Abstract
Rapidly growing interest in using nanoparticles (NPs) for biomedical applications has increased concerns about their safety and toxicity. In comparison with bulk materials, NPs are more chemically active and toxic due to the greater surface area and small size. Understanding the NPs’ mechanism of toxicity, together with the factors influencing their behavior in biological environments, can help researchers to design NPs with reduced side effects and improved performance. After overviewing the classification and properties of NPs, this review article discusses their biomedical applications in molecular imaging and cell therapy, gene transfer, tissue engineering, targeted drug delivery, Anti-SARS-CoV-2 vaccines, cancer treatment, wound healing, and anti-bacterial applications. There are different mechanisms of toxicity of NPs, and their toxicity and behaviors depend on various factors, which are elaborated on in this article. More specifically, the mechanism of toxicity and their interactions with living components are discussed by considering the impact of different physiochemical parameters such as size, shape, structure, agglomeration state, surface charge, wettability, dose, and substance type. The toxicity of polymeric, silica-based, carbon-based, and metallic-based NPs (including plasmonic alloy NPs) have been considered separately.
Similar content being viewed by others
Introduction
Nanomaterials have leveraged technological advances due to their enhanced performance and tunable properties compared to their bulkier counterparts. Various revolutionary developments have been made in the medical field since “nanotechnology” was presented [1,2,3]. NPs can be classified into carbon, inorganic, organic (excluding inorganic-based or carbon-based NMs), composite, and bio-based particles on a nanometric scale, and they can be produced in different morphologies with different methods.
Throughout the tremendous progress obtained in nanotechnology during the last decades, as a result of an extensive range of applications in medical fields, NPs have attracted a large amount of interest. Medical applications of NPs include molecular imaging and cell therapy, tissue engineering, targeted drug delivery, cancer treatment, wound healing, gene transfer, anti-bacterial applications, and most recently, as a treatment for COVID-19 [3,4,5,6,7]. Currently, hundreds of thousands of tons of nanomaterials are fabricated worldwide. For instance, it has been estimated that AgNPs will increase from 360–450 tons annually to 800 tons by 2025 [8, 9]. The enlarged fabrication and application of NPs have enhanced the possibility of their potential for unintended adverse health effects.
NPs provide enhanced properties of the original material, including high surface area, reactivity, and sensitivity [10]. However, due to their tiny size and high chemical reactivity, the probability of their cell uptake and interactions with biomolecules and tissues has increased [11]. Considering the interactions of NPs with cellular elements such as the plasma membrane, macromolecules, or organelles, distinctive biological responses can be triggered by different NPs [12]. Nevertheless, for NPs to move into biomedical applications, it is essential that their undesirable properties and toxicity can be avoided [13]. NPs can directly damage cells and organelles and induce unregulated cell signaling, which can lead to DNA damage by releasing toxic ions and generating reactive oxygen species (ROS). Cytotoxicity of NPs is directly related to their physiochemical characteristics, including size, structure, shape, agglomeration state, surface charge, chemistry, dose, and substance type.
Because of the prominence of NPs and their medicinal applications, multiple review papers have been written on the subject. Wang et al. thoroughly reviewed many synthesis methodologies for metal NCs that use distinct biomolecules as stabilizers for detecting several biological analytes. They also emphasized on the fluorescence quenching and enhancement processes of biosensors for various analytes. Additionally, they highlighted the obstacles and possibilities for prospective biological sensing applications [14]. Recently, research has favored using lipid or polymer-based nanoparticles as robust and adaptable delivery vehicles. Lipid nanoparticles (LNPs) have appeared as one of the most attractive and widely utilized mRNA carriers, particularly in mRNA vaccines. Pardi et al. concentrated further on this application of NPs. They present a complete summary of mRNA vaccines and address upcoming opportunities and obstacles in moving this highly potential field to an extensive therapeutic application using the lipid nanoparticles [15].
The recent COVID-19 pandemic proved the importance of mRNA vaccines. In discussing the technology behind mRNA vaccines, Chaudhary et al. focused mainly on LNPs and other delivery carriers. They also discussed critical topics for the future implementation of this groundbreaking vaccine technology and provided an overview of mRNA vaccines against numerous infectious viruses [16].
The first aim of this review is to overview NPs’ classification, morphology, manufacturing, characterization, and properties. Then, their biomedical engineering application, including molecular imaging and cell therapy, tissue engineering, targeted drug delivery, cancer treatment and wound healing, gene transfer, anti-bacterial applications, and anti-SARS-CoV-2 vaccines, are comprehensively reviewed. Later, the mechanism of cytotoxicity in NPs and the critical parameters affecting the toxicity of NPs will be highlighted.
Nanostructured materials
NPs and their fabrication, characterization, and applications have attracted attention during the last decades through the tremendous progress in nanotechnology. This phenomenon is because the physical, chemical, and mechanical properties of the materials [17, 18], such as melting point, chemical reactivity of materials, optical properties, and thermal conductivity, change in this scale. According to the typical definition of a nanoscale, nanomaterials (NMs) are “materials with small dimensions with building units sized between 1 and 1000 nm in at least one dimension” [19]. The European Commission described the term “nanomaterial” as “a natural, incidental or manufactured material containing particles where at least 50% of the particles have one or more dimensions in size range of 1–100 nm” [20]. In ISO/TS 80,004, the nanomaterial is outlined as a “material with any external dimension in the nanoscale or having an internal structure or surface structure in the nanoscale,” with nanoscale being described as the “length range approximately from 1 to 100 nm” [21] To have a better idea about the size of objects in nanoscale, Fig. 1 shows multiple natural micro and nanostructures in different size scales.
Comparing the size of NMs (in a logarithmical length scale) to biological objects and “nano” and “micro” sizes definitions (the concept of this figure is adapted from [19], which is distributed under the Creative Commons Attribution)
Because of their exceptional physicochemical features, engineered nanomaterials (ENMs) have also been produced for imaging, drug delivery, diagnostics, and medical treatment applications. Comprehension of nanomaterial interaction with nanomedicine has kept nanomedicines’ function and ultimate efficiency from being suitable for clinical use. Thus, recent advances in investigating the aforementioned interaction of nanomedicines were described by Wang et al., who emphasized on the driving force and redox reaction at the nano-bio interface as the significant parameters governing the functionalities and toxicities of nanomaterials for diagnosis and treatment [22].
Moreover, nanoparticles have been used to detect and treat different viruses, such as COVID-19. An antiviral nanoparticle with long, malleable linkers that imitate heparan sulfate proteoglycans has been produced. The nanoparticles’ high forces (190 pN) permanently distort the virus. Gold nanoparticles (AuNPs) are another nanoparticle with several uses in antiviral medications and improved virus detection [23].
Classification
In the past two decades, different classifications of NMs have been introduced [20, 24,25,26,27]. One approach is to organize the NMs based on their origin or whether they are natural or synthetic (engineered) [26]. Another method is classifying NMs based on their dimensions, including 3D, 2D, 1D, and 0D NMs [19, 27]. However, the most practical classification of NMs was introduced by Tuominen et al. [24], who categorized the NMs into five groups: metal-based materials, carbon-based materials, polymeric particles, dendrimers, and composites. This classification has been modified to the following five categories [25, 28]:
-
(1)
Carbon-based NMs like carbon nanotubes (CNT), graphene, and carbon nanofibers. These NMs are composed of carbon and can have different morphologies, such as spheres, hollow tubes, and ellipsoids.
-
(2)
Inorganic-based NMs include non-carbon NPs, nanostructured materials like metals (Cu, Ag, and Au NPs), and metal oxides.
-
(3)
Organic-based NMs are mainly made from organic matter, excepting inorganic-based or carbon-based NMs. This category includes polymer NPs, nanocellulose, and nanostarch.
-
(4)
Composite-based NMs include multiphase materials which have one phase on the nanoscale dimension. This category can range from simple systems like NPs combined with large bulk-type materials (e.g., composite nanofibers) to more complex structures like mixed metal oxides. Any carbon-based, inorganic-based, or organic-based NMs combined with bulk materials like polymer, metal, or ceramic can form a composite.
-
(5)
Bio-based NMs are mainly comprised of biomaterials like nanobacteria and enzymes.
Morphology
Controlling the shape and morphology of NPs is a critical factor in exploiting their properties for their use in novel technologies. NPs with various morphologies and dimensions can be synthesized using different methods, depending on the required properties. Nonporous (0D), nanorods and nanofibers (1D), graphene nanosheets and carbon nanotubes (2D), and nanowires and nanowire networks (3D) are the most common nanomaterial morphologies [28].
Due to the widespread of COVID-19, mRNA vaccines have become a hot topic in the biotechnology and pharmaceutical sectors. The mRNA LNP’s nanostructural properties, when coupled with an LNP delivery method, resemble those of viral systems and moving endogenic, chylomicrons contain lipids regarding size, lipid envelope, and, for viral systems, the interior genomic material that relates to their application as delivery carriers for vaccines and other medicative [29, 30].
Manufacturing
Nanoparticle (NP) and nanomaterial manufacturing methods can be generally divided into two main categories, i.e., (1) bottom-up and (2) top-down approaches, as summarized in Fig. 2 [31,32,33]. The top-down synthesis method is relying on a destructive approach involving decomposing a larger molecule into smaller units, which are then transformed into proper NPs. Methods like grinding and milling, chemical etching, electro-explosion, and other decomposition techniques are based on the top-down approach. In the bottom-up synthesis or building-up approach, NPs are built from moderately simpler substances. Sedimentation and reduction techniques are examples of this method. Biochemical synthesis, sol–gel, green synthesis, and spinning are all included in the bottom-up synthesis approach. As a recent example of this category, intense green fluorescence was seen in papain-encapsulated platinum nanoclusters generated by Chang et al. through a biomineralization method, with maximal excitation and emission wavelengths of 380 and 490 nm, correspondingly. The novel family of fluorophores known as fluorescent metal nanoclusters has a widespread use in fields as diverse as biosensors, bioimaging, catalysis, and cutting-edge medicine [34].
Typical synthetic methods for NPs for the a top-down and b bottom-up approaches (adapted from reference [32] with permission)
In contrast, Gram-negative bacteria, such as E. coli, did not show an optical emission when stained with papain-Pt NCs. In contrast, cells of Gram-positive bacteria, such as Bacillus subtilis, could generate detectable green fluorescence. These findings suggest that papain-Pt NCs may help recognize Gram-positive bacteria as fluorescent label-free nanoparticles [34].
Total synthesis, in which favorite organic biomolecules might be synthesized from primary precursors with atomic accuracy and with known stepwise processes, is another example that fits this description. Recent advancements in the introduction and development of complete synthesis pathways and strategies for atomically accurate metal nanoclusters (NCs) were highlighted by Yao et al. Total synthesis of metal NCs is necessary for reliably achieving their practical uses since their molecular-like characteristics are strongly regulated by their size and composition [35].
Characterization
The major challenge in research on nanostructured materials is the characterization of nano-scale reinforcement materials. To control the parameters that contribute to, or inhibit the NMs’ efficiency, facilitate the fabrication, production, and design of the next generation of nanostructured materials and enhance their procedures and properties, the development of methodologies, techniques, and tools for nanomaterial characterization is a critical step. Quantifying the compositions and variations in the physical form of NPs and having access to the proper techniques to describe size and size distribution, the chemical characteristics, shape, and specific surface area are of critical importance in the field of NMs [24, 36,37,38]. The commonly used analytical methods in nanomaterial characterization, along with their abbreviations and controlling parameters, have been reviewed elsewhere [24].
