CFU/mL for the bacteria and 3 × 108 CFU/mL for the pathogenic yeast.
First, the liquid nutritional broth was mixed with the fixed microorganisms in the designated test tubes, and an overnight incubation at 37 °C was followed by the antibiofilm test [45]. After incubation, all the treated and untreated tubes were discarded, and the rings in tubes under investigation were cleansed with phosphate buffer saline (PBS; pH 7.0). Finally, deionized water was used to repeatedly cleanse the tubes [46].
Before being completely rinsed with deionized water, the adhering microorganisms in the investigated tubes must be fixed with 3.5% sodium acetate (5 mL) for around 15 min. The cleaned tubes with the fixed microbial biofilm must then be stained with 0.15% crystal violet (CV; 5 ml) for around 15 min in order to assess the semi-qualitative antibiofilm activity of the synthesised samples. Finally, the semi-quantitative antibiofilm capability of the produced samples was evaluated by dissolving the CV-labeled microbial cells in ethanol solution (5 mL). After employing the UV–Vis., the dissolver CV’s O.D. was analysed and measured. The following equation was used to quantify the microbial biofilm hindrance % using a fixed wavelength spectroscopy technique (570 nm) (1).
2.6 Growth Curve Method
The effect of the synthesised D-AgNs on the kinetic growth curve of S. aureus (the most sensitive bacteria) was examined using the Huang et al. technique [47]. The inoculum of the examined bacteria was standardized to 0.5 McFarland in 2 × 108 CFU/mL prior to testing. Then, the investigated bacterial sample from the examined tube was mixed with synthetic D-AgNs. To obtain the standard growth curve and assess the impact of the synthesised samples on the kinetic growth of the tested S. aureus, the final spectrum and relationship between the average of duplicate assignments and time (hours) were carried out in the test procedure. The O.D. at a fixed wavelength (600 nm) was measured every two hours for approximately 24 h.
2.7 Potential Effect of UV Illumination
The D-AgNs (at concentration of 10 µg/mL) was combined with the adjusted bacterial cells, which were adapted to standard 0.5 McFarland (2 × 108 CFU/mL). After mixing, all tubes were exposed to UV radiation. The antimicrobial effectiveness of the UV-irradiated samples was then tested in triplicate against the most delicate microbe (S. aureus) using the optical density method [48], and compared to the control samples that had not been exposed to UV radiation.
The tubes under investigation were divided into tubes with D-AgNs but no UV radiation and tubes with D-AgNs and UV radiation. They were exposed at different periods (0, 15, 30, 45, 60, and 75 min). Note that the treated samples produced turbidity, which was detected at a fixed wavelength of 600 nm. The evaluated samples were selected with the UV-lamp source at 37 °C (disturbance of 7.0 mW cm2). After employing the equation, the method developed by Abd Elkodous et al., [48] was applied to determine the inhibition percentage (Equ. 1).
2.8 Effect of the Synthesized D-AgNs on Protein Leakage from Bacterial Cell Membranes
The assay of protein leakage was evaluated in triplicate to ascertain the hypothesized bacterial response mechanism of the synthesized samples. The bacterial inoculums were fixed using the standard 0.5 McFarland procedure (2 × 108 CFU/mL) and then combined with various concentrations of D-AgNs.
D-AgNs-free broth and culture were used to prepare the negative control. After being incubated at 37 °C for 5 h, all test samples were separated by centrifugation, which was conducted for 20 min at 5000 rpm [49]. The separated supernatant from the tested samples (100 µL) was mixed with a Bradford reagent solution (1 mL), and after 10 min of incubation at 37 °C in the dark, O.D. at a fixed wavelength (595 nm) was measured, and calculated [49].
2.9 Reaction Mechanism Determination by SEM imaging
To prepare the bacterial samples for SEM imaging, S. aureus cells must first be thoroughly washed with PBS several times before being fixed with a glutaraldehyde solution (3.5% concentration). The ethanol solution was added to the fixed S. aureus cells for 30 min at room temperature, followed by several PBS rinses and air drying. Placing the immobilized bacteria (S. aureus) on the aluminium stump was the first step in SEM imaging [50]. The surface morphology and any changes in the untreated and treated bacterial cells exposed to 10 µg/mL D-AgNs were examined using SEM imaging.
2.10 Statistical Analysis
After using the ONE-WAY ANOVA (at P = 0.05) and Duncan's techniques, the statistical analysis of the acquired results was looked according to the following standard reference [51]. The acceptable results were examined using SPSS software version 15.
3 Results and Discussion
3.1 UV–Vis. Absorption Analysis
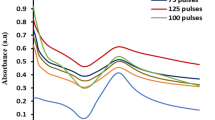
UV–Vis spectroscopy is the most effective method for explaining the creation of metal nanoparticles via the Surface Plasmon Resonance (SPR) phenomena. The optical absorption spectra of the unirradiated and gamma irradiated Ag+/PVP solutions are shown in Fig. 1.
No surface Plasmon resonance (SPR) absorption of Ag Ns was seen for samples not exposed to radiation. In contrast, SPR in the 350–600 nm range following γ–irradiation indicates the presence of Ag Ns, which was discovered to be size and shape-dependent [52].
In the current study, SPR broad bands appeared at 350–600 nm were observed, with a strong peak centered at 489 nm undergoing a significant red shift as the irradiation dose was increased. Furthermore, the intensity of the absorption bands increases significantly with increasing irradiation dose indicating the growth and high yeild of silver nanoparticles. Also, two weak shoulder peaks at 390 and 406 nm was appeared.
The strong broad peak centered at 489 nm is attributed to in-plane quadrupole resonances of silver decahedrons while the weak shoulder peaks at 390 and 406 nm are attributed to the out-of-plane quadrupole resonance and dipole resonance excited on the sharp vertexes and edges of silver decahedrons. In other words, the strong peak attributed to the longitudinal dipole SPR modesand the shoulders assigned to the transverse dipolar SPR modes of silver decahedrons [53, 54].
3.2 XRD Analysis
X-ray crystallography confirmed the crystalline phase of synthesized nanoparticles. The XRD patterns of a 100 kGy irradiated D-AgNs are shown in Fig. 2.
