Abstract
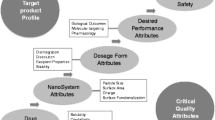
The purpose of this review is to discuss the challenges associated with the development of nanoparticle-based quality drug products in adhering to the principles of quality by design (QbD) and defining appropriate quality parameters towards successful product development. With the advent of nanotechnology into the pharmaceutical field, the novel field of nanomedicine was born. Due to their unique properties in terms of size, conformation and targeted delivery, nanomedicines are able to overcome many drawbacks of conventional medicine. As nano-sized formulations have made their way into more and more therapies, it has became clear that these very unique properties create hurdles for nanomedicines in successfully traversing the regulatory pathways and there is a need to develop nanomedicines in a more controlled and consistent fashion. The elements of a QbD methodology explained in this review enable the development of nano-based formulations in a way that maximizes the possibility of success. The identification of critical quality attributes (CQA) of the drug product and its intermediates are discussed in detail with a focus on nanomaterial-based formulations. In conclusion, QbD and the identification and specification of CQAs at its core are critical to the design, development and growth of nanomaterials in pharmaceuticals.





Similar content being viewed by others
Abbreviations
- ADME:
-
Absorption, Distribution, Metabolism and Excretion
- ANDA:
-
Abbreviated new drug application
- API:
-
Active pharmaceutical ingredients
- CE:
-
Coating efficiency
- CMC:
-
Chemistry, Manufacturing and Controls
- CPP:
-
Critical process parameter
- CQA:
-
Critical quality attribute
- DLS:
-
Dynamic light scattering
- DS:
-
Design space
- EDAX:
-
Energy dispersive analysis of X-ray
- EE:
-
Encapsulation efficiency
- ELISA:
-
Enzyme-Linked immunosorbent assay
- EU-NCL:
-
European nanomedicine characterization lab
- FDA:
-
Food and drug administration (United States)
- FMEA:
-
Failure mode and effects analysis
- GRAS:
-
Generally regarded as safe
- ICH:
-
International conference on harmonisation
- IND:
-
Investigational new drug
- IVIVC:
-
In Vitro In Vivo correlation
- MPS:
-
Mononuclear phagocyte system
- NCI:
-
National cancer institute
- NCL:
-
National characterization lab
- NIR:
-
Near Infra-Red
- NNI:
-
National nanotechnology initiative
- NTA:
-
Nanoparticle tracking analysis
- PALS:
-
Phase analysis light scattering
- PAT:
-
Process analytical technology
- PCR:
-
Polymerase chain reaction
- PDI:
-
Polydispersity index
- PEG:
-
Polyethylene glycol
- PK/PD:
-
Pharmacokinetics/Pharmacodynamics
- QbD:
-
Quality by design
- QbT:
-
Quality by testing
- QTPP:
-
Quality target product profile
- PKM:
-
Process knowledge map
- PLA:
-
Polylactic acid
- PLGA:
-
Poly(Lactic-co-Glycolic Acid)
- PSMA:
-
Prostate specific membrane antigen
- SANS:
-
Small Angle neutron scattering
- SAXS:
-
Small angle X-ray scattering
- SEC:
-
Size exclusion chromatography
- TOF-SIMS:
-
Time-of-flight secondary ion mass spectrometry
- TRPS:
-
Tunable resistive pulse sensing
- XPS:
-
X-ray photoelectron spectroscopy
References
Anselmoand AC, Mitragotri S. Nanoparticles in the clinic. Bioeng Transl Med. 2016;1:10–29.
D'Mello SR, Cruz CN, Chen ML, Kapoor M, Lee SL, Tyner KM. The evolving landscape of drug products containing nanomaterials in the United States. Nat Nanotechnol. 2017;12:523–9.
U.S. Food Drug and Administration: Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology. https://www.fda.gov/RegulatoryInformation/Guidances/ucm257698.htm (accessed 26 March 2019).
International Conference on Harmonisation, ICH Q8(R2): Pharmaceutical Development. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Step4/Q8_R2_Guideline.pdf (accessed March 22 2019).