Properties
The high aspect ratio (surface to volume) and quantum effects are the main features that result in the NP’s different or enhanced performance or properties over their bulky counterparts [36, 37, 39]. The importance of a high aspect ratio, and its significant effect on the properties of NPs, makes the surface of NPs a critical element. A high aspect ratio means NMs feature a higher proportion of surface atoms to interior atoms [39]. Consequently, even the simplest NPs will have a surface chemistry different from their core. NPs feature high surface energy and reactivity due to their high aspect ratio. Accordingly, NPs are much more reactive than larger particles with the same mass because of their larger surface area, which is where growth and catalytic chemical reactions occur [39]. If the active surface of the NP is not protected by using other components (capping agents), the interconnection between molecules will reduce the surface energy. Aggregation is a direct outcome of these interactions [39]. The capping agents are employed explicitly in synthesizing colloidal NPs to control NP aggregation, growth, and structural properties. They usually affect the process by stabilizing either charge or steric. The presence of these agents as the essential component of most NPs can bring extra complexity to the system, and they need to be carefully reviewed when studying the properties of the NPs [39,40,41].
Quantum effects control electrical, magnetic, and optical properties at the nanoscale level. This is particularly important in quantum dots to accurately obtain the predetermined properties. This unique feature can benefit various applications, including quantum computing, solar cells, and medical imaging [39, 42].
It is also shown that for bioactive components, better bioavailability, greater uptake, and higher concentration in the body can be obtained by nanosizing, compared to the larger ingredients [43]. This will have implications for environmental and health effects and can bring a revolutionary change in producing novel biomaterials with tailored characteristics and functions for targeted biomedical applications.
The unusual physiochemical properties of the engineered NPs, which happen at the lower end of the nanoscale (1–10 nm), raise many concerns. It may not be possible to use average assumptions about NPs, and more importantly, the unique chemical reactivity and behavior of NPs can lead to the exhibition of toxic effects.
Biomedical applications of NPs
In recent years, the biomedical applications of NPs (Fig. 3) have attracted a tremendous amount of interest due to the unique characteristics and properties of NPs compared to their bulky counterparts (as described in detail in the previous parts). This section discusses the main biomedical applications of NPs, including molecular imaging and cell therapy, tissue engineering, biosensing, targeted drug delivery, magnetic NP-based hyperthermia cancer treatment, wound healing, gene therapy, anti-bacterial treatments, and COVID-19 treatments that will be reviewed.
Molecular imaging and cell therapy
NP-based imaging technologies are considered noninvasive methods for real-time observation for a wide range of applications, including drug improvement, assessing efficacy treatment, diagnosis, and therapy. For in vivo cell tracking, cells need to be surface labeled with particular markers or used as imaging agents to monitor physiological or pathological development at the molecular or cellular level within a living organism [44,45,46]. Different imaging modalities are available to visualize the labeled cells, including magnetic resonance imaging (MRI), bioluminescent imaging (BLI), positron emission tomography (PET), and single photon emission tomography (SPECT) [45,46,47]. The NP-based imaging modalities have different applications based on the resolution, penetration depth, quantitative, longitudinal tracking, and cost [44].
One of the critical usages of NP-based imaging techniques is in exogenous cell treatment to interchange, fix, or enrich the biological functions of damaged tissue or a diseased organ. The main challenges in developing an effective cell therapy are to evaluate the exact location, analyze the distribution, and study the long-term perseverance of transplanted cells. Traditionally, histological analysis has been performed to monitor therapeutic cells, but this method is invasive and requires multiple tissue biopsies [45]. NP-based imaging technologies are noninvasive methods to track and visualize the fate and function of transplanted cells [44, 45, 48, 49]. In stem cell transplantation, to monitor the survival, location, migration, and differentiation of the cells in therapeutic processes, NP-based labeling agents can be employed to understand their fates in vivo fully. Using magnetic NPs, Kim et al. [50] studied the influence of human adipose-derived stem cells (hASCs) transplantation on Parkinson’s disease. For in vivo tracking of the NP-loaded hASCs, they used a maestro imaging system and magnetic resonance imaging (MRI) [50]. This study demonstrated strong hASCs signals in the brains of Parkinson’s disease model mice with MRI [50]. To promote ultrasound imaging in tracking therapeutic cells by making the injected therapeutic cells more noticeable, Chen et al. employed Stöber silica contrast agents for mesenchymal stem cell labeling. This labeling can enhance the ultrasound intensity of therapeutic cells with NPs [49]. In another study, they also proposed the application of exosome-like silica NPs for labeling stem cells for regenerative medicine imaging due to their ultrasound impedance mismatch.
Gene transfer
The research on nonviral gene transfer NPs is increasing, and a recent review discussed the current challenges, opportunities, and clinical applications [7]. The FDA has recently approved multiple formulations of these NPs for various purposes. They have the following advantages: safety, flexible cargo-carrying capacity, tissue- and cell-specific targeting, and ease of manufacturing. There have been clinical successes with NPs containing RNA, and more studies are looking at NPs containing DNA.
Previously, the primary approach for gene editing was using viral vectors, including retroviruses, adenoviruses, and adeno-associated viruses (AAVs). These methods have been successful; however, concerns over transfection, safety, and immunogenicity have increased the interest in NPs in gene editing. Nonviral delivery, or delivery with NPs, appears to address these concerns. To encapsulate DNA or RNA into the NPs, the NPs must have positively charged amine groups to interact with the negatively charged DNA or RNA. In addition, different types of NPs are better for different types of cargo. For example, natural material polysaccharides can be used with 50–100 nm DNA or RNA, whereas lipids (or lipid NPs) can be used with 30–200 nm DNA, RNA, or ribonucleoproteins (RNPs) [7].
The physical properties of the NPs can be modified to target specific organ systems. Still, intravenous delivery can also be used if the NP is to be used to target many organs. One study looked at the different surface properties of various NPs and found that NPs could be delivered to specific targets (lung, spleen, or liver) based on the surface properties [51].
CRISPR-Cas9 has been studied widely in recent literature and has the ability for gene editing, but there have been some potential setbacks, such as delivery and off-target effects. Since the delivery of NPs can be controlled, NPs can be used to counteract the setbacks encountered with the CRISPR-Cas9 technology [7].
Tissue engineering
Apart from NP-based cell targeting and labeling, as addressed in the previous section, NPs have a wide range of tissue engineering applications. NPs can enable the design and fabrication of biocompatible scaffolds that mimic tissue-specific microenvironments by providing appropriate tensile strength, releasing biological factors, and ultimately promoting the creation of implantable tissues [52, 53].
For both substrate and support roles, it is required to have a porous scaffold for tissue growth. This porous structure creates the virtual spatial configuration, which allows cells to grow into proper anatomical form. The main requirements for such platforms are biocompatibility and biodegradability to prevent growth inhibition and to enable ultimate replacement with functional tissue. Conventional biodegradable polymeric or ceramic scaffolds are limited in many terms, such as lacking desired mechanical strength. Instead, we can use NP-modified composite platforms, offering enhanced properties for a smooth tissue rejuvenation [53].
To increase the cellular survival rate of implanted constructs, the physicochemical interactions between the natural in vivo environment and the embedded structure can be improved using NPs. Moreover, nano-composite-based scaffolds are promising materials to aid bone regeneration by enhancing biocompatibility and mechanical stability. For instance, the scaffolds made of 45S5 bioactive glass, gelatin, and manganese-doped mesoporous bioactive glass NPs have shown promising potential for bone regeneration. Another study showed that nano-biocomposite scaffolds containing silver NPs could be employed for bone tissue engineering applications [54]. The polymeric scaffolds containing nanoscale organic and inorganic materials can improve the surface and mechanical properties required to promote musculoskeletal tissue regeneration [53, 55].
Cell attachment, differentiation, integration, survival, and many other aspects of tissue engineering depend on the properties of nanostructures that are used as scaffolds. The NP-based scaffolds can show different features depending on the applications. For example, electrical conductivity is an essential functionality for neural tissues, while solid mechanical properties are crucial in bone and cartilage cells. CNTs (carbon nanotubes) are electrically conductive and have excellent chemical stability and mechanical strength. Hence, they are ideal candidates to be used as scaffolds in tissue engineering. Nanocomposites of PLA-based scaffolds with CNTs and micro-hydroxyapatite (HA) particles show improved differentiation and attachment of mesenchymal stromal cells (MSCs) to osteoprogenitors in the bone engineering [56]. Scaffolds containing metallic NPs have shown increased mechanical strength, improved tissue formation capacity, and cell attachment [53]. It is also shown that we can significantly increase tensile strength using various NPs based on iron, aluminoxane, and titanium. They will improve the alkaline phosphatase activity, collagen synthesis, and calcium deposition by osteoblasts [57,58,59]. Researchers recently designed cellulose nanofibers by incorporating hydroxyapatite (HAp) and silver (Ag) NPs and using cellulose acetate. It has been shown that these scaffolds, aside from their antibacterial properties, possess significant potential in both soft and hard tissue engineering [60]. Conductive carbonized nanofibers loaded with gold NPs have been synthesized and proven to be used for bone tissue engineering applications [61].
NP-based delivery systems also are the leading players in tissue engineering to deliver genetic components, bioactive molecules, and growth factors. For instance, biological signaling molecules instruct the fate of cells in cardiac tissue regeneration and stimulate neovascularization in the myocardial infarction area; the delivery control of these molecules can be significantly improved using rate-program NPs [62, 63]. Accordingly, significant attention has been drawn to the growth factor (GF)-loaded polymeric NPs [64]. In bone tissue engineering, nanomaterial carriers are used to overcome the limitations of contemporary biomaterials; low cell membrane permeability, weak pharmacokinetics, low physiological stability, and non-explicit targeting are some limitations. Nanomaterial carriers can stabilize bioactive components via surface adhesion and encapsulation. These carriers can also aid cell entry, targeted and controlled drug delivery, and release [53]. Figure 4 summarizes the application of NPs for regenerative medicine and tissue engineering.
NPs Applications in tissue engineering (AC represents “alternating current,” QDs mean “quantum dots,” and PEG is polyethylene glycol). Adapted from reference [52] distributed under the Creative Commons Attribution
Targeted drug delivery
Recently, a significant amount of interest has been placed on using nanosized carriers like polymeric NPs, nanocrystals, and liposomes in the controlled delivery of various drugs and biocomponents [65,66,67,68,69,70]. The goal is to use these NMs to obtain higher therapeutic efficacy and weaker side effects by delivering drugs in the optimum dosage range. NPs either encapsulate the medications (in the case of mesoporous, hollow, or polymeric NPs), or the drugs are grafted on their surface [44, 71, 72].
Ideal NPs in drug delivery applications have low cytotoxicity, long-standing physical stabilities, and high loading capacity. Again, the goal is to minimize or avoid the therapeutic agents’ side effects by targeted delivery to pathologic tissues [72, 73]. Today, there are several kinds of NPs used for this purpose. The most common ones are polymeric nanoparticles, solid lipid nanoparticles, liposomes, dendrimers, inorganic nanoparticles, and nanotubules [72].
Using NPs in drug delivery started with lipid NPs. Solid lipid NPs (SLNs), nanostructured lipid carriers (NLCs), liposomes, and micelles are placed in this category. SLNs contain lipids in the solid state, while NLCs, the second-generation SLNs, control solid lipids and liquid lipids with a higher drug loading capacity [73]. In ocular drug delivery, SLNs load the antibiotic tobramycin for bacterial infection treatment [74]. SLNs are also suitable for peptide delivery; the presence of the lipid matrix in SLNs results in higher stability, prevents proteolytic degradation, and can improve the controlled release [75]. In SLNs, the lipid component, concentration of surfactant, homogenization factors, and final sizes of particles can be used to control drug release. There are some concerns and disadvantages to using SLNs in drug delivery. The hydrophilic drugs can potentially get partitioned in the aqueous phase in the preparation step, and that is one of the main disadvantages of these NPs, which may weaken the drug loading and may cause the drugs to be expulsed from the matrix after the polymorphic transition during storage [73].
A study was managed to study the likelihood of improving brain targeting affinity and temazepam-loaded nanostructured lipid carriers (NLCs) bioavailability. According to the results, for brain-mediated drug delivery, temazepam-NLCs are successful carriers. Due to their sustained release, they can be used in the outpatient management of insomnia [76].