The XRD pattern of the as-prepared D-AgNs shows five peaks, which correspond to the (111), (200), (220), (311), and (222) diffraction peaks of the face cubic crystal (fcc) Ag, respectively. This pattern contains no impurities, and indicates that pure crystalline Ag can be obtained under the current synthetic conditions (JCPDS File No. 04-0783) [15, 55].
This indicates that the product's polycrystalline nature is quantitatively similar to previously reported structures. No diffraction peaks from other impurities were detected, indicating that the particles obtained are pure Ag with an fcc structure [56].
The Debye-Scherer Eq. (2) was applied to the width of the (111) peak to determine the average crystal size:
The wavelength of x-ray radiation is = 0.154056 nm, the geometric factor or Scherrer constant is k = 0.893 and β is the full angular width at half maximum (FWHM) of the XRD peak. The thickness of the silver nano-decahedral, as assessed directly from the HRTEM image below, coincides with the calculated average crystallite size of the Ag nano-decahedral, which was determined to be 15.99 nm. Decahedral nanoparticles had a crystallite size of 15.99 nm, corresponding to a broadening of the (111) peak [15].
3.3 HRTEM Analysis
One of the most valuable technologies for analyzing the structure, size, and shape of synthesized nanoparticles directly is HRTEM. Figure 3a–d depicts the HRTEM micrograph of the D-AgNs, which reveals distinct, uniformly decahedral forms that are differentiated. The solid lines in (Fig. 3d) represent the size distribution histogram of D-AgNs fitted with a Gaussian curve, and the peak point of the curve denotes the average diameter. The form of the Gaussian peak supported the limited size distribution of D-AgNs. The size distribution ranged from 15 to 30 nm, and the average size was around 19 nm, as seen from the histogram plot. This result is following the results obtained from XRD analysis.
3.4 SEM and EDX Elemental Analysis
SEM imaging detects and verifies the surface morphology and homogeneity of the produced D-AgNs. Figure 4a–c displays SEM picture of D-AgNs in various sizes that correspond to creating the spherical decahedral shape. The D-AgNs texture formation is eliminated, and the SEM data demonstrate a uniform NPs exterior. PVP was typically used to locate D-AgNs, visible as bright NPs mixed with the stabilizer.
Analysis of the EDX spectrophotometer revealed the presence of an Ag element suggestive of D-AgNs. In Fig. 4c, an intense signal was picked up by the EDX analysis from the Ag region of D-AgNs with (PVP). The elemental side picture of the D-AgNs highlights the formation of Ag NPs. The average percentage of elemental silver was 93.88% in metallic D-AgNs, which typically exhibit a conventional optical absorption peak between 3 and 4 keV. Moreover, elemental analysis of the PVP polymer revealed that silver had the highest amount, followed by C, O, and N (for PVP chemical structure).
3.5 FTIR Analysis
In order to investigate the interactions of PVP molecule with D-AgNs, ATR-FTIR spectroscopy was performed. It was shown in Fig. 5, D-AgNs exhibit the same feature of PVP matrix, but the intensity of the interaction peaks was changed. Spectral comparison indicates a shift of a peak from 1654 cm−1 in the pure PVP to 1638 cm−1 in the PVP containing D-AgNs. This band is attributed to C = O stretching of PVP structure and its shift to lower energies should be due to the chemical interaction between PVP molecules and D-AgNs surface [38, 57].
The effects of D-AgNs on the vibrational modes, which result from the coordination of D-AgNs, were indicated by decreases in intensities and a shifting of the bands to lower wavenumbers [58]. The shift of the pyrrolidone structure’s C–N bending vibration to 1289 cm−1 can be attributed to the band at 1282 cm−1. A bond weakening caused by the partial donation of an electron pair from the carbonyl oxygen in PVP to the vacant orbital of D-AgNs, which lowers the electron density of the carbonyl bond and subsequently the vibration energy, is indicated by the more remarkable red shift of the amide C–O group (band at 1654 cm−1) compared to the C–N (at 1282 cm−1). Although N has a higher ability to donate electrons than O due to its lower electronegativity (the bond strength between C and O is 798.9 kJ mol−2 compared to 304.6 kJ mol−2 for C and N), the steric effect of the pyrrolidone ring influenced the type of coordination bonding in this instance [59].
3.6 Mechanism of D-AgNs Formation
In general, the gamma radiolysis process of aqueous solution involves creating a large number of homogenously distributed reducing agents [60, 61]. Solvated electrons (e− aq) and hydrogen radicals (H.) reduce Ag+ ions to the zerovalent state. After that, larger clusters of silver atoms were produced due to the coalescence of silver atoms created during the irradiation process. In the presence of PVP as a capping and shape directing agent, the growth process and formation of Ag decahedrons occurs through an edge-selective particle fusion mechanism and gradually assembling into a decahedron [53, 54]. ((Eqs. (3–6)) and Scheme 1)
3.7 Antimicrobial Behavior
Antibiotics and antiseptics were developed over time to treat microbial infectious diseases, but when they were used carelessly, microbial resistance increased, and became ineffective [62]. New and innovative green nanocomposites must be created as an excellent antimicrobial agents to control the spread of microbial infectious diseases to solve the persistent issue [63].
The antimicrobial ZOI behavior of the synthesized D-AgNs was evaluated using the chosen agar-disc diffusion experiment [64] at a fixed concentration (10 µg/mL). Following analysis of the ZOI data, it was noted that the synthesized D-AgNs have antibacterial capability against the tested microorganisms, particularly against Staphylococcus aureus and Escherichia coli. The tested sample are active against S. aureus (25.6 ± 0.2259 mm ZOI; 0.019 µg/mL MIC) and C. albicans (22.2 ± 0.1599 mm ZOI; 0.039 µg/mL MIC), as shown in Table 1. In particular, D-AgNs possessed the highest effect against all the examined bacteria and unicellular fungi.
Following analysis of the ZOI data, it should be highlighted that, compared to the published paper, the D-AgNs were more effective against Gram-positive bacteria than against Gram-negative bacteria [65]. D-AgNs antibacterial potency was compared with the positive controls (AX & NS), and the results obtained showed that the synthesized D-AgNs more active than the AX and NS. As an illustration, the D-AgNs had an inhibition effect on S. aureus (25.6 mm) and C. albicans (22.2 mm), but the standard controls AX and NS had a reduced impact on the same bacteria as 6.5 mm and 6.5 mm, respectively.