Workshop on Implementation of ICH Q8/Q9/Q10. http://www.ich.org/fileadmin/Public_Web_Site/Training/GCG_-_Endorsed_Training_Events/APEC_LSIF_JCCT_workshop_Beijing__China_Dec_08/Day_1/Regulatory_perspective.pdf (accessed August 13 2019).
CFR - Code of Federal Regulations Title 21 CHAPTER I--FOOD AND DRUG ADMINISTRATION DEPARTMENT OF HEALTH AND HUMAN SERVICES, SUBCHAPTER D--DRUGS FOR HUMAN USE, PART 312 -- INVESTIGATIONAL NEW DRUG APPLICATIONl Subpart B--Investigational New Drug Application (IND). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=312.23 (accessed August 13 2019).
U.S. Food and Drug Administration Guidance Document: Exploratory IND Studies. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/exploratory-ind-studies (accessed August 13 2019).
U.S. Food and Drug Administration Guidance Document: Content and Format of Investigational New Drug Applications (INDs) for Phase 1 Studies of Drugs, Including Well-Characterized, Therapeutic, Biotechnology-derived Products. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/content-and-format-investigational-new-drug-applications-inds-phase-1-studies-drugs-including-well (accessed August 13 2019).
U.S. Food and Drug Administration GUIDANCE DOCUMENT: INDs for Phase 2 and Phase 3 Studies Chemistry, Manufacturing, and Controls Information. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/inds-phase-2-and-phase-3-studies-chemistry-manufacturing-and-controls-information (accessed August 13 2019).
Pandey AP, Karande KP, Sonawane RO, Deshmukh PK. Applying quality by design (QbD) concept for fabrication of chitosan coated nanoliposomes. J Liposome Res. 2014;24:37–52.
Pramod K, Tahir MA, Charoo NA, Ansari SH, Ali J. Pharmaceutical product development: a quality by design approach. Int J Pharm Invest. 2016;6:129–38.
U. S. Food and Drug Administration: Quality by design for ANDs: an example for immediate-release dosage forms. http://www.fda.gov/downloads/Drugs/.../UCM304305.pdf (accessed March 22 2019).
International Conference of Harmonisation, ICH Q9: Quality Risk Management. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q9/Step4/Q9_Guideline.pdf (accessed March 22 2019).
International Conference on Harmonisation, ICH Q10:Pharmaceutical Quality System. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q10/Step4/Q10_Guideline.pdf (accessed August 13 2019).
Raw AS, Lionberger R, Yu LX. Pharmaceutical equivalence by design for generic drugs: modified-release products. Pharm Res. 2011;28:1445–53.
Troiano G, Nolan J, Parsons D, Van Geen Hoven C, Zale S. A quality by design approach to developing and manufacturing polymeric nanoparticle drug products. AAPS J. 2016;18:1354–65.
Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14:282–95.
Trynda-Lemiesz L. Paclitaxel-HSA interaction. Binding sites on HSA molecule Bioorg Med Chem. 2004;12:3269–75.
Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, et al. Understanding pharmaceutical quality by design. AAPS J. 2014;16:771–83.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–51.
Tyner KM, Wokovich AM, Doub WH, Buhse LF, Sung LP, Watson SS, et al. Comparing methods for detecting and characterizing metal oxide nanoparticles in unmodified commercial sunscreens. Nanomedicine (Lond). 2009;4(145–159):145–59.
U. Bulbake, S. Doppalapudi, N. Kommineni, and W. Khan. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics. 9:(2017).
Pattni BS, Chupin VV, Torchilin VP. New developments in liposomal drug delivery. Chem Rev. 2015;115:10938–66.
A. Lechanteur, V. Sanna, A. Duchemin, B. Evrard, D. Mottet, and G. Piel. Cationic Liposomes Carrying siRNA: Impact of Lipid Composition on Physicochemical Properties, Cytotoxicity and Endosomal Escape. Nanomaterials. 8:(2018).