Liposomes are also Lipid-based NPs containing a central aqueous core plus one or more concentric phospholipid bilayers. Liposome vesicles, which can be unilamellar or multilamellar, feature advantages like biocompatibility, biodegradability, and low toxicity. Moreover, hydrophobic and hydrophilic compounds can be entrapped in liposomes to prevent the compound’s decomposition, resulting in compounds being released to the targeted areas [72, 73]. The functionality of liposomes to carry small molecules and biologically relevant macromolecules has been widely investigated [77, 78]. Several examples of liposome-based drug delivery systems currently present in clinical trials include Daunoxome and Doxil, which are encapsulated versions of the anti-cancer chemotherapy drug doxorubicin in liposomes [71]. Recently, to enhance the stability of actively loaded liposomal doxorubicin (DOX), researchers designed a method as nanobowl stabilization of liposomes [79].
After the promising results obtained using these lipid-based drug delivery NPs, an increasing attraction has drawn towards polymeric NPs and led to the development of many different compositions, including polysaccharide- and polypeptide-based NPs, dendrimers, and drug conjugates [71]. Polymeric NPs, such as polymeric micelle, solid polymeric NPs, polymersome, nanoshell, polymer-drug conjugates, and dendrimer, are extensively used for controlled drug delivery [71, 72, 80] and have different structures and morphologies in the core and corona. For example, polymeric micelles with dimensions between 10 and 100 nm are diblock copolymers containing amphiphilic hydrophilic-hydrophobic blocks [81]. They have a corona-core structure comprising a central hydrophobic core plus a hydrophilic corona in an aqueous medium [81]. Hydrophobic drugs can be accommodated in the hydrophobic core of the polymeric micelle via physical entrapping (solubilization). At the same time, the exterior hydrophilic surface contributes to the stability and protection of the therapy [81,82,83]. It has been proved that encapsulation of tamoxifen citrate (TMX) into micelles increased cytotoxicity in MCF-7 cancer cells by facilitating cellular uptake.
The targeted delivery system can be designed by encapsulating/protecting the therapeutic molecules within the core or by conjugating them to the NP’s surface. Different methods can be utilized for corona formation to functionalize the NP’s surface. Functionalizing the surface of NPs can lead to higher residual time within the blood and lower nontargeted distribution. The functionalization can also target specific tissues or antigens with a targeting ligand such as aptamer, peptide, and fragments of antibody [71]. Targeted polymeric therapeutic NPs can be used in improving the drug efficacy in cancer therapy or facilitating drug penetration in a group of diseases such as neurodegenerative disorders (NDs) [80, 84], cardiovascular diseases [61, 64], and osteoporosis and viral infections.
Inorganic NPs are another group of NMs primarily investigated and employed in drug delivery. In magnetic NPs, drugs can be carried either by conjugating to the NP surface or encapsulating inside the NP. Hence, these NPs need to be functionalized and coated with a biocompatible layer, such as gold or polymer. The drug/NP complex will be directed to specific sites by applying an external magnetic field. The release process is controlled through the activity of enzymes or by altering osmolality, pH, or temperature [5, 85]. Iron oxide NPs are the most common inorganic magnetic NPs used for targeted delivery, mainly because of their exceptional characteristics, including chemical stability, non-toxicity, biocompatibility, high saturation magnetization, and high magnetic susceptibility [5, 86]. Cu0.3Zn0.5Mg0.2Fe2O4 magnetic NPs as drug delivery agents for ibuprofen have been studied and showed sustained drug release up to 72 h [87]. Recently, magnetic iron oxide NP-hollow mesoporous silica spheres were designed to deliver anti-cancer drugs triggered by pH changes [88]. Metallic NPs such as gold (Au) exhibit unique properties such as exceptional electronic and optical properties, chemical inertness, and the ability for surface functionalization, which results from the presence of negative surface charges. These features allow NPs to be functionalized with organic molecules and conjugated with ligands, antibodies, or drug molecules for active or passive drug delivery [89]. The chemical inertness also provides good biocompatibility in vitro and in vivo [5, 89]. Sulaiman et al. loaded hesperidin anti-cancer drugs on gold NPs and proved that they inhibit the secretion of IL-1β, IL-6, and TNF [90]. In another study, folic acid-modified methotrexate-conjugated gold NPs showed high cytotoxicity to folate receptor-positive tumor cells [91].
Dendrimers are hyper-branched, 3D globular macromolecules comprising a well-defined central core surrounded by many arms attached to it. The dendritic molecular architecture is characterized by unique features, such as functional groups on the surface, that make them a promising candidate for targeted delivery applications [92, 93]. The functionality of dendrimers can be enhanced by modifying these terminal groups on the surface. The drug is either grafted on the branched interior or attached to the dendrimer surface. Different studies have shown the application of dendrimers for delivering macromolecules, DNA, and hydrophobic drugs [73, 94, 95]. Chanphaia et al. studied folic acid-dendrimer NPs containing doxorubicin (Dox), tetracycline (Tet), and tamoxifen (Tam) and demonstrated that the order of drug stability was doxorubicin > tetracycline > tamoxifen [96]. In 2021, based on amphiphilic peptide dendrimers, safe and effective cancer drug-delivery nanosystems were established to encapsulate doxorubicin (the anti-cancer drug) efficiently. Compared to free doxorubicin, the dendrimer-based drug delivery system increased intracellular uptake of the drug in drug-resistant breast cancer cells [97].
Carbon nanotubes (CNTs), due to their distinct characteristics, have gained tremendous attention as promising drug carriers. Their unique physicochemical properties, such as enhanced cellular uptake and easy surface functionalization with drugs, bioactive peptides, nucleic acids, and proteins, display superior efficacy and low toxicity [98, 99]. CNTs are widely used in designing anticancer drug delivery systems as they can selectively direct the treatment towards the tumors and overcome the lack of selectivity as one of the limitations of anticancer therapies [100]. Recently, single-walled carbon nanotubes, capable of releasing Doxorubicin in a controlled manner through pH changes, have been designed and enhance the pharmacological efficacy of drugs [101]. In another study, researchers demonstrated that chitosan-silica-coated carbon nanotubes are efficient for oral breast cancer therapy as a pH-based drug delivery [102]. However, unlike other nanocarriers such as micelles and liposomes, CNTs have recently emerged, and more examinations are needed to confirm their safety [72, 73, 103].
Recently, NPs have been used to mimic cellular membranes and thus further improve drug delivery. The NPs can be coated with red blood cell membranes, white blood cell membranes, platelet membranes, cancer cell membranes, bacterial membrane vesicles, and mesenchymal stem cell membranes [104].
Anti-SARS-CoV-2 vaccines
Over the past two years, vaccines have been rapidly developed against the SARS-CoV-2 virus. There have been approximately 150 vaccines produced since the start of the pandemic. Various techniques have been used, such as protein subunit vaccines (Novavax), inactivated virus vaccines (Sinovac), viral vector (adenovirus) vaccines (Astra-Zeneca, Johnson & Johnson, Reithera and Sputnik), and mRNA vaccines (Pfizer-BioNTech and Moderna) [3, 105]. The Pfizer-BioNTech and Moderna vaccines utilize a lipid nanoparticle that encapsulates an mRNA sequence translated into a full-length spike protein [3]. These vaccines have a low cost and have been able to be rapidly developed with an efficacy of 87.5–95% and 94.5–100% for Pfizer and Moderna, respectively. These vaccines can be rapidly developed because they are synthetically produced, unlike other vaccines. One issue with mRNA vaccines is the instability of the mRNA, which requires it to be stored at shallow temperatures. Despite this drawback, mRNA has been successfully used within a lipid nanoparticle that keeps it stable and allows delivery of the mRNA intramuscularly. After injection, the mRNA is transported into the cytoplasm and is translated into a spike protein. This spike protein induces T-helper cells to produce cytokines that cause plasma cells in the body to produce antibodies to these viral spike proteins. These antibodies are responsible for the immunity the virus provides, and it has been suggested that immunity should last from six to nine months [3]. The ionizable lipids used in these vaccines are heptadecan-9-yl 8-((2-hydroxyethyl) (6-oxo-6-(undecyloxy) hexyl) amino) octanoate (Lipid H (SM-102)) and ((4-hydroxybutyl) azanediyl) bis(hexane-6,1-diyl) bis (2-hexyldecanoate) (ALC-0315) for the Moderna and Pfizer-BioNTech vaccines, respectively. These two vaccines also contain 1,2-stearoyl-sn-glycerol-3-phosp (DSPC), which is a lipid that helps improve the stability of the lipid nanoparticle due to its geometry and melting temperature [106].
Magnetic NP-based hyperthermia cancer treatment
Hyperthermia, or a slight increase in a tumor’s temperature, has been used during the last decades combined with radiotherapy to increase the susceptibility of cancer cells to chemotherapy and radiation. However, this treatment method has certain drawbacks. For instance, other healthy tissues can also be damaged as tumor temperature cannot often uniformly increase to 42–45 °C, which is required for the direct cytotoxicity [107, 108]. NPs are promising candidates, and they provide a uniform and less invasive tumor-concentrated hyperthermia method with maximum damage to cancer cells and minimal injury to normal cells.
Magnetic NPs, especially iron oxide and gold NPs [109] are the most common NPs utilized in hyperthermia. In magnetic hyperthermia treatment, the activation of magnetic NPs by AMFs (alternating magnetic field) will remotely induce local heat, stimulating a temperature rise in the location of tumoral cells in targeted organs and tissues. To increase this technique’s efficiency, the magnetic NPs have to be systematically coated and functionalized to specifically target the desired area inside the body [110].
Arriortua et al. [111] used a peptide of arginine-glycine-aspartate (RGD) and functionalized Fe3O4 NPs to target αvβ3 integrin receptors in angiogenic cancer cells. It has been demonstrated that the selectivity of magnetic NPs has been enhanced for tumor treatment with a hyperthermia [111]. Recently, researchers observed that through surface functionalization and coatings with different biopolymers, such as chitosan and dextran, the temperature of Fe3O4 NPs can be adjusted to the desired therapeutic (hyperthermia) [112]. This temperature change is possible through surface functionalization and coatings with different biopolymers such as chitosan and dextran [112]. Gold NPs and carbon nanotubes have been primarily investigated to be used for hyperthermia cancer treatments, where near-infrared (NIR) and radiofrequency fields have been utilized to generate hyperthermia [5, 72, 113,114,115,116,117].
Wound healing
Metal NPs, including selenium, silver, platinum, gold, aurum, copper, and palladium NPs, along with their oxide compounds like iron oxide, zinc oxide, titanium dioxide, and tantalum oxide NPs, potentially have some therapeutic effects on accelerating wound healing [118,119,120]. Apart from being ideal candidates for drug and gene delivery (as discussed previously), metal NPs provide outstanding properties for wound healing, such as antibacterial activity and healing stimulation.
For instance, silver NPs prevent scar growth by stimulating rapid wound closure and epidermal re-epithelization via the proliferation of keratinocytes. Moreover, silver NPs, due to their anti-inflammatory features, are capable of accelerating wound healing by protecting cells from the negative influence of elevated ROS [121]. However, recently, it has been proved that a time-frame inflammation should be present for optimum wound healing since early inflammation is beneficial and continued inflammation is unfavorable to burn wound healing. Hence, a short delay in silver NPs application provides optimal burn wound healing because topical silver NPs treatment immediately after burn injury significantly suppresses early inflammation [122].
Zinc oxide and gold NPs can also promote wound healing due to their antimicrobial activity [121, 123,124,125]. Vedhanayagam et al. studied the influence of different shapes of zinc oxide NPs, including sphere, needle, rod, hexagonal, star, flower, doughnut, circular disk, and cube, on wound healing. They reported that the spherical shape of zinc dioxide (ZnO) in a cross-linked collagen scaffold provides enhanced re-epithelization and a faster collagen deposition [126]. Gold NPs can enhance epithelialization, vascularization, and collagen deposition [127]. It has been proved that topical gold NPs could accelerate wound healing on mouse skin through anti-inflammatory and antioxidation effects [128]. Mahmoud et al. investigated the effect of different shapes (rods and spheres) and surface modifications (neutral, cationic, and anionic-charged polymers) of gold NPs on wound healing by loading them into a thermosensitive hydrogel. They reported that gold nanorods with cationic poly allyl amine hydrochloride provide a better environment for optical wound healing [129].