The sample purity, unique shape, and composition may all significantly affect how the antimicrobial properties are assessed. Fundamentally, there is a connection between the physicochemical properties of D-AgNs and the ZOI results; the appropriate physical and chemical features, the nanoscale form, purity, and good distribution give the D-AgNs antimicrobial behavior more potency than synthetic disinfectants. It enables more interaction with the microbial cells, hence enhancing the character of contact and death [66]. The strength, stability, and distinctive shape of the D-AgNs and their encouraging physicochemical properties are further raised factors that increase their antimicrobial activity [67].
According to some of the literature’s suggested reaction mechanisms, the D-AgNs alter the bacterial surface and cellular behavior, ultimately changing the membrane permeability and triggering the production of oxidative pressure response genes in the bacterial cell because hydrogen peroxide is formed. According to the reference below, the species that was most negatively impacted as reactive oxygen species (ROS), which disseminated into the cells of the bacteria that had been treated [68]. Finally, the elevated inhibition and killing of the pathogenic microbes are made possible by the close relationship between the nanoscale composite (D-AgNs) and microbial cells.
3.8 Antibiofilm Activity
Exopolysaccharide molecules from some specific pathogenic microbes have been discovered to have the ability to form biofilms [46]. A tube approach was developed to evaluate the antibiofilm properties of the synthesized D-AgNs [69].
A negative results for biofilm inhibition of the tested S. aureus was shown by a noticeable whitish-yellow matt at the air–liquid interface attached to the designed tubes’ walls and stained blue following CV treatment. The absence of the synthesized D-AgNs (that serve as the control) is evident in the observed unfavorable results. In contrast, a favorable outcome for the synthesized D-AgNs ability to suppress growth was shown by a faint blue or absence of any CV color.
Bacterial ring growth was also restricted, and the synthesized D-AgNs demonstrated antibiofilm properties. UV–Vis. spectroscopy at a fixed wavelength is used to measure semi-quantitative antibiofilm inhibition % (fixed at 570.0 nm). After dissolving the stained microbial matt with ethanol, a detailed procedure is initiated, and the O.D. is evaluated to determine the inhibition percentage using an Eq. (1). For the synthesized D-AgNs, the inhibition percentage is determined. According to Table 2, S. aureus (89.58%), E. coli (80.35%), P. aureginosa (78.45%), and C. albicans (77.90%) showed the highest levels of biofilm inhibition.
Figure S1 presents a review diagram concerning the antibiofilm activity of the D-AgNs (as inhibition %) toward various pathogenic microbes.
The synthesized D-AgNs prevent the formation of biofilm during their irreversible adhesion phase [69]. However, it has not yet been confirmed if the synthesized D-AgNs automatically affect the structure of the biofilm. Several aspects, including antibacterial activity, physicochemical properties, penetration capabilities, and other chemical results regarding the charge connection, metal NPs (Ag), and practical decahedron shape, may influence our knowledge of the inhibitory percentage [70]. It was discovered that the synthesized D-AgNs prevented the development of biofilm and microbial growth. Exopolysaccharide secretion can be controlled in order to avoid the formation of biofilms, which is a good thing [46].
There is a clear-cut difference in MICs results (Table 2) and percent biofilm inhibition (Table 2) of Gram-negative and Gram-positive bacteria as well as fungi. The noted differences was seen in case of tested E. coli where the antibiofilm activity was about 80.35% at relatively higher MIC (0.159 µg/ml), when compared with the tested P. aeruginosa with where the antibiofilm activity was about 75.45% at relatively lower MIC (0.039 µg/ml). The main resonse was due to the capacity of the tested microbe to creat biofilm as a protctive layers which increased in some bacteria and decresed in others, so the antimicrobial potential results may be in some cases not with the line of antibiofilm results as indicated in the following reference [71].
3.9 Growth Curve Assay
Figure 6 shows the results of a kinetic analysis of how the D-AgNs (10 µg/mL) affected the growth and reproduction of the tested S. aureus. The untreated control S. aureus’ growth kinetics were normal, and the observed O.D. value at a particular wavelength (600 nm) was 2.78. Positively, a discernible influence on the kinetic growth curve was seen after adding D-AgNs, and the measured O.D. was reported at 0.125, showing the future inhibitory effect on the kinetics of S. aureus growth. D-AgNs have additional suppressing potential, and the specialimpact was brought on the antimicrobial D-AgNs’ catalytic potential [72].
The production of ROS on the surface of NPs may occur as a fatal factor for bacterial cells [73, 74]. The investigated microbes can be destroyed by the unique ROS produced by D-AgNs, which also causes protein oxidation, bacterial DNA damage, and lipid peroxidation. Additionally, because S. aureus cells have a negative charge, the release of positive ions like (Ag+) intensifies the fatal interaction between the D-AgNs and the pathogen's membrane. The synthesized D-AgNs had a suitable reactivity due to their nanoscale, stability, purity, surface charge, and chemical arrangement, which could increase the interactions with more pathogenic bacteria.
It is important to note that the results of the antibacterial activity as measured by the ZOI and growth curve testing were associated and showed that the synthesized D-AgNs had an inhibitory effect on the tested S. aureus’s growth kinetics (0.125) even at low concentrations.
According to the article published by Xu et al. [75], E. coli's membrane developed abnormalities, and the bacteria died after being exposed to nanocomposite treatment and UV light for about 80 min. According to another study [76], newly synthesized nanocomposites strongly exhibited antibacterial behaviors against the tested strains of E. coli and S. aureus.
3.10 Effect of UV on the Antimicrobial Potential
The antibacterial potential of the synthesized D-AgNs considerably increased with the extension of the UV exposure period and correlated with the deactivation of the pathogenic S. aureus cells following UV illumination, as shown in Fig. 7. During the exhibition period, positive impacts on S. aureus growth and expansion were seen. The UV assay results demonstrated that UV exposure of D-AgNs had a favourable impact on bacterial cell proliferation when compared to the untreated control. Results of the UV experiment showed that bacterial growth is affected and reduces following UV illumination of the synthesized nanocomposites (D-AgNs). D-AgNs are more susceptible to photo-activation from UV radiation than the control sample. After being excited by UV radiation, D-AgNs make an ideal biocidal agent.