Bunker A, Magarkar A, Viitala T. Rational design of liposomal drug delivery systems, a review: combined experimental and computational studies of lipid membranes, liposomes and their PEGylation. Biochim Biophys Acta. 2016;1858:2334–52.
Mathaes R, Winter G, Engert J, Besheer A. Application of different analytical methods for the characterization of non-spherical micro- and nanoparticles. Int J Pharm. 2013;453:620–9.
B.J. Ree, J. Lee, Y. Satoh, K. Kwon, T. Isono, T. Satoh, and M. Ree. A Comparative Study of Dynamic Light and X-ray Scatterings on Micelles of Topological Polymer Amphiphiles. Polymers. 10:(2018).
Park SJ, Choo GH, Hwang SJ, Kim MS. Quality by design: screening of critical variables and formulation optimization of Eudragit E nanoparticles containing dutasteride. Arch Pharm Res. 2013;36:593–601.
Xu X, Costa AP, Khan MA, Burgess DJ. Application of quality by design to formulation and processing of protein liposomes. Int J Pharm. 2012;434:349–59.
Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–55.
Championand JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103:4930–4.
Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A. 2008;105:11613–8.
Black KC, Wang Y, Luehmann HP, Cai X, Xing W, Pang B, et al. Radioactive 198Au-doped nanostructures with different shapes for in vivo analyses of their biodistribution, tumor uptake, and intratumoral distribution. ACS Nano. 2014;8:4385–94.
Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:16014.
Ruozi B, Belletti D, Tombesi A, Tosi G, Bondioli L, Forni F, et al. AFM, ESEM, TEM, and CLSM in liposomal characterization: a comparative study. Int J Nanomedicine. 2011;6:557–63.
Robson AL, Dastoor PC, Flynn J, Palmer W, Martin A, Smith DW, et al. Advantages and limitations of current imaging techniques for characterizing liposome morphology. Front Pharmacol. 2018;9:80.
Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–15.
Caracciolo G, Farokhzad OC, Mahmoudi M. Biological identity of nanoparticles in vivo: clinical implications of the protein Corona. Trends Biotechnol. 2017;35:257–64.
Zhang XQ, Xu X, Bertrand N, Pridgen E, Swami A, Farokhzad OC. Interactions of nanomaterials and biological systems: implications to personalized nanomedicine. Adv Drug Deliv Rev. 2012;64:1363–84.
Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010;145:182–95.
Yameen B, Choi WI, Vilos C, Swami A, Shi J, Farokhzad OC. Insight into nanoparticle cellular uptake and intracellular targeting. J Control Release 2014;190:485–99.
Walkey CD, Olsen JB, Guo H, Emili A, Chan WC. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J Am Chem Soc. 2012;134:2139–47.
Kersting R, Breitenstein D, Hagenhoff B, Fartmann M, Heller D, Grehl T, et al. Surface characterization of nanoparticles: different surface analytical techniques compared. Surf Interface Anal. 2013;45:503–5.
Smith AM, Johnston KA, Crawford SE, Marbella LE, Millstone JE. Ligand density quantification on colloidal inorganic nanoparticles. Analyst. 2016;142:11–29.
Shen S, Wu Y, Liu Y, Wu D. High drug-loading nanomedicines: progress, current status, and prospects. Int J Nanomedicine. 2017;12:4085–109.
Brown CK, Friedel HD, Barker AR, Buhse LF, Keitel S, Cecil TL, et al. FIP/AAPS joint workshop report: dissolution/in vitro release testing of novel/special dosage forms. AAPS PharmSciTech. 2011;12:782–94.
Siewert M, Dressman J, Brown CK, Shah VP. Fip, and Aaps. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms AAPS PharmSciTech. 2003;4:E7.
Petschauer JS, Madden AJ, Kirschbrown WP, Song G, Zamboni WC. The effects of nanoparticle drug loading on the pharmacokinetics of anticancer agents. Nanomedicine (Lond). 2015;10(447–463):447–63.