Polymeric NPs based on cellulose, hyaluronic acid, chitosan, and alginate can also exhibit good re-epithelialization and antibacterial properties for improved wound-healing applications [130,131,132,133].
Anti-bacterial applications
Antimicrobial drugs, nanosized inorganic antimicrobial agents, in particular, have been more popular in recent years owing to their lack of side effects, tolerance to heat, and low rate of drug resistance [134]. It has also been reported that silver, gold, copper, and platinum nanoparticles, as well as those made of a variety of metal oxides, such as silver oxide, copper oxide, and zinc oxide, are effective anti-bacterial [135, 136].
Polymeric NPs using chitosan have become increasingly popular due to their high biocompatibility and biodegradability. Chitosan is derived from chitin, found in the cell wall of fungi, and prepared via deacetylation. It is approved by the FDA for various applications and has been reviewed recently for multiple applications, including anti-bacterial capabilities [137]. The preparation process and the molecular weight of chitosan play an essential role in reducing its toxicity of chitosan. Since the preparation process does not require any toxic solvents, the final products are more biocompatible. The toxicity can further be reduced by reducing the molecular weight of chitosan.
Common target microorganisms for chitosan (and combinations of chitosan) are Staphylococcus aureus, Escherichia coli, Salmonella typhi, and Pseudomonas aeruginosa [137]. The exact mechanism of the anti-bacterial effects of chitosan has been debated, but several theories have been proposed. It has been suggested that the amine groups (when positively charged) cause increased cell permeability and, thus, cell death. This theory is substantiated by the fact that antimicrobial activity decreases when the pH is not favorable for the protonation of the amine group. Another proposed approach is that chitosan can suppress the activity of mRNA by binding to DNA.
Chitosan can be used alone for anti-bacterial properties, or it can be used with higher effectiveness when combined with specific metal oxides [137]. One such application is the use of hydrogels for skin wounds and the prevention of bacterial infections. These are biodegradable, biocompatible, and less toxic than previous treatments.
An additional important use of chitosan NPs is with antimicrobial peptides (AMPs). Antimicrobial resistance is a widespread problem, and AMPs could help solve this issue. Unfortunately, there are problems with AMPs, such as low stability and adverse interactions with other molecules. NPs could help with these two issues and thus have become an important area of study. One study specifically looked at temporin B, a small, cationic AMP with antibacterial, antiviral, and antifungal properties. Temporin B was encapsulated using chitosan nanoparticles, and the results showed that temporin B was released linearly and had continuous anti-bacterial activity against Staphylococcus epidermidis [138].
Biosensing applications
A biosensor is described via its bioreceptor element specified to related analytes. These analytes are frequently of biological origins, like bacteria or viruses’ DNAs or proteins formed by a diseased or contaminated live immune system (antibody, antigen). Simple compounds like glucose or pollutants may also be analytes when a bioreceptor with exceptional sensitivity is present. One of the significant issues in developing biosensors is the effective signal collection of biorecognition (transduction). Such transducers transfer the bio interaction into measurable signals. To raise sensitivity and decrease the limit of detection to single molecules, nanomaterials are intriguing options owing to the potential to immobilize an expanded amount of bioreceptors at concentrated quantities and also to operate as transducers. Among such nanomaterials, AuNPs, semiconductor quantum dots, polymer NPs, carbon nanotubes, nanodiamonds, and graphene are actively investigated [139].
Recent advances in multidisciplinary sciences have developed a new kind of fluorescent NPs: biomolecule-assisted fluorescent metal nanoclusters (NCs). The advantages of these NCs as a biosensor over other fluorescent materials are their simple manufacturing, ultrasmall size, tunability of fluorescence characteristics, and high biocompatibility [14].
Proteins, DNA, and targeted cells may all be detected via fluorescence thanks to hybrid bi-layer nanostructures that integrate distinct fluorescent inorganic compounds. Combinatorial characteristics and synergistic effects are present in the resulting nanoparticles, allowing for combination therapies with precise targeting, biomedical imaging, and amplified diagnostic and catalytic effects [140].
Even though NPs have found widespread use, there are still some significant problems that must be solved. In the pre-clinical, clinical, and production stages of new product management, the FDA has prioritized determining the dispersion of NPs carriers in the body after systemic administration via different methods. The evolution of imaging techniques that can track biodistribution over time is related to a second worry. The third problem is how mass is transferred between different body parts. For this reason, the fourth and fifth priority highlight the need to create new mathematical and computer-based simulations that will add to a “periodic table” of NPs for estimating danger and reward. The development of criteria or reference materials and agreement analysis methodologies that may serve as baselines for creating innovative class materials ranks as a close sixth. An analytical toolset for nanopharmaceuticals production, complete with a specification sheet of toxicologic, safety, and biodistribution qualities generated by standard, established methodologies, will be given as the end aim [141].
Mechanism of cytotoxicity
A rapidly growing interest in using NPs in biomedical engineering increased concerns about biocompatibility and cytotoxicity. The first section explains that NMs behave significantly differently than bulky materials due to their surface-to-volume ratio. These parameters also directly affect the chemical reactivity of materials and their toxicity. Various factors such as size, surface area, aggregation, the bulk chemistry of the material, surface functionalization, shape, and porosity can impact their toxicity; however, the role of different parameters on the toxicity of NPs will be discussed further. Generally, the higher chemical reactivity of NPs, due to their greater surface area to volume ratio, increases the surface interaction of NPs with biological system [142]. Consequently, using NMs for biomedical applications has risks, considering the uncertainties of the NP properties that can pose health risks.
Existing studies in animals and humans showed that different parameters, including the persistence of the material and the duration of the nanomaterial exposure in the body, can increase the health risks induced by NPs. NPs, due to their small size, can enter cells through receptor-mediated endocytosis or direct penetration. As a result, they can translocate into different organelles. Thus, before their application, to get reliable results, various tests such as mitochondrial metabolism, cell metabolism, cell membrane integrity, and lysosomal membrane integrity have to be conducted [143].
Understanding the toxicity mechanism of NPs will help researchers to design NPs with lower side effects. Several central mechanisms exist to explain NPs’ cytotoxicity, such as direct cell damage by their entry to the cell and interaction with intracellular components or indirect damage due to the generation of radical species [142]. For example, NPs contribute not only to lipid peroxidation but also to the destruction of the cell, lysosomal, and nuclear membranes and cause the release of their contents. Toxic ions released from their dissolution can interact with cells’ DNA or impair essential enzyme functions. However, the creation of reactive oxygen species (ROS) is another mechanism of toxicity for NPs, which can also damage enzymes or the DNA of cells [144]. Yet, the production of excessive amounts of (ROS) is the leading cause of NPs’ toxicity [145, 146].
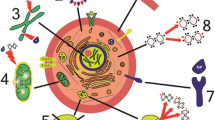
Living organs are surrounded by molecules containing oxygen atoms that may participate in chemical reactions with redox systems, essential ions, biomolecules, etc. [147]. Inducing ROS production has been observed in different materials, such as silica and metal oxide NPs and carbon nanotubes [148,149,150]. As a result, this mechanism causes severe cell dysfunction due to impairment of the mitochondria, the cell plasma membrane, and the nucleus; in the end, cells go through one of the two possible paths: suffering from DNA impairment or initiating cell-death signaling (apoptosis or necrosis).
ROSs can originate from two primary sources. The first group of sources stems from extracellular factors such as exposure to NMs, microbial infection, environmental pollutants, and radiation exposure [151]. The second group of authorities is related to intracellular-like endoplasmic reticulum stress and mitochondria (Fig. 5) [152]. The generated ROS can lead to genotoxicity and cell death due to their toxicity. The human body can handle moderate levels of ROS, and it is essential for cell function [153,154,155]. However, high ROS levels are a sign of oxidative stress that may cause cancer, cardiovascular, neurological, respiratory, and kidney disease due to the triggering of several reactions, such as enzyme dysfunction, gene mutation, lipid peroxidation, and mitochondria membrane damage (Fig. 6).
Schematic diagram showing sources of ROS generation and the negative effect of ROS in the body. The general concept is adapted from reference [152], distributed under the Creative Commons Attribution
To better understand the mechanism of ROS formation, the reaction of iron oxide NPs (as NMs representatives) in the biological microenvironment can be considered an example. The production of ROS due to the presence of NPs can be explained by the Fenton and Harber-Weiss reactions [156]. The first step starts with the Fenton reaction, followed by reducing the ferric (Fe3+) ion into the ferrous (Fe2+) ion. The Fenton reaction between H2O2 and Fe2 + is the first step in generating ROS and produces hydroxide (OH−) and hydroxyl radical [157]. The second step is called the Haber–Weiss reaction, which produces superoxide (•O2 −) catalyzed by iron ions and •OH (hydroxyl radicals) from H2O2 (hydrogen peroxide [158, 159].
Among ROS, hydroxyl radicals are considered the most potent oxidant [160]. The ROS induced by NPs can polymerize the proteins and result in mutation of the DNA [146].
When NPs enter the cell, they can interact with intracellular components like lipids, nucleic acids, and proteins [161] (Fig. 6). The interaction of NPs with proteins, depending on their size, forms a combined structure, such as NP-protein corona on the surface of the NPS, that can influence cellular uptake and the accumulation of the NPs [161, 162]. Soft corona complexes are formed when NPs are surrounded by a loose protein layer (∼ 2–3 protein molecules thick). Challenging corona complexes are formed when the NPs are surrounded by strongly bounded protein fraction (Fig. 7) [163, 164].
NPs and protein composition (adapted from reference [162] with permission)
Various forces, such as van der Waals’ interactions, hydrogen bonds, and electrostatic and hydrophobic interactions, can govern the interactions between NPs and proteins. The interactions between NPs and proteins are affected by the surface properties of NPs, the properties of proteins, and the biological microenvironment [162, 163]. Generally, the interaction between NPs and nucleic acids is the same as that of NPs and proteins [165].
Factors that affect the cytotoxicity of NPs
As discussed in previous sections, the physical and chemical characteristics of NPs strongly influence their biochemical characteristics and toxicity. The toxicity effect of the physical and chemical properties of NPs, such as size, shape, agglomeration state, coatings, functionalization, surface charge, structure, material type, dose, and concentration, will be discussed separately.
Impact of the size of NPs on toxicity
Although different physical and chemical parameters affect the NPs-cells interaction, the critical physical property of the NPs is their size [166, 167]. Nanoparticle size directly affects the distribution, diffusion, and physiological response of cells [168]. The size and surface area of NPs play a critical role in their cell penetration ability since the large surface area ensures efficient adsorption of NPs. Specifically, as the particles decrease in size, the surface area will exponentially increase so that the smaller NPs are more reactive in the biological environment and have a larger potential catalytic surface for chemical reactions [169, 170]. NPs with sizes of 1 to 100 nm can quickly enter cells because their size is comparable to the thickness of cell membranes, around 10 nm. For instance, gold NPs with a size of 10–16 nm can be found in the cytoplasm, but gold NPs with less than 6 nm can enter the cell nucleus. Hence, smaller NPs can be more toxic [171]. Generally, NPs are internalized more efficiently when they have an optimum size of 50 nm. However, several studies have indicated that smaller NPs with the size of about 15–30 nm or larger NPs with the size of 70–240 nm show lower cellular uptake rate [11, 172, 173].
Additionally, there are six significant pathways for nanomedicines to enter cells according to their size: phagocytosis, macropinocytosis, caveolin, clathrin-mediated as well as independent endocytosis, and direct cell infiltration [174].
The optimum sizes for NMs used in bio-applications and uptake properties during phagocytosis are more significant than 10 nm and smaller than 100 nm. Zhao et al. proved that mesoporous silica NPs (MSNPs) (∼ 100 nm) could be adsorbed on the surface of human red blood cells, while larger MSNs (∼ 600 nm) induce local membrane deformation (Figs. 8 and 9) [175]. Although NPs with different sizes have been used in biomedical applications, the type of application determines the required size of NPs. For example, NPs in the range of 50 nm are useful for drug delivery and cellular uptake [176]. The size variation of NPs will affect various organs in the body and which organism responds upon exposure.