Generally, most of the synthesized nanocomposites contributed to the formation of ROS following the acceptance of photons from the UV illumination process [77]. The created ROS (as hydrogen peroxide) after the UV excitation may be the promising factor for the bacterial biocidal process after interacting with the bacterial membrane. Finally, the infusion of the created ROS within the bacterial cell caused the formation of stable hydroxyl free radicals, and the death of the bacterial cell was noted [73, 74].
3.11 Determination of Protein Leakage from Bacterial Cell Membranes
The protein leakage assay has been used to estimate how many bacterial proteins have leaked from the bacterial cells (bacterial cell-free supernatant). It was noted that, 1.0 mg/mL of the synthesized D-AgNs caused 259.25 µg/mL of protein to be released from the cell, as shown in Fig. 8, which demonstrated that the amount of bacterial protein released from the cell is directly proportional to the increasing concentrations of the synthesized D-AgNs. The result outcomes validated the catalytic role of the synthesized D-AgNs as an antibacterial agent and showed the positive impacts on the bacterial cells. The currently available data supported the existence of the protein molecules in the bacterial cytoplasm and validated their leakage from the S. aureus membrane. D-AgNs perform noticed spots around the examined bacterial cells and the S. aureus membrane.
According to the noted findings, D-AgNs enhanced bacterial membrane leakage and changed the permeability of bacterial cell membranes. The bacteria also perished as a result of interference with the plasma membrane brought on by protein leakage, which was brought on by the treatment with the synthesized D-AgNs, which weaken the bacterial cell's line defence [78]. According to concentration dependence, Azam et al. [79] identify the mode of action, which finally affected bacterial protein leakage and subsequently destroyed the plasma membrane of some chosen harmful bacteria.
3.12 Reaction Mechanism Determination by SEM Analysis
The SEM imaging method is regarded as the confirmatory test for determining the bacterial reaction mechanism, as shown in Fig. 9. The tested bacteria had a regular shape, standard number, and conformation with typical surface morphology, as shown in Fig. 9 a, which was supported by the control S. aureus employed for SEM imaging. Positively, by the treating with the synthesized D-AgNs, abnormalities such as the irregular shape of the S. aureus cells as illustrated in Fig. 9b and malformations such as the semi-lysis of the outer surface and deformations of the S. aureus cells were found. The D-AgNs also partially lysed the cells, and there was a discernible decrease in the total number of viable cells. On the other hand, spots near the treated bacterial cell were observed, and the protein leakage assay confirmed this.
The layers of D-AgNs were observed across the treated cells may have been caused by the charge attraction between the positively charged Ag+ and the negatively charged bacterial cell. According to the literature, the reactive D-AgNs surface may be responsible for the reaction mechanism and the inhibitory potential of the synthesized D-AgNs.
Due to the breakdown of the bacterial membrane caused by the synthesized D-AgNs attachment to the bacterial cells through charge attraction, the bacterial membrane may change, and modifying the bacterial permeability. According to a schematic representation in Fig. 10, the reaction of bacterial cells appears to be the creation of genes causing oxidative stress as a result of the beneficial action of the produced ROS from the surface of the D-AgNs. We understand that the synthesized D-AgNs begin their activity after adhering to the microbial surface by charge attraction, allowing the bacterial membrane to leak, resulting in the formation of holes and cavities (Fig. 10), and subsequently stopping the ions transport from and inside the bacterial cells [80], as shown in Fig. 10. The primary microorganelles in the treated bacterial cell are damaged due to the ROS produced and penetrated inside the cell in response to the synthesized D-AgNs (like DNA and plasmid) [81]. Eventually, the negative impact and stress of the generated ROS lead to genotoxicity and cellular toxicity (Fig. 10) [80]. Finally, a comparative analysis of existing antibacterial efficiency of Ag NP (spherical) with the synthesized decahedron structured Ag NPs was indicated in Table 3.
Schematic description of the four main steps of antibacterial action of PVP@D-AgNs, where: 1. PVP@D-AgNs adhere to the bacterial cell’s surface and result in membrane breakdown, endocytosis, the creation of endosomes, and altered transport potential. 2. PVP@D-AgNs create and increase ROS, which suggests that the bacterial cell wall is weakening. 3. PVP@D-AgNs prevent ions from entering and leaving the bacterial cell. 4. PVP@D-AgNs enter bacterial cells and interact with cellular organelles, affecting the function of the corresponding cellular components and triggering cell lysis. PVP@D-AgNs could act as a transporter to effectively release Ag+ ions into the cytoplasm and different bacterial layers, where the presence of proton motive force would cause the pH to drop below 3.2 and encourage the release of Ag+ ions
4 Conclusions
To date, γ-irradiation has played a crucial role in the development and modification of D-AgNs because of its ability to fabricate average volumes with different shapes without relying on complex or expensive equipment. However, further improvements in γ-radiosynthesis technology will allow the production of stable metal nanoparticles with enhanced physical, chemical and biological properties and improved economic efficiency.The preparation of D-AgNs is very common, so the challenge in this research is to control the shape of the D-AgNs formed. Shape-controlled PVP capped D-AgNs have been synthesized using γ-irradiation technique at room temperature. By varying the irradiation dose, particles in the shape of decahedrons have been formed. The results of the X-ray diffraction, the HRTEM analysis and the corresponding histogram of as-prepared nanocomposites showed a relatively narrow size distribution of silver decahedral nanocomposite (D-AgNs). The FTIR results represented chemical interaction between PVP molecules and D-AgNs surface. SEM data show a homogenous NPs surface, and the synthesized D-AgNs texture formation is cleared. The produced materials demonstrate a performance in deactivating a variety of bacteria, including S. aureus, P. aeruginosa, P. mirabilis, and C. albicans. The results are active against S. aureus (25.6 ± 0.2259 mm ZOI; 0.019 µg/mL MIC) and C.albicans (22.2 ± 0.1599 mm ZOI; 0.039 µg/mL MIC), and D-AgNs in particular had the strongest effect against all the examined bacteria and unicellular fungus. Due