Balzus B, Colombo M, Sahle FF, Zoubari G, Staufenbiel S, Bodmeier R. Comparison of different in vitro release methods used to investigate nanocarriers intended for dermal application. Int J Pharm. 2016;513:247–54.
D’Souza S. A review of in vitro drug release test methods for nano-sized dosage forms. Adv Pharm. 2014;2014.
International Conference of Harmonisation, ICH Q1A(R2): Stability Testing of New Drug Substances and Products. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1A_R2/Step4/Q1A_R2__Guideline.pdf (accessed March 22 2019).
Vetten MA, Yah CS, Singh T, Gulumian M. Challenges facing sterilization and depyrogenation of nanoparticles: effects on structural stability and biomedical applications. Nanomed: Nanotechnol, Biol Med. 2014;10:1391–9.
C. Fornagueraand C. Solans. Methods for the In Vitro Characterization of Nanomedicines-Biological Component Interaction. Journal of personalized medicine. 7:(2017).
Grimm JC, Zhang F, Magruder JT, Crawford TC, Mishra M, Rangaramanujam KM, et al. Accumulation and cellular localization of nanoparticles in an ex vivo model of acute lung injury. J Surg Res. 2017;210:78–85.
Roberts MS, Mohammed Y, Pastore MN, Namjoshi S, Yousef S, Alinaghi A, et al. Topical and cutaneous delivery using nanosystems. J Control Release 2017;247:86–105.
Phan N, Hong JJ, Tofig B, Mapua M, Elashoff D, Moatamed NA, et al. A simple high-throughput approach identifies actionable drug sensitivities in patient-derived tumor organoids. Commun Biol. 2019;2:78.
Date AA, Hanes J, Ensign LM. Nanoparticles for oral delivery: design, evaluation and state-of-the-art. J Control Release. 2016;240:504–26.
Shenand J, Burgess DJ. In vitro-in vivo correlation for complex non-oral drug products: where do we stand? J Control Release. 2015;219:644–51.
Dobrovolskaiaand MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–78.
X. Xu, M.A. Khan, and D.J. Burgess. A quality by design (QbD) case study on liposomes containing hydrophilic API: II. Screening of critical variables, and establishment of design space at laboratory scale. Int J Pharm. 423:543–553 (2012).
Curic A, Reul R, Moschwitzer J, Fricker G. Formulation optimization of itraconazole loaded PEGylated liposomes for parenteral administration by using design of experiments. Int J Pharm. 2013;448:189–97.
Ingvarsson PT, Yang M, Mulvad H, Nielsen HM, Rantanen J, Foged C. Engineering of an inhalable DDA/TDB liposomal adjuvant: a quality-by-design approach towards optimization of the spray drying process. Pharm Res. 2013;30:2772–84.
Kumar S, Gokhale R, Burgess DJ. Quality by design approach to spray drying processing of crystalline nanosuspensions. Int J Pharm. 2014;464:234–42.
Cun D, Jensen DK, Maltesen MJ, Bunker M, Whiteside P, Scurr D, et al. High loading efficiency and sustained release of siRNA encapsulated in PLGA nanoparticles: quality by design optimization and characterization. Eur J Pharma Biopharm. 2011;77:26–35.
Shah RB, Zidan AS, Funck T, Tawakkul MA, Nguyenpho A, Khan MA. Quality by design: characterization of self-nano-emulsified drug delivery systems (SNEDDs) using ultrasonic resonator technology. Int J Pharm. 2007;341:189–94.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editor: Joshua Reineke
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
For a Special Thematic Issue of Pharmaceutical Research – “Nanomedicines in Cancer” edited by Dr. Joshua Reineke and Dr. Joseph Nicolazzo
Rights and permissions
About this article
Cite this article
Rawal, M., Singh, A. & Amiji, M.M. Quality-by-Design Concepts to Improve Nanotechnology-Based Drug Development. Pharm Res 36, 153 (2019). https://doi.org/10.1007/s11095-019-2692-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2692-6