SEM visualization of the interaction of MSNPs with red blood cells. A Red blood cells incubated for 2 h at room temperature with PBS, B red blood cells with 100 μg mL–1 of MSNPs with an average size of 122 nm, and C red blood cells with 100 μg mL.–1 of MSNPs with an average size of 531 nm (adapted from reference [175] with permission)
Transmission electron (TEM) images of the interaction of red blood cells with MSNPs with average sizes of 122 nm (top) and average sizes of 531 nm (bottom) (adapted from reference [175] with permission)
Consequently, the size of NPs determines how and where the body reacts to them [177]. Moreover, the size is a different parameter for cells to define clear foreign particles [178]. Depending on their size, the particles that are not clear will be translocated or accumulated in the body. With the clearance activity of kidneys, particles less than 5 nm in size will be removed from the body.
Due to the effects mentioned above on NP size and its health-related outcomes, scientists believe that size is the first parameter to be considered in characterizing NPs’ toxicity. It has been shown that TiO2 NPs with smaller sizes (20 nm) can cause severe inflammatory responses in the lungs compared to larger particles (250 nm) [19, 179]. There are different techniques for measuring the size of NPs, including dynamic light scattering, time of flight methods, laser diffraction, differential mobility analysis, surface area measurements, impaction methods, and application of microscopy [180].
Impact of particle shape on toxicity
The shape of the NPs (e.g., spheres, cylinders, cubes, sheets, pillar and rod-like, needle-like, and plate-like) is another determinant factor of cytotoxicity, and in a “safer by design” context, NPs shape should be carefully taken [181]. For instance, spherical NPs, in comparison to nanotubes, nanofibers, plate-like, and needle-like NPs, exhibit less toxicity [182].
The impact of the shape of cerium oxides (CeO2) NPs has been studied with macrophages from the RAW264.7 cell line. Results showed that rod-like NPs, dose-dependently, produced more tumor necrosis factor-alpha (TNF-α) and ROS [181], while in cubic/octahedral NPs, the release of TNF-α and ROS did not vary [181]. TEM images of differently shaped NPs are depicted in Fig. 10. As can be seen, the possibility of agglomeration is weak for all samples [181].
Different structures of CeO2 NPs with different concentrations and volumes (R = rod-shaped, O = octahedron-shaped) (adapted from reference [181] with permission)
In another study, particle–cell association and cellular uptake were investigated on BEAS-2B cells after synthesizing the hydroxyapatite NPs with four shapes, including needle, plate, sphere, and rod shapes. Results show that the most significant cell death in BEAS-2B cultures was observed in needle-shaped and plate-shaped NPs [182].
Another study looked at the impact of the shape and size of zinc oxide nanoparticles on the toxicity of Raphidocelis subcapitata, a freshwater microalga [183]. R. subcapitata was chosen as a representative organism of freshwater algae because there is less tendency of this algae to aggregate and, thus, they are sensitive to toxic substances. This study used two different sizes of spherical zinc oxide nanoparticles and two different sizes of rod-shaped nanoparticles. The spherical nanoparticles were found to be more toxic than the rod-shaped nanoparticles. Concerning the rod-shaped nanoparticles, an increase in the size of the nanoparticles resulted in decreased toxicity.
Impact of agglomeration state of NPs on toxicity
Particle surface reactivity and surface area, which, apart from the size, are also related to single particle aggregation, play a significant role in the cytotoxicity of the NPs [184]. Even though most of the studies address the possible adverse effects and cytotoxicity of single NPs, we should consider that NPs (NPs) tend to aggregate. Several hundred nm in diameter, these structures exhibit different physicochemical properties and mechanisms of interaction with cells. Cells can uptake NPs and form aggregates inside the cell (Fig. 11). However, it has been shown that the growth rate of HeLa cells increases when the aggregates become too large to enter the cell and adhere to the cell surface [184, 185]. In another study, researchers showed that compared to single and monodisperse NPs, uptake of aggregated NPs with HeLa cells decreased by 25% while MDA-MB 435 cells increased by 100%. Tripathy et al. researched to evaluate the toxicity impact of zinc oxide NPs aggregation on RAW 264.7 murine macrophages. Firstly, they concluded that modulating the NP aggregation depends on the concentration of particles. Secondly, they showed that low-concentration zinc oxide NPs’ aggregates, compared to high concentration, led to more cell apoptosis in RAW 264.7 cell (Fig. 12). These contrasting results highlight the need to evaluate the interaction of aggregates with cells per each case.
TEM image of MDA-MB-435 and HeLa cells incubated with single AuNPs and 98 nm aggregates. a HeLa cells treated with monodisperse AuNPs, b Hela cells with aggregates, c MDA-MB-435 cells incubated with monodisperse, d MDA-MB-435 cells incubated with aggregates. Adapted from reference [186] with permission
The effect of concentration of zinc oxide NPs aggregates on the viability of murine macrophage RAW 264.7 cells (adapted from reference [187] with permission)
Impact of the structure of NPs on toxicity
The cytotoxic effects of NPs depend on the crystalline form of the nanomaterial. The cytotoxicity and genotoxicity of two crystalline forms of TiO2 NPS, anatase and rutile, in Balb/3T3 mouse fibroblasts, were evaluated [188]. The crystalline form had a significant impact on toxicity. Cell uptake was higher in the anatase form (although internalizing seemed to be size-dependent more than crystallinity), while cyto- and genotoxicity appeared in the rutile structure. In addition, the neoplastic transformation with multiple genomic changes that cause cancer was more prominent rutile NP (e type-III foci formation). In addition, rutile titania may cause more genotoxic effects due to producing more ROS species (more photocatalytic). The morphology of the two different structures is shown in Fig. 13 [188].
TEM micrographs of A egg-shaped TiO2 NPs An-10 and 11–18 nm size and B elongated Ru-10 particles with 10–35 nm (adapted from reference [188] with permission)
In another study, the toxicity of TiO2 NPs with different types of crystal lattice was evaluated using a human bronchial epithelium cell line. It has been proven that while NPs with an anatase-like crystal structure are nontoxic, rutile-like crystal structures can trigger oxidative damage to DNA and the growth of micronuclei [189].
Impact of NP surface energy and wettability on toxicity
Wettability and surface properties can govern the toxicity and biodistribution of NPs in vivo and in vitro. Larger contact areas create a higher chance of adsorption. Three types of mesoporous silica NPs (BMSs) were synthesized by employing heterocyclic amino acid derivatives, including C16-L-poline, C16-L-histidine, and C16-L-tryptophan. Then, cellular uptake, morphology, structure, and wettability of NPs were studied. The surface wettability of samples followed this sequence: silica NPs templated by C16-L-tryptophan (Trp-BMS) > C16-L-histidine (His-BMS) > C16-L-poline (Pro-BMS). This study demonstrated that a smaller contact angle or higher wettability would result in more considerable protein adsorption. Among three samples, silica NPs templated by C16-L-tryptophan exhibited the highest wettability due to the large amount of –OH groups and, consequently, the highest protein adsorption. The degradation analysis results also proved that the degradation rate was by the wettability. Hence, the NPs with the highest wettability showed faster degradation. All three samples (silica NPs templated by C16-L-histidine, C16-L-poline, or C16-L-tryptophan) showed increased uptake in the brain even though the blood–brain barrier prevents the entry of the majority of drugs. However, they exhibited the lowest distribution amount in the brain due to the super-hydrophilicity surface of silica NPs templates by C16-L-tryptophan. According to the histopathology of animals’ major organs, as seen in Fig. 14, no abnormality or structural changes were observed in the heart, liver, spleen, lungs, and kidney compared to the control group.
Histopathology of heart, liver, spleen, lung, and kidney organs in control and treated animals with His-BMS, Trp-BMS, and Pro-BMS (adapted from reference [190] with permission)
Impact of coatings, functionalization, and surface charge of NPs on toxicity
The properties, behavior, and environmental fate of NPs can be enhanced by functionalizing their surface by applying biocompatible coatings. The behavior and property change in NPS depend on both types of coating material and layer thickness. For illustration, coating NPs with biocompatible conjugates and substances decrease the toxicity of silver NPs in a time- and dose-dependent manner. The toxic effects of uncoated silver NPs with different sizes (20, 40, 60, and 80 nm) and coated silver NPs with other coatings (standard citrate and polyvinylpyrrolidone (PVP)) and different sizes (10, 50, and 75 nm) on J774A.1 macrophage and HT29 epithelial cells were investigated. Their analyses proved three main results [191]. First, the cell viability was decreased by 20–40% at a concentration of 1 µg/ml in samples exposed to uncoated Ag NPs with the sizes of 20 and 40 nm and decreased by 30–40% in samples exposed to uncoated Ag NPs with the sizes of 60 and 80 nm. Hence, smaller particles are more toxic, and the effects are size-dependent. Second, the cell viability was decreased at 25 µg/ml or higher concentrations in samples exposed to coated Ag NPs, and the results were also size-dependent. Therefore, coated Ag NPs are more biocompatible compared to uncoated Ag NPs. Third, in a size-dependent manner at 1 µg/ml, uncoated Ag-NPs increased superoxide dismutase (SOD) activity and decreased glutathione (GSH) content, while even at the highest test concentrations, non-significant changes in GSH and SOD were observed in samples exposed to coated Ag NPs [191]. It can be concluded that coated Ag-NPs are less toxic than uncoated Ag-NPs. And last, the toxicity of Ag NPs is size- and coating-dependent, as citrate-coated Ag NPs are less toxic than PVP-coated Ag NPs [191]. Furthermore, the cytotoxicity of ZnO NPs can be reduced by incorporating titanium oxide shell coatings, which prevents the release of zinc ions.
Drug delivery systems can also benefit from NPs coating. When applying NPs for target drug delivery, they should be invisible for phagocytizing cells. In this case, coating NPs with biodegradable polymeric surfactants is useful. These coatings can provide enriched surfaces with stealth proteins for inhibiting cell uptake and controlling the unspecific protein adsorption and aggregation tendency (Fig. 15) [192].
Coating of NPs with functional polymeric surfactants. (Adapted from reference [192] with permission)
Another surface determinant parameter of NP, which controls their interactions with biological systems, is their surface charge, which can be modified by grafting differently charged polymers [193, 194].
For example, the effect of size and surface charge on the cellular uptake of NPs has been studied through the development of polymeric NPs with carboxymethyl chitosan (negatively charged NPs) and chitosan hydrochloride grafted NPs (positively charged NPs). According to the results, NPs with negative charges and smaller particle sizes have more tendency toward gathering in tumors [168]. The effect of the surface charge of gold NPs on their toxicity has been studied. Compared to neutral NPs, positively and negatively charged gold NPs displayed toxicity at lower doses [193].
Impact of NP dose on toxicity
High doses of NPs can cause a disproportionate decrease in cell viability. Kim et al. proved that monodisperse spherical silica NPs (SNPs) cytotoxicity depends on their dosage [195]. In another study, cobalt NPs on the viability of macrophages in vitro were investigated. The result suggested that local adverse biological effects can be caused by increased production of cobalt NPs in vivo due to excessive metal-on-metal implant wear [196].
The toxicity of TiO2 NPs with different dose ranges (0.1–100 mg/ml) and different sizes (18, 30, and 87 nm, which TEM confirms) was also assessed through exposure to human lung and colon cells. All of the samples induced a dose-dependent cytotoxicity via decreased cell viability, and the smaller size nanoparticles (18 nm) produced a significant toxic effect even at the lowest dose [197].
Impact of substance type of NPs on toxicity
During the last decade, a wide range of NPs has been used for several biomedical applications. The source of nanomaterials can be metallic and non-metallic materials.
While non-metallic NPs include polymeric NPs, silica-based NPs, and carbon-based NPs, metallic NPs include gold, silver, copper, aluminum oxide, zinc oxide, Iron oxide, and Titanium oxide.