to UV illumination’s activation, bacterial development in the UV display came to a stop in its smallest form. D-AgNs were more likely to be photoactivated than the control because UV presentation was chosen to boost that chance. From the protein leakage assay, it was revealed that the amount of cellular protein released from S. aureus is directly correlated to the concentration of D-AgNs (at different concentrations), which was found to be 259.25 µg/ml (at concentration of 1.0 mg/mL), proving the antibacterial properties of the D-AgNs and explaining the formation of holes in the S. aureus cell membrane resulting in the oozing out of the proteins from the S. aureus cytoplasm. From the data obtained, the synthesized sample (D-AgNs) possessed an excellent antimicrobial, and antibiofilm activities which prompted its use in various disciplines, including the disinfection of wastewater from harmful pathogenic microbes, and in biomedical application specially when utilizing to inhibit the UTI-causing bacteria and unicellular fungi.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
G.S. El-Sayyad, D. Elfadil, M.S. Gaballah, D.M. El-Sherif, M. Abouzid, H.G. Nada, M.S. Khalil, M.A. Ghorab, Implication of nanotechnology to reduce the environmental risks of waste associated with the COVID-19 pandemic. RSC Adv. 13(18), 12438–12454 (2023)
B. Khodashenas, H.R. Ghorbani, Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 12(8), 1823–1838 (2019)
A. Zielińska, E. Skwarek, A. Zaleska, M. Gazda, J. Hupka, Preparation of silver nanoparticles with controlled particle size. Procedia Chem. 1(2), 1560–1566 (2009)
S.M. Gamboa, E. Rojas, V. Martínez, J. Vega-Baudrit, Synthesis and characterization of silver nanoparticles and their application as an antibacterial agent. Int. J. Biosen. Bioelectron 5, 166–173 (2019)
K. Ali, S. Zaidi, A.A. Khan, A.U. Khan, Orally fed EGCG coronate food released TiO2 and enhanced penetrability into body organs via gut. Biomater. Adv. 144, 213205 (2023)
K. Ali, Q. Saquib, M.A. Siddiqui, J. Ahmad, A.A. Al-Khedhairy, J. Musarrat, Anti-cancer efficacy of Aloe vera capped hematite nanoparticles in human breast cancer (MCF-7) cells. J. Drug Deliv. Sci. Technol. 60, 102052 (2020)
K. Ali, B. Ahmed, M.S. Khan, J. Musarrat, Differential surface contact killing of pristine and low EPS Pseudomonas aeruginosa with Aloe vera capped hematite (α-Fe2O3) nanoparticles. J. Photochem. Photobiol., B 188, 146–158 (2018)
K. Ali, F. Abul Qais, S. Dwivedi, E.M. Abdel-Salam, S.M. Ansari, Q. Saquib, M. Faisal, A.A. Al-Khedhairy, M. Al-Shaeri, J. Musarrat, Titanium dioxide nanoparticles preferentially bind in subdomains IB, IIA of HSA and minor groove of DNA. J. Biomol. Struct. Dyn. 36(10), 2530–2542 (2018)
M. Baláž, N. Daneu, Ľ Balážová, E. Dutková, Ľ Tkáčiková, J. Briančin, M. Vargová, M. Balážová, A. Zorkovská, P. Baláž, Bio-mechanochemical synthesis of silver nanoparticles with antibacterial activity. Adv. Powder Technol. 28(12), 3307–3312 (2017)
S. Ibrahim, Z. Ahmad, M.Z. Manzoor, M. Mujahid, Z. Faheem, A. Adnan, Optimization for biogenic microbial synthesis of silver nanoparticles through response surface methodology, characterization, their antimicrobial, antioxidant, and catalytic potential. Sci. Rep. 11(1), 770 (2021)
S. Elbasuney, G.S. El-Sayyad, Silver nanoparticles coated medical fiber synthesized by surface engineering with bio-inspired mussel powered polydopamine: an investigated antimicrobial potential with bacterial membrane leakage reaction mechanism. Microb. Pathog. 169, 105680 (2022)
M. Rycenga, C.M. Cobley, J. Zeng, W. Li, C.H. Moran, Q. Zhang, D. Qin, Y. Xia, Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem. Rev. 111(6), 3669–3712 (2011)
W. Lewandowski, M. Fruhnert, J. Mieczkowski, C. Rockstuhl, E. Górecka, Dynamically self-assembled silver nanoparticles as a thermally tunable metamaterial. Nat. Commun. 6(1), 1–9 (2015)
P.D. Marcato, N. Durán, New aspects of nanopharmaceutical delivery systems. J. Nanosci. Nanotechnol. 8(5), 2216–2229 (2008)
M. Abdel Maksoud, A. Awed, R. Sokary, M. Bekhit, Effect of gamma irradiation on the free-standing polyvinyl alcohol/chitosan/Ag nanocomposite films: insights on the structure, optical, and dispersion properties. Appl. Phys. A 127(8), 1–11 (2021)
L. Sun, A. Liu, X. Tao, Y. Zhao, A green method for synthesis of silver nanodendrites. J. Mater. Sci. 46, 839–845 (2011)
P. Mdluli, N. Revaprasadu, Time dependant evolution of silver nanodendrites. Mater. Lett. 63(3–4), 447–450 (2009)
C. Cazan, A. Enesca, L. Andronic, Synergic effect of TiO2 filler on the mechanical properties of polymer nanocomposites. Polymers 13(12), 2017 (2021)
M. Teodorescu, M. Bercea, S. Morariu, Biomaterials of PVA and PVP in medical and pharmaceutical applications: perspectives and challenges. Biotechnol. Adv. 37(1), 109–131 (2019)
M. Teodorescu, M. Bercea, Poly (vinylpyrrolidone)–a versatile polymer for biomedical and beyond medical applications. Polym.-Plast. Technol. Eng. 54(9), 923–943 (2015)
N. Huang, S. Radiman, H. Lim, P. Khiew, W. Chiu, K. Lee, A. Syahida, R. Hashim, C. Chia, γ-Ray assisted synthesis of silver nanoparticles in chitosan solution and the antibacterial properties. Chem. Eng. J. 155(1–2), 499–507 (2009)
B.D. Du, D.V. Phu, N.N. Duy, N.T.K. Lan, V.T.K. Lang, N.V.K. Thanh, N.T.P. Phong, N.Q. Hien, Preparation of colloidal silver nanoparticles in poly (N-vinylpyrrolidone) by γ-irradiation. J. Exp. Nanosci. 3(3), 207–213 (2008)
A.C. Dhayagude, A. Das, S.S. Joshi, S. Kapoor, γ-Radiation induced synthesis of silver nanoparticles in aqueous poly (N-vinylpyrrolidone) solution. Colloids Surf., A 556, 148–156 (2018)
M. Saif-Elnasr, S. El-Ghlban, A.I. Bayomi, G.S. El-Sayyad, M.S. Maghraby, Gallic acid and/or cerium oxide nanoparticles synthesized by gamma-irradiation protect cisplatin-induced nephrotoxicity via modulating oxidative stress, inflammation and apoptosis. Arch. Biochem. Biophys. 740, 109594 (2023)
Z. Ali, O. Ghazy, G. Meligi, H. Saleh, M. Bekhit, Copper nanoparticles: Synthesis, characterization and its application as catalyst for p-nitrophenol reduction. J. Inorg. Organomet. Polym Mater. 28, 1195–1205 (2018)
D. Clifford, C. Castano, J. Rojas, Supported transition metal nanomaterials: nanocomposites synthesized by ionizing radiation. Radiat. Phys. Chem. 132, 52–64 (2017)
G. Flores-Rojas, F. López-Saucedo, E. Bucio, Gamma-irradiation applied in the synthesis of metallic and organic nanoparticles: a short review. Radiat. Phys. Chem. 169, 107962 (2020)
M. Bekhit, A.O. Abo El Naga, M. El Saied, M.I. Abdel Maksoud, Radiation-induced synthesis of copper sulfide nanotubes with improved catalytic and antibacterial activities. Environ. Sci. Pollut. Res. 28, 44467–44478 (2021)
T. Jarzembowski, A. Daca, M.A. Dębska-Ślizień, (2018) Urinary Tract Infection: The Result of the Strength of the Pathogen, or the Weakness of the Host, BoD–Books on Demand.
S.V. Sánchez, N. Navarro, J. Catalán-Figueroa, J.O. Morales, Nanoparticles as potential novel therapies for urinary tract infections. Front. Cell. Infect. Microbiol. (2021). https://doi.org/10.3389/fcimb.2021.656496
A.L. Flores-Mireles, J.N. Walker, M. Caparon, S.J. Hultgren, Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 13(5), 269–284 (2015)
M. Rawal, A. Singh, M.M. Amiji, Quality-by-design concepts to improve nanotechnology-based drug development. Pharm. Res. 36(11), 1–20 (2019)
Y. Hiyama, T. Sato, S. Takahashi, S. Yamamoto, Y. Fukushima, C. Nakajima, Y. Suzuki, S.-I. Yokota, N. Masumori, Sitafloxacin has a potent activity for eradication of extended spectrum β-lactamase-producing fluoroquinolone-resistant Escherichia coli forming intracellular bacterial communities in uroepithelial cells. J. Infect. Chemother. 26(12), 1272–1277 (2020)
M.M. Al-Ansari, P. Dhasarathan, A. Ranjitsingh, L.A. Al-Humaid, Challenging multidrug-resistant urinary tract bacterial isolates via bio-inspired synthesis of silver nanoparticles using the inflorescence extracts of Tridax procumbens. J. King Saud Univ.-Sci. 32(7), 3145–3152 (2020)
P. Singha, J. Locklin, H. Handa, A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 50, 20–40 (2017)
R. Thombre, K. Jangid, R. Shukla, N.K. Dutta, Alternative therapeutics against antimicrobial-resistant pathogens. Front. Microbiol. 10, 2173 (2019)
G.S. El-Sayyad, H.S. El-Bastawisy, M. Gobara, A.I. El-Batal, Gentamicin-assisted mycogenic selenium nanoparticles synthesized under gamma irradiation for robust reluctance of resistant urinary tract infection-causing pathogens. Biol. Trace Elem. Res. 195(1), 323–342 (2020)
N. Sheikh, A. Akhavan, M. Kassaee, Synthesis of antibacterial silver nanoparticles by γ-irradiation. Physica E 42(2), 132–135 (2009)
E. Álvarez-Fernández, A. Cancelo, C. Díaz-Vega, R. Capita, C. Alonso-Calleja, Antimicrobial resistance in E. coli isolates from conventionally and organically reared poultry: a comparison of agar disc diffusion and Sensi Test Gram-negative methods. Food Control 30(1), 227–234 (2013)
A.H. Hashem, A.M.A. Khalil, A.M. Reyad, S.S. Salem, Biomedical applications of mycosynthesized selenium nanoparticles using Penicillium expansum ATTC 36200. Biol. Trace Elem. Res. (2021). https://doi.org/10.1007/s12011-020-02506-z
E. Asín, A. Isla, A. Canut, A.R. Gascón, Comparison of antimicrobial pharmacokinetic/pharmacodynamic breakpoints with EUCAST and CLSI clinical breakpoints for Gram-positive bacteria. Int. J. Antimicrob. Agents 40(4), 313–322 (2012)
A.I. El-Batal, H.G. Nada, R.R. El-Behery, M. Gobara, G.S. El-Sayyad, Nystatin-mediated bismuth oxide nano-drug synthesis using gamma rays for increasing the antimicrobial and antibiofilm activities against some pathogenic bacteria and Candida species. RSC Adv. 10(16), 9274–9289 (2020)
A.H. Ashour, A.I. El-Batal, M.I.A.A. Maksoud, G.S. El-Sayyad, S. Labib, E. Abdeltwab, M.M. El-Okr, Antimicrobial activity of metal-substituted cobalt ferrite nanoparticles synthesized by sol–gel technique. Particuology 40, 141–151 (2018)
G.D. Christensen, W.A. Simpson, A.L. Bisno, E.H. Beachey, Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 37(1), 318–326 (1982)
A.K. Suresh, D.A. Pelletier, W. Wang, J.-W. Moon, B. Gu, N.P. Mortensen, D.P. Allison, D.C. Joy, T.J. Phelps, M.J. Doktycz, Silver nanocrystallites: biofabrication using Shewanella oneidensis, and an evaluation of their comparative toxicity on gram-negative and gram-positive bacteria. Environ. Sci. Technol. 44(13), 5210–5215 (2010)
M.A. Ansari, H.M. Khan, A.A. Khan, S.S. Cameotra, R. Pal, Antibiofilm efficacy of silver nanoparticles against biofilm of extended spectrum β-lactamase isolates of Escherichia coli and Klebsiella pneumoniae. Appl. Nanosci. 4(7), 859–868 (2014)
W. Huang, J.-Q. Wang, H.-Y. Song, Q. Zhang, G.-F. Liu, Chemical analysis and in vitro antimicrobial effects and mechanism of action of Trachyspermum copticum essential oil against Escherichia coli. Asian Pac J Trop. Med. 10(7), 663–669 (2017)
M. Abd Elkodous, A.M. El-Khawaga, M.I.A. Abdel Maksoud, G.S. El-Sayyad, N. Alias, H. Abdelsalam, M.A. Ibrahim, M.A. Elsayed, G. Kawamura, Z. Lockman, W.K. Tan, A. Matsuda, Enhanced photocatalytic and antimicrobial performance of a multifunctional Cu-loaded nanocomposite under UV light: theoretical and experimental study. Nanoscale 14(23), 8306–8317 (2022)
H. Agarwal, A. Nakara, S. Menon, V. Shanmugam, Eco-friendly synthesis of zinc oxide nanoparticles using Cinnamomum tamala leaf extract and its promising effect towards the antibacterial activity. J. Drug Deliv. Sci. Technol. 53, 101212 (2019)