It is worth mentioning that in low concentrations, metallic NPs could be toxic to most bacteria and yeast, so due to this antimicrobial behavior and also because of the possibility of synthesizing highly active metal NPs, they have been widely used in a wide range of applications [198, 199]. Due to their antimicrobial properties, metals have been used for disease treatment since the beginning. Moreover, a feature that distinguishes metallic NPs from other NPs is their image contrast characteristic in targeted cancer therapy and radiotherapy [200]. Although researchers have been focused on the toxicity of metallic NPs, this topic has yet to be fully elucidated as several variables are involved. Multiple side effects have been observed in utilizing metallic NPs in both in vitro and in vivo experimentations, including ROS, cell death, DNA injury, and mitochondrial dysfunction, reported [201].
For example, ZnO NPs prompted much more significant cytotoxicity than non-metal NPs, including carbon black (CB), single-wall carbon nanotube, and silicon dioxide (SiO2) [202]. This section will discuss important types of metallic and non-metallic NPs.
Polymeric NPs
Based on the matrix polymer extraction source of polymeric NPs, they are divided as either natural or synthetic. The most common synthetic polymers are polylactide acid (PLA), poly(lactide-co-glycolide) (PLGA), hyaluronic acid, poly(d,l-lactide), polyglycolide, and polycaprolactone (PCL). At the same time, chitosan is a widely used natural polymer in the polymeric NPs [203]. The application of these sorts of NPs is rapidly growing in nano-bio materials, such as drug delivery. Like other kinds of NMs, polymeric NPs possess beneficial and detrimental features. For instance, they offer such adaptability in customizing their size, surface functionality, morphology, biodegradability, and chemical composition. However, researchers face the challenge of controlling their monodispersed size distribution and surface charge [204]. Moreover, polymeric NPs can carry a broad range of drugs to different body parts, which is considered an excellent advantage for biomedical applications [205, 206].
Different types of polymeric NPs utilized for biomedical applications, including polymeric micelles, solid polymeric NPs, dendrimers, polymer conjugates, polymer-lipid hybrid systems, polyplexes, and polymersomes. The therapeutic agent can be added to polymeric NPs through two different methods: (1) surface conjugating to NPs or (2) encapsulating within the polymer center [207]. As an example of polymeric NPs, Alibolandi et al. developed a PEG–PLGA nanopolymer capable of carrying DOX for target drug delivery applications [208]. They have used SK-MES-1 and A59 cells for in vitro experimentations and the SK-MES-1 tumor model for in vivo practices to demonstrate the effect of this DOX-loaded nanopolymer in lung cancer treatment. They have found that DOX-NPs, aptamer-conjugated (Apt-DOX-NP), increase the uptake of NPs and cause inhibiting tumor growth in nude mice.
However, in another paper, Alibolandi et al. unveiled local toxicities of PLGA-based NPs due to the high toxicity of stabilizers used for the formulation of PLGA NPs [209].
A review on polymeric NPs discussed the in vitro and in vivo toxicological studies using polymeric NPs and suggested that polymeric NPs increase the bioavailability of the drugs loaded into the NP and are non-toxic. They conclude that polymeric nanogels are stable in the serum, have a high potential to encapsulate a drug, and are customizable in size [210]. One study successfully used PLGA nanoparticles to load triterpenoids into various human cell lines (hepatoma, colorectal epithelial adenocarcinoma, and retinoblastoma cell lines) and found an increase in cell viability [211]. Another study looked at the incorporation of dexibuprofen, an enantiomer of ibuprofen, into PEGylated-PLGA nanospheres and found a decreased toxicity level compared to the drug alone when inserted into the ocular mucosa. In vivo and in vitro studies [212] confirmed this.
Silica-based NPs
Silica-based NPs are the leading group of NMs used in biomedical engineering. They are usually used as cell markers, drug carriers, imaging moieties, and gene transfection agents [212]. Silica NPs have unique properties such as availability of surface modification, ease of synthesis, relatively inert chemical composition, and robust mechanical properties [213]. The critical factor in the application of silica NPs is their crystallinity level. If silica NPs have an amorphous structure, they can be safely used in biomedical applications such as drug delivery [214]. While crystalline silica NPs can cause different diseases, such as lung cancer [215]. Mesoporous silica has been used extensively as it is famous for its mechanical and biochemical solidity and excellent properties [216,217,218].
A toxicity study of silica NPs by Kim et al. employed monodisperse spherical silica NPs to verify their size and dose effect on the cytotoxicity in epithelial and fibroblast cells [195]. They studied oxidative stress, cellular uptake, cell viability, and membrane disruption. In addition to size and dose, they found that the toxicity of monodisperse spherical silica NPs is also highly dependent on the cell type. Their study showed ROS generation and cell death ensued when monodisperse spherical silica NPs were exposed to A549 cells. Moreover, they discovered that the particles with a size of 20 nm were rather harmful compared to those with a size of 60 nm. In another study, Docter et al. used amorphous silica NPs to investigate the potential cytotoxic effects on the protein corona [219]. The MTT assay evaluated the cytotoxicity with epithelial GI tract Caco-2 cells. The amorphous silica NPs were characterized in terms of hydrodynamic diameter, colloidal stability of the amorphous silica NPs distribution, and negative zeta potential. It was shown that apart from particle size, surface area and surface characteristics of NPs directly affect their toxicity. In 2017, Orlando et al. utilized mesoporous silica NPs in vitro experimentations to specify the size and dose effects on toxicity. They have concluded that the toxicity of mesoporous silica NPs depends on the size and cellular uptake. In addition, particles at doses less than 0.25 mg/mL and with a diameter of less than 30 nm are safe for biomedical applications.
Carbon-based NPs
Carbon-based nanomaterials have attracted significant interest in biomedical applications due to their excellent chemical, mechanical, thermal, electrical, and optical properties. Graphene oxide, carbon nanotubes, graphene quantum dots, and fullerene are examples of carbon-based NPs [220,221,222,223,224]. However, the toxic effect of carbon-based NPs has been proven in previous studies. For instance, it has been showed that single-walled carbon nanotubes can decrease the cell growth of human HEK293 cells by diminishing the ability of cellular adhesion and stimulating apoptosis.
The low risk of antimicrobial resistance characterizes carbon-based nanomaterials. This is because their antibacterial mechanism is mechanical (by membrane damage). Hence, it has been proposed that carbon-based nanomaterials can be a promising antiviral agent to combat COVID-19 with low or no toxicity to humans in the Microbial-Resistant Era [224].
The effects of single-walled carbon nanotubes (SWCNTs) on human HEK293 cells were analyzed to explore SWCNTs biocompatibility. Results indicated that SWCNTs could prevent HEK293 cell proliferation, decrease cell adhesive ability in a dose- and time-dependent manner, and induce cell apoptosis [225]. After studying the side effects of carbon-based NPs in vivo on mice models, they have proven that they are biocompatible with liver function enzymes. No significant alteration was observed in the liver, lung, and spleen organs [226].
Gold-based NPs
Colloidal gold (Au), frequently called the gold NPs, was first produced by Michael Faraday and has been employed in medical fields since the early 1920s [227]. Gold-based NPs are synthesized by various methods and can exist in different shapes, like gold nanorods, spherical nanocages, tripods, and tetrapods. Multiple studies have demonstrated the preparation of gold-based NPs [228]. Gold-based NPs can deliver biomolecules such as biopolymers, peptides, DNA, and proteins to different body parts. Hence, they can be used as delivery vehicles for DNA, drugs, genes, siRNAs, and also for therapeutic applications [229,230,231].
Sometimes gold-based NPs are used for coating and modifying the polymeric carriers [232]. In one study, Zhang et al. used gold NPs for coating polyethylene glycol for cancer radiation therapy [233]. They studied the polyethylene glycol-coated gold NPs size’s effect on radio sensitization and found that 12.1 and 27.3 nm sizes were the best for radiotherapy and drug delivery. They have also found that these NPs did not cause kidney and spleen damage.
Generally, gold-based NPs have a small size, so they can quickly enter cells. Various parameters like size, dosage, or even route of an administration play crucial roles in gold-based NPs’ toxicity [234]. For example, Mironava et al. described the size and concentration effects of gold NPs on the cell functions [235]. They found that gold NPs are harmful to skin fibroblasts. Additionally, the size, concentration, and exposure time of NPs can influence the toxicity rate.
They showed that when gold-based NPs interact with primary dermal fibroblasts, clathrin-mediated endocytosis is the pathway for cell infiltration of the 45-nm gold NPs. In comparison, the particles below 13 nm enter through the phagocytosis [235].
In another study, it has been proved that 1.4-nm gold-based NPs can cause quick cell death predominantly by necrosis within 12 h, while 1.2-nm gold-based NPs affect predominantly programmed cell death by apoptosis. The cellular response is size dependent, so 1.4-nm particles cause essentially rapid cell death by necrosis within 12 h. Closely related particles 1.2 nm in diameter affect mostly programmed cell death by apoptosis [236, 237]. During in vivo cytotoxicity experiments of 12.5-nm-sized gold-based NPs, researchers studied the bioaccumulation and toxic effects of different doses (40, 200, and 400 μg/kg/day). They proved that, with the dose administered, gold levels in the blood did not rise, while there was a proportional increase in gold in all the organs. However, no toxicity was observed in organ morphology, blood biochemistry, and tissue histology while trapped in the lungs, kidneys, brain, spleen, and liver.
In 2017, Woźniak et al., for the first time, evaluated the toxicity of different morphology of gold-based NPs, including spherical (~ 10 nm), nanorods (~ 41 nm length), nanoprisms (~ 160 nm), nanostars (~ 240 nm), and nanoflowers (~ 370 nm) with various concentrations (1, 6, 8, 16, 32, 100, and 300 µM) and proved that gold nanorods and nanospheres are more toxic than flower, star, and prism gold nanostructures [238]. Li et al. evaluated the biodistribution of gold-based NPs ranging from 6.2 to 61.2 nm in vivo. According to their results, particles with larger sizes (42.5 and 61.2 nm) were distributed mainly in the liver and spleen. At the same time, little or none were found in the heart, kidney, and lung, while smaller ones (6.2 and 24.3 nm) accumulated in the liver, spleen, and other organs [239].
The successful application of gold NPs depends on their uptake and possible toxicity in the liver, their leading site for accumulation [240]. In 2021, the cytotoxic effects of gold NPs with different size (~ 15 nm and 60 nm) and shape (nanospheres and nanostars) were investigated in human HepaRG cells or primary rat hepatocytes (PRH). Their results proved that the highest toxicity was related to spherical particles with a size of 15 nm. Hence, the cellular uptake of gold NPs depends on the shape, size, and the type of cellular model [240].
Silver NPs
Silver is usually utilized in the nitrate form; the antibacterial and anti-fungal characteristics, conductivity, optical properties, and diagnostic capability of silver NPs make them the most appealing particles in potential applications in the biotechnology [241]. Similar to gold NPs, silver has been utilized for wounds, burn injuries, and microbial infections [242]. Recently, it has been discovered that due to the increased efficiency of drug delivery by silver NPs (producing anti-tumorigenic effects and enhancing the effectiveness of drug delivery), these particles help improve the efficacy of cancer treatments [243].
Silver NPs may be toxic or non-toxic depending on their synthesis method (chemical, physical and biological). For example, with the chemical reduction action of some reducing factors or physical processes such as evaporation–condensation, the generated NPs are toxic. However, caused NPs from a group of methods are non-toxic and valuable for biomedical applications. These methods include green methods (use of biological entities) such as template applications (viral DNA and diatoms), utilizing plants and plant extracts, and the use of microorganisms (actinomycetes, bacteria, yeasts, and fungi). They are non-toxic and valuable for biomedical applications [244].
Silver NPs can affect cellular metabolic activity, generate ROS, and cause mitochondrial dysfunction, cell death, and DNA damage. Similar to other NPs, the toxicity, mitochondrial damage, and ROS levels of these kinds of NPs depend on different factors such as size and shape [245, 246]. Several factors have been offered to explain the cytotoxicity mechanism of silver NPs; however, unleashing silver ions is considered the main factor according to most researchers [247].