M. Relucenti, G. Familiari, O. Donfrancesco, M. Taurino, X. Li, R. Chen, M. Artini, R. Papa, L. Selan, Microscopy methods for biofilm imaging: focus on SEM and VP-SEM pros and cons. Biology 10(1), 51 (2021)
A.M. Brown, A new software for carrying out one-way ANOVA post hoc tests. Comput. Methods Programs Biomed. 79(1), 89–95 (2005)
K. Djeddou, M. Bouloudenine, H. Soualah Alila, M. Bououdina, Formation of silver nanoparticles by a novel irradiation method without a reducing agent and their impact on four pathogenic bacterial strains. J. Inorg. Organomet. Polym. Mater. 30(8), 3095–3104 (2020)
Y. Gao, P. Jiang, L. Song, J. Wang, L. Liu, D. Liu, Y. Xiang, Z. Zhang, X. Zhao, X. Dou, Studies on silver nanodecahedrons synthesized by PVP-assisted N, N-dimethylformamide (DMF) reduction. J. Cryst. Growth 289(1), 376–380 (2006)
T.T.H. Pham, N.D. Dien, X.H. Vu, T.T. Tran, N.X. Ca, N. Van Truong, P.M. Tan, H. Van, P. Van Do, Synthesis and in-depth study of the mechanism of silver nanoplate and nanodecahedra growth by LED irradiation for SERS application. J. Electron. Mater. 49, 5009–5027 (2020)
D. Chen, X. Qiao, X. Qiu, J. Chen, R. Jiang, Large-scale synthesis of silver nanowires via a solvothermal method. J. Mater. Sci.: Mater. Electron. 22(1), 6–13 (2011)
W. Li, X. Xu, W. Li, Y. Zhao, M. Chen, Green synthesis of micron-sized silver flakes and their application in conductive ink. J. Mater. Sci. 53(9), 6424–6432 (2018)
A. Abdelghany, A. Oraby, M. Farea, Influence of green synthesized gold nanoparticles on the structural, optical, electrical and dielectric properties of (PVP/SA) blend. Physica B 560, 162–173 (2019)
B. Saraswathi, V. Patil, S. Halse, M. Kalasad, Optical and structural studies of PVP capped silver oxide nanoparticles. Mater. Today: Proc. 67, 290–294 (2022)
N. Nikolić, J. Spasojević, A. Radosavljević, M. Milošević, T. Barudžija, L. Rakočević, Z. Kačarević-Popović, Influence of poly (vinyl alcohol)/poly (N-vinyl-2-pyrrolidone) polymer matrix composition on the bonding environment and characteristics of Ag nanoparticles produced by gamma irradiation. Radiat. Phys. Chem. (2022). https://doi.org/10.2139/ssrn.4142153
M.S.N. Salleh, R.R. Ali, K. Shameli, M.Y. Hamzah, R.M. Kasmani, M.M. Nasef, Interaction insight of pullulan-mediated gamma-irradiated silver nanoparticle synthesis and its antibacterial activity. Polymers 13(20), 3578 (2021)
M. Bekhit, M.N. Abu el-naga, R. Sokary, R.A. Fahim, N.M. El-Sawy, Radiation-induced synthesis of tween 80 stabilized silver nanoparticles for antibacterial applications. J. Environ. Sci. Health, Part A 55(10), 1210–1217 (2020)
A. Russell, Biocide use and antibiotic resistance: the relevance of laboratory findings to clinical and environmental situations. Lancet. Infect. Dis. 3(12), 794–803 (2003)
T. Jin, Y. He, Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodborne pathogens. J. Nanopart. Res. 13(12), 6877–6885 (2011)
M. Abd Elkodous, G.S. El-Sayyad, I.Y. Abdelrahman, H.S. El-Bastawisy, A.E. Mohamed, F.M. Mosallam, H.A. Nasser, M. Gobara, A. Baraka, M.A. Elsayed, A.I. El-Batal, Therapeutic and diagnostic potential of nanomaterials for enhanced biomedical applications. Colloids Surf. B: Biointerfaces 180, 411–428 (2019)
M.I.A.A. Maksoud, G.S. El-Sayyad, A.H. Ashour, A.I. El-Batal, M.A. Elsayed, M. Gobara, A.M. El-Khawaga, E.K. Abdel-Khalek, M.M. El-Okr, Antibacterial, antibiofilm, and photocatalytic activities of metals-substituted spinel cobalt ferrite nanoparticles. Microb. Pathog. 127, 144–158 (2019)
K. Karthik, M.M. Naik, M. Shashank, M. Vinuth, V. Revathi, Microwave-assisted ZrO 2 nanoparticles and its photocatalytic and antibacterial studies. J. Cluster Sci. 30(2), 311–318 (2019)
K. Karthik, S. Dhanuskodi, C. Gobinath, S. Prabukumar, S. Sivaramakrishnan, Fabrication of MgO nanostructures and its efficient photocatalytic, antibacterial and anticancer performance. J. Photochem. Photobiol., B 190, 8–20 (2019)
Z.-X. Tang, B.-F. Lv, MgO nanoparticles as antibacterial agent: preparation and activity. Braz. J. Chem. Eng. 31(3), 591–601 (2014)
C. Ashajyothi, K.H. Harish, N. Dubey, R.K. Chandrakanth, Antibiofilm activity of biogenic copper and zinc oxide nanoparticles-antimicrobials collegiate against multiple drug resistant bacteria: a nanoscale approach. J. Nanostruct. Chem. 6(4), 329–341 (2016)
H.-J. Park, H.Y. Kim, S. Cha, C.H. Ahn, J. Roh, S. Park, S. Kim, K. Choi, J. Yi, Y. Kim, Removal characteristics of engineered nanoparticles by activated sludge. Chemosphere 92(5), 524–528 (2013)
J.A. Garza-Cervantes, C.E. Escárcega-González, E.D. Barriga Castro, G. Mendiola-Garza, B.A. Marichal-Cancino, M.A. López-Vázquez, J.R. Morones-Ramirez, Antimicrobial and antibiofilm activity of biopolymer-Ni, Zn nanoparticle biocomposites synthesized using R. mucilaginosa UANL-001L exopolysaccharide as a capping agent. Int. J. Nanomed. 14, 2557–2571 (2019)
Y. Zhang, T.P. Shareena Dasari, H. Deng, H. Yu, Antimicrobial activity of gold nanoparticles and ionic gold. J. Environ. Sci. Health, Part C 33(3), 286–327 (2015)
R.M. Fathy, A.Y. Mahfouz, Eco-friendly graphene oxide-based magnesium oxide nanocomposite synthesis using fungal fermented by-products and gamma rays for outstanding antimicrobial, antioxidant, and anticancer activities. J. Nanostruct. Chem 11, 301–321 (2021)
A. Joe, S.-H. Park, D.-J. Kim, Y.-J. Lee, K.-H. Jhee, Y. Sohn, E.-S. Jang, Antimicrobial activity of ZnO nanoplates and its Ag nanocomposites: insight into an ROS-mediated antibacterial mechanism under UV light. J. Solid State Chem. 267, 124–133 (2018)
Y. Xu, Q. Liu, M. Xie, S. Huang, M. He, L. Huang, H. Xu, H. Li, Synthesis of zinc ferrite/silver iodide composite with enhanced photocatalytic antibacterial and pollutant degradation ability. J. Colloid Interface Sci. 528, 70–81 (2018)
I. Matai, A. Sachdev, P. Dubey, S.U. Kumar, B. Bhushan, P. Gopinath, Antibacterial activity and mechanism of Ag–ZnO nanocomposite on S. aureus and GFP-expressing antibiotic resistant E. coli. Colloids Surf. B: Biointerfaces 115, 359–367 (2014)
L.F. Gaunt, C.B. Beggs, G.E. Georghiou, Bactericidal action of the reactive species produced by gas-discharge nonthermal plasma at atmospheric pressure: a review. IEEE Trans. Plasma Sci. 34(4), 1257–1269 (2006)
H.Y. Mostafa, G.S. El-Sayyad, H.G. Nada, R.A. Ellethy, E. Zaki, Promising antimicrobial and antibiofilm activities of Orobanche aegyptiaca extract-mediated bimetallic silver-selenium nanoparticles synthesis: effect of UV-exposure, bacterial membrane leakage reaction mechanism, and kinetic study. Arch. Biochem. Biophys. 736, 109539 (2023)
Z. Azam, A. Ayaz, M. Younas, Z. Qureshi, B. Arshad, W. Zaman, F. Ullah, M.Q. Nasar, S. Bahadur, M.M. Irfan, Microbial synthesized cadmium oxide nanoparticles induce oxidative stress and protein leakage in bacterial cells. Microb. Pathog. 144, 104188 (2020)
M.A. Maksoud, G.S. El-Sayyad, A.M. El-Khawaga, M. Abd Elkodous, A. Abokhadra, M.A. Elsayed, M. Gobara, L. Soliman, H. El-Bahnasawy, A. Ashour, Nanostructured Mg substituted Mn–Zn ferrites: a magnetic recyclable catalyst for outstanding photocatalytic and antimicrobial potentials. J. Hazard. Mater. 399, 123000 (2020)
A.H. Hashem, G.S. El-Sayyad, Antimicrobial and anticancer activities of biosynthesized bimetallic silver-zinc oxide nanoparticles (Ag–ZnO NPs) using pomegranate peel extract. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04126-8
A.I. El-Batal, F.M. Mosallam, G.S. El-Sayyad, Synthesis of metallic silver nanoparticles by fluconazole drug and gamma rays to inhibit the growth of multidrug-resistant microbes. J. Cluster Sci. 29(6), 1003–1015 (2018)
B. Buszewski, V. Railean-Plugaru, P. Pomastowski, K. Rafińska, M. Szultka-Mlynska, P. Golinska, M. Wypij, D. Laskowski, H. Dahm, Antimicrobial activity of biosilver nanoparticles produced by a novel Streptacidiphilus durhamensis strain. J. Microbiol. Immunol. Infect. 51(1), 45–54 (2018)
M. Mahmood, M. Abid, M.F. Nazar, M.N. Zafar, M.A. Raza, M. Ashfaq, A.M. Khan, S.H. Sumrra, M. Zubair, The wet chemical synthesis of surfactant-capped quasi-spherical silver nanoparticles with enhanced antibacterial activity. Mater. Adv. 1(7), 2332–2338 (2020)
J.Y. Cheon, S.J. Kim, Y.H. Rhee, O.H. Kwon, W.H. Park, Shape-dependent antimicrobial activities of silver nanoparticles. Int. J. Nanomed. 14, 2773–2780 (2019)
Acknowledgements
The authors thanks National Center for Radiation Research and Technology (NCRRT), Egyptian Atomic Energy Authority (EAEA) for the possibility to use their equipment and facilities during gamma irradiation process.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MB: suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. GSE: suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. RS: suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
Not applicable.
Research Involving Human Participation and/or Animals
Not applicable.
Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bekhit, M., El-Sayyad, G.S. & Sokary, R. Gamma Radiation-Induced Synthesis and Characterization of Decahedron-Like Silver Nanostructure and Their Antimicrobial Application. J Inorg Organomet Polym 33, 2906–2923 (2023). https://doi.org/10.1007/s10904-023-02718-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02718-5