Li et al. validated that cell necrosis can be caused by silver ions dissolved from silver NPs due to the overload of intracellular sodium and calcium. Moreover, pulmonary inflammation also can be triggered through elevating mitochondrial-related contents of necrotic cells [248].
Kumari et al. synthesized rectangular, hexagonal, penta, and spherical silver NPs [249]. They observed that the antimicrobial activity of NPs is directly related to their size and shape. As expected, 2–5 nm spherical-shaped NPs displayed excellent antibacterial efficacy.
In another study in 2017, Makama et al. studied the size impacts of silver NPs on cytotoxicity with different surface coating/charges in an in vitro assay [250]. They used macrophage cells (RAW 264.7) at 0–200 mg/ml concentrations to systematically assess the effects of silver NP properties. This research tested viability, ATP production, tumor necrosis factor (TNF)-a induction, ROS, and uptake dynamics. NPs with a size of 20 nm and bovine serum albumin (BSA)-coated were considerably more toxic. They have shown that the effect of surface coating/charge on toxicity is significant and this parameter also has to be considered. Skandalis et al. developed a new silver NP synthesis method to reduce the number of silver ions [251]. They introduced a biological pathway using various plant extracts to synthesize silver NPs. Cost-effectiveness and being environment-friendly are the essential properties of their method for synthesizing silver NPs.
However, due to the contradictory results on silver NPs’ potential toxicity, in 2021, Andreoli et al. conducted a study. They introduce a standardized in vitro approach to evaluate the probable toxicity of AgNPs and to acquire consistent and comparable results [252].
Plasmonic Alloy NPs
While individual elements have been studied in the use of nanoparticles, there have been recent developments in alloying plasmonic elements with additional plasmonic or non-plasmonic elements [253]. A plasmon is a quantum of plasma oscillation and can be promoted by electromagnetic light, and with nanoscale metals, the plasmons are referred to as localized surface plasmons (LSPs). Most elements have a poor plasmonic response, except for gold or silver, due to oxidation or degradation. Still, when these elements are combined with an additional feature, properties can be changed to suit a particular application. Some motivations for producing such NPs are surface accessibility, biocompatibility, chemical stability, sustainability, photocatalytic activity, chemical selectivity, and magnetism. These nanoalloys form via metallic bolding and have various applications, such as photothermal effects for nanomedicine applications, plasmon-enhanced catalysis, and surface-enhanced Raman spectroscopy (SERS) labeling. Another critical application of plasmonic nanoalloys is improving biosensors. Ag and Au are combined in these applications due to Ag’s corrosion and instability but Au’s high biocompatibility. One study looked at the ideal combination of Ag and Au to detect human immunoglobulin and found it to be \({Au}_{0.6}{Ag}_{0.4}\) [254].
Conclusion
NPs exhibit unique features that make them a powerful tool for biomedical applications, such as molecular imaging and cell therapy, tissue engineering, targeted drug delivery, cancer treatment, wound healing, and most recently, as a vaccine delivery method for COVID-19. NPs can diagnose a disease, its stage, and location and carry a therapeutic agent for the tumor. NPs can simulate the natural nanometer size scale of extracellular matrix (ECM) components, and their size is almost similar to peptides and small proteins. They can simply be uptaken by cells and diffuse across membranes due to their small size and associated sizeable surface-to-volume ratio. Incorporating NPs into biomaterials and scaffolds by creating innovative nanocomposite materials can aid wound healing through their antimicrobial, anti-inflammatory, and pro-angiogenic properties. They also can facilitate wound healing by promoting protein synthesis and influencing collagen deposition.
Nevertheless, the direct or indirect interaction of NPs with cells can damage cells’ structure or organelles or disrupt cell survival and signaling. Consequently, only a limited number have reached the clinical stage. Cyto-genotoxicity of NPs stems from three main routes, including (1) direct NPs damage to the cell surface and organelles, (2) generation of toxic ions and their interaction with the cell’s DNA, and (3) oxidative stress generation because of ROS production and their damage to enzymes or DNA. Different factors, including size, shape, structure, agglomeration state, surface characteristics, dose, and type of material, can affect the cytotoxicity of NPs. Aside from the mentioned factors, responding cell type and exposure time have proven to affect the toxicity of NPs. Hence, all aspects should be considered simultaneously to evaluate the cytotoxicity of NPs for biomedical applications. In vitro and in vivo cytotoxicity testings are an indispensable part of the development of NPs to engineer NPs that minimalize cytotoxicity to a range of potentially exposed cells.
Despite the tremendous advantages of nanoparticles and their applications, some possible drawbacks still need to be addressed first, such as a lack of advanced equipment for precise and scalable nanomaterial production, difficulty analyzing its efficacy and safety, and specific constraints of particular materials. Factors such as low cost and high effectiveness, biocompatibility, potential to penetrate biological barriers, and stability in physiological conditions should be considered to facilitate nanoparticle production.
Research institutions and businesses should support scale-up efforts to bring their innovations in this area to market so that society may reap the full benefits of its many applications. LNPs, like nanosuspensions, may be lyophilized and spray-dried in a later manufacturing step. LNPs products must still adhere to all other industry requirements, such as using a clean room, specialized manufacturing equipment, machine authentication, flooring, safety, and employee instruction. Authorities have recently provided explicit definitions for liposome regulatory rules, which may give a comprehensive grasp of SLNs. Clinical trials also point to the potential arrival of these technologies on the pharmaceutical market shortly.
References
Khan I, Saeed K, Khan I (2019) Nanoparticles: properties, applications and toxicities. Arab J Chem 12(7):908–931
Oktaviani O (2021) Nanoparticles: properties, applications and toxicities. J Latihan. 1(2):11–20
Mascellino MT et al (2021) Overview of the main anti-SARS-CoV-2 vaccines: mechanism of action, efficacy and safety. Infect Drug Resist 14:4501–4502
Prasad G (2009) Biomedical applications of nanoparticles. Safety of nanoparticles. Springer, pp 89–109
McNamara K, Tofail SA (2017) Nanoparticles in biomedical applications. Adv Phys X 2(1):54–88
Bharathala S, Sharma P (2019) Biomedical applications of nanoparticles. Nanotechnology in modern animal biotechnology. Elsevier, pp 113–132
Kavanagh EW, Green JJ (2022) Toward Gene Transfer Nanoparticles as Therapeutics. Adv Healthc Mater 11(7):e2102145
Pulit-Prociak J, Banach M (2016) Silver nanoparticles–a material of the future…? Open Chem 14(1):76–91
Skiba MI, Vorobyova VI, Pivovarov A, Makarshenko NP (2020) Green synthesis of silver nanoparticles in the presence of polysaccharide: optimization and characterization. J Nanomaterials 2020:1–10
Ealia SAM, Saravanakumar M (2017) A review on the classification, characterisation, synthesis of nanoparticles and their application. In: IOP conference series: materials science and engineering, vol. 263, no. 3. IOP Publishing, p 032019
Foroozandeh P, Aziz AA (2018) Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res Lett 13(1):1–12
Krpetić Ž, Anguissola S, Garry D, Kelly PM, Dawson KA (2014) Nanomaterials: Impact on Cells and Cell Organelles. In: Capco D, Chen Y (eds) Nanomaterial. Advances in Experimental Medicine and Biology, vol 811. Springer, Dordrecht
Nimesh S, Chandra R (2011) Theory, techniques and applications of nanotechnology in gene silencing (1st edn). River Publishers, p 218
Wang B et al (2019) Biomolecule-assisted synthesis and functionality of metal nanoclusters for biological sensing: a review. Materials Chemistry Frontiers 3(9):1722–1735
Pardi N et al (2018) mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discovery 17(4):261–279
Chaudhary N, Weissman D, Whitehead KA (2021) mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat Rev Drug Discovery 20(11):817–838
Wu Q et al (2020) Mechanical properties of nanomaterials: a review. Nanotechnol Rev 9(1):259–273
Yu X, Zhan Z (2014) The effects of the size of nanocrystalline materials on their thermodynamic and mechanical properties. Nanoscale Res Lett 9(1):1–6
Buzea C, Pacheco II, Robbie K (2007) Nanomaterials and nanoparticles: sources and toxicity. Biointerphases 2(4):MR17–MR71
EU-Commission, Commission recommendation of 18 October 2011 on the definition of nanomaterial text with EEA relevance (2011) Commission, E.(Ed.), Brussels, BE, p. 2.
Standards IOf (2010) Nanotechnologies-Vocabulary-Part 1: Core Terms. ISO Geneva.
Wang Y, Cai R, Chen C (2019) The nano–bio interactions of nanomedicines: understanding the biochemical driving forces and redox reactions. Acc Chem Res 52(6):1507–1518
Tang Z et al (2020) A materials-science perspective on tackling COVID-19. Nat Rev Mater 5(11):847–860
Tuominen M, Schultz E (2010) Environmental aspects related to nanomaterials-A literature survey
Samyn P et al (2018) nanoparticles and nanostructured materials in papermaking. J Mater Sci 53(1):146–184
Nowack B, Bucheli TD (2007) Occurrence, behavior and effects of nanoparticles in the environment. Environ Pollut 150(1):5–22
Pokropivny V, Skorokhod V (2007) Classification of nanostructures by dimensionality and concept of surface forms engineering in nanomaterial science. Mater Sci Eng, C 27(5–8):990–993
Jeevanandam J et al (2018) Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 9:1050
Buschmann MD et al (2021) Nanomaterial delivery systems for mRNA vaccines. Vaccines 9(1):65
Kanasty R et al (2013) Delivery materials for siRNA therapeutics. Nat Mater 12(11):967–977
Wang Y, Xia Y (2004) Bottom-up and top-down approaches to the synthesis of monodispersed spherical colloids of low melting-point metals. Nano Lett 4(10):2047–2050
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13(10):2638–2650
Lancaster CA et al (2020) Uniting top-down and bottom-up strategies using fabricated nanostructures as hosts for synthesis of nanomites. The Journal of Physical Chemistry C 124(12):6822–6829
Chang X et al (2021) Fluorescent papain-encapsulated platinum nanoclusters for sensing lysozyme in biofluid and gram-positive bacterial identification. Sens Actuators, B Chem 345:130363
Yao Q et al (2018) Toward total synthesis of thiolate-protected metal nanoclusters. Acc Chem Res 51(6):1338–1348
Abbasi S et al (2009) Rheological properties and percolation in suspensions of multiwalled carbon nanotubes in polycarbonate. Rheol Acta 48(9):943
Abbasi S, Carreau PJ, Derdouri A (2010) Flow induced orientation of multiwalled carbon nanotubes in polycarbonate nanocomposites: rheology, conductivity and mechanical properties. Polymer 51(4):922–935
Abbasi S, Derdouri A, Carreau PJ (2011) Properties of microinjection molding of polymer multiwalled carbon nanotube conducting composites. Polym Eng Sci 51(5):992–1003
Society R (2004) Nanoscience and nanotechnologies: opportunities and uncertainties. Royal Society.
Phan CM, Nguyen HM (2017) Role of capping agent in wet synthesis of nanoparticles. J Phys Chem A 121(17):3213–3219
Niu Z, Li Y (2013) Removal and utilization of capping agents in nanocatalysis. Chem Mater 26(1):72–83
Loss D (2009) Quantum phenomena in nanotechnology. Nanotechnology 20(43):430205
World Health Organization (2010) FAO/WHO expert meeting on the application of nanotechnologies in the food and agriculture sectors: potential food safety implications: meeting report. World Health Organization
Xu C et al (2011) Nanoparticle-based monitoring of cell therapy. Nanotechnology 22(49):494001
Nguyen PK, Nag D, Wu JC (2010) Methods to assess stem cell lineage, fate and function. Adv Drug Deliv Rev 62(12):1175–1186
Chen ZY, Wang YX, Lin Y, Zhang JS, Yang F, Zhou QL, Liao YY (2014) Advance of molecular imaging technology and targeted imaging agent in imaging and therapy. BioMed Res Int 2014:819324
So P-W et al (2010) Efficient and rapid labeling of transplanted cell populations with superparamagnetic iron oxide nanoparticles using cell surface chemical biotinylation for in vivo monitoring by MRI. Cell Transplant 19(4):419–429
Ni J-S et al (2020) Nanoparticle-based cell trackers for biomedical applications. Theranostics 10(4):1923
Chen F, Jokerst JV (2020) Stem cell tracking with nanoparticle-based ultrasound contrast agents. Methods Mol Biol (Clifton, NJ) 2126:141
Kim KY, Chang K-A (2021) Therapeutic potential of magnetic nanoparticle-based human adipose-derived stem cells in a mouse model of Parkinson’s disease. Int J Mol Sci 22(2):654
Cheng Q et al (2020) Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat Nanotechnol 15(4):313–320
Kim E-S et al (2014) Emerging nanotechnology approaches in tissue engineering and regenerative medicine. Int J Nanomed 9(Suppl 1):1
Walmsley GG et al (2015) Nanotechnology in bone tissue engineering. Nanomedicine 11(5):1253–1263
Hasan A et al (2018) Nano-biocomposite scaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modified cellulose nanowhiskers for bone tissue engineering applications. Int J Biol Macromol 111:923–934
Amini AR, Laurencin CT, Nukavarapu SP (2012) Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 40(5):363–408
Ciapetti G et al (2012) Enhancing osteoconduction of PLLA-based nanocomposite scaffolds for bone regeneration using different biomimetic signals to MSCs. Int J Mol Sci 13(2):2439–2458
Tran N, Webster TJ (2011) Increased osteoblast functions in the presence of hydroxyapatite-coated iron oxide nanoparticles. Acta Biomater 7(3):1298–1306
Goto K et al (2005) Bioactive bone cements containing nano-sized titania particles for use as bone substitutes. Biomaterials 26(33):6496–6505
Horch RA et al (2004) Nanoreinforcement of poly (propylene fumarate)-based networks with surface modified alumoxane nanoparticles for bone tissue engineering. Biomacromol 5(5):1990–1998
Sofi HS et al (2021) Regenerated cellulose nanofibers from cellulose acetate: incorporating hydroxyapatite (HAp) and silver (Ag) nanoparticles (NPs), as a scaffold for tissue engineering applications. Mater Sci Eng, C 118:111547
Nekounam H et al (2020) Simple and robust fabrication and characterization of conductive carbonized nanofibers loaded with gold nanoparticles for bone tissue engineering applications. Mater Sci Eng, C 117:111226
Izadifar M et al (2015) Optimization of nanoparticles for cardiovascular tissue engineering. Nanotechnology 26(23):235301
Izadifar M et al (2014) Rate-programming of nano-particulate delivery systems for smart bioactive scaffolds in tissue engineering. Nanotechnology 26(1):012001
Zhang S, Uludağ H (2009) Nanoparticulate systems for growth factor delivery. Pharm Res 26(7):1561
Kashkooli FM, Soltani M, Souri M (2020) Controlled anti-cancer drug release through advanced nano-drug delivery systems: static and dynamic targeting strategies. J Control Release 327:316–349
Kashkooli FM et al (2021) Nexus between in silico and in vivo models to enhance clinical translation of nanomedicine. Nano Today 36:101057
Kashkooli FM, et al (2021) Enhanced drug delivery to solid tumors via drug-loaded nanocarriers: an image-based computational framework. Front Oncol 11.
Soltani M et al (2021) Enhancing clinical translation of cancer using nanoinformatics. Cancers 13(10):2481
Hassanzadeganroudsari M et al (2020) Targeted nano-drug delivery system for glioblastoma therapy: in vitro and in vivo study. J Drug Deliv Sci Technol 60:102039
Hassanzadeganroudsari M et al (2019) Enhancing anti-cancer efficacy of carboplatin by PEGylated poly (butyl cyanoacrylate) nano-particles. J Drug Deliv Sci Technol 54:101218
Alexis F et al (2008) Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm 5(4):505–515
De Crozals G et al (2016) Nanoparticles with multiple properties for biomedical applications: a strategic guide. Nano Today 11(4):435–463
Maximilien J et al (2015) Nanoparticles in biomedical applications. Measuring Biological Impacts of Nanomaterials. Springer, pp 177–210
Cavalli R et al (2002) Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. Int J Pharm 238(1–2):241–245
Almeida AJ, Runge S, Mu¨ller RH (1997) Peptide-loaded solid lipid nanoparticles (SLN): influence of production parameters. Int J Pharm 149(2):10
E Eleraky N et al (2020) Nanostructured lipid carriers to mediate brain delivery of temazepam: design and in vivo study. Pharmaceutics. 12(5):451
Hwang SY et al (2012) Effects of operating parameters on the efficiency of liposomal encapsulation of enzymes. Colloids Surf, B 94:296–303
He J et al (2013) Hydrodynamically driven self-assembly of giant vesicles of metal nanoparticles for remote-controlled release. Angew Chem 125(9):2523–2528
Chen Z-J et al (2020) Nanobowl-supported liposomes improve drug loading and delivery. Nano Lett 20(6):4177–4187
Banik BL, Fattahi P, Brown JL (2016) Polymeric nanoparticles: the future of nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol 8(2):271–299
Hussein YH, Youssry M (2018) Polymeric micelles of biodegradable diblock copolymers: enhanced encapsulation of hydrophobic drugs. Materials 11(5):688
Agrawal RD et al (2020) Polymeric micelle as a nanocarrier for delivery of therapeutic agents: a comprehensive review. J Drug Deliv Ther. 10(1-s):191–195
Gorain B et al (2020) Polymeric micelle-based drug delivery systems for tuberculosis treatment. Nanotechnology Based Approaches for Tuberculosis Treatment. Elsevier, pp 175–191
Goldsmith M, Abramovitz L, Peer D (2014) Precision nanomedicine in neurodegenerative diseases. ACS Nano 8(3):1958–1965
Gao J, Xu B (2009) Applications of nanomaterials inside cells. Nano Today 4(1):37–51
Vangijzegem T, Stanicki D, Laurent S (2019) Magnetic iron oxide nanoparticles for drug delivery: applications and characteristics. Expert Opin Drug Deliv 16(1):69–78
Ansari M et al (2017) Synthesis and characterization of Cu0. 3Zn0. 5 Mg0. 2Fe2O4 nanoparticles as a magnetic drug delivery system. J Magnetism Magn Mater. 439:67–75
Teng Y et al (2020) Magnetic iron oxide nanoparticle-hollow mesoporous silica spheres: fabrication and potential application in drug delivery. Curr Appl Phys 20(2):320–325
Siddique S, Chow JC (2020) Gold nanoparticles for drug delivery and cancer therapy. Appl Sci 10(11):3824
Sulaiman GM et al (2020) Hesperidin loaded on gold nanoparticles as a drug delivery system for a successful biocompatible, anti-cancer, anti-inflammatory and phagocytosis inducer model. Sci Rep 10(1):1–16
Yücel O et al (2020) Folic acid-modified methotrexate-conjugated gold nanoparticles as nano-sized trojans for drug delivery to folate receptor-positive cancer cells. Nanotechnology 31(35):355101
Nikzamir M, Hanifehpour Y, Akbarzadeh A et al (2021) Applications of dendrimers innanomedicine and drug delivery: a review. J Inorg Organomet Polym 31:2246–2261
Sherje AP et al (2018) Dendrimers: a versatile nanocarrier for drug delivery and targeting. Int J Pharm 548(1):707–720
Lee CC et al (2006) A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc Natl Acad Sci 103(45):16649–16654
Haensler J, Szoka FC Jr (1993) Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug Chem 4(5):372–379
Chanphai P, Thomas T, Tajmir-Riahi H (2021) Application and biomolecular study of functionalized folic acid-dendrimer nanoparticles in drug delivery. J Biomol Struct Dyn 39(3):787–794
Zhu D et al (2021) A self-assembling amphiphilic peptide dendrimer-based drug delivery system for cancer therapy. Pharmaceutics 13(7):1092
Bianco A, Kostarelos K, Prato M (2005) Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol 9(6):674–679
Wong BS et al (2013) Carbon nanotubes for delivery of small molecule drugs. Adv Drug Deliv Rev 65(15):1964–2015
Fabbro C et al (2012) Targeting carbon nanotubes against cancer. Chem Commun 48(33):3911–3926
Jagusiak A et al (2020) Controlled release of doxorubicin from the drug delivery formulation composed of single-walled carbon nanotubes and Congo red: a molecular dynamics study and dynamic light scattering analysis. Pharmaceutics 12(7):622
El-Shahawy AAG, Elnagar N, Zohery M, Abd Elhafeez MS, El-Dek SI (2021) Smart nanocarrier-based chitosan@ silica coated carbon nanotubes composite for breast cancer treatment approach. Int J Polymeric Mater Polymeric Biomater 71(12):910–922
Zhang W, Zhang Z, Zhang Y (2011) The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res Lett 6(1):555
Imran M, Jha LA, Hasan N, Shrestha J, Pangeni R, Parvez N, Mohammed Y, Jha SK, Paudel KR (2022) "Nanodecoys"- Future of drug delivery by encapsulating nanoparticles in natural cell membranes. Int J Pharm 621:121790.
Ismail AA (2022) SARS-CoV-2 (Covid-19): A short update on molecular biochemistry, pathology, diagnosis and therapeutic strategies. Ann Clin Biochem 59(1):59–64
Hou X et al (2021) Lipid nanoparticles for mRNA delivery. Nat Rev Mater 6(12):1078–1094
Jha S, Sharma PK, Malviya R (2016) Hyperthermia: role and risk factor for cancer treatment. Achiev Life Sci 10(2):161–167
Bettaieb A, Wrzal PK, Averill-Bates DA (2013) Hyperthermia: Cancer treatment and beyond. Cancer Treatment-Conventional and Innovative Approaches, pp 257–283.
Cherukuri P, Glazer ES, Curley SA (2010) Targeted hyperthermia using metal nanoparticles. Adv Drug Deliv Rev 62(3):339–345
Saifullah S et al (2020) Surface functionalized magnetic nanoparticles for targeted cancer therapy and diagnosis. Metal Nanoparticles for Drug Delivery and Diagnostic Applications. Elsevier, pp 215–236
Arriortua OK et al (2018) RGD-Functionalized Fe3O4 nanoparticles for magnetic hyperthermia. Colloids Surf, B 165:315–324
Jamir M et al (2021) Effect of surface functionalization on the heating efficiency of magnetite nanoclusters for hyperthermia application. J Alloy Compd 854:157248
Giustini AJ et al (2010) (2010) Magnetic nanoparticle hyperthermia in cancer treatment. Nano Life 1(01n02):17–32
Chatterjee DK, Diagaradjane P, Krishnan S (2011) Nanoparticle-mediated hyperthermia in cancer therapy. Ther Deliv 2(8):1001–1014
Kaur P et al (2016) Hyperthermia using nanoparticles–promises and pitfalls. Int J Hyperth 32(1):76–88
Bañobre-López M, Teijeiro A, Rivas J (2013) Magnetic nanoparticle-based hyperthermia for cancer treatment. Rep Pract Oncol Radiother 18(6):397–400
Curley SA, Cherukuri P, Briggs K, Patra CR, Upton M, Dolson E, Mukherjee P (2008) Noninvasive radiofrequency field-induced hyperthermic cytotoxicity in human cancer cells using cetuximab-targeted gold nanoparticles. J Exp Ther Oncol 7(4):139
Shurygina IA, Shurygin MG (2017) Nanoparticles in Wound Healing and Regeneration. Metal Nanoparticles in Pharma. Springer, pp 21–37
Naderi N et al (2018) Nanoparticles in wound healing; from hope to promise, from promise to routine. Frontiers in bioscience (Landmark edition) 23:1038–1059
Oyarzun-Ampuero F et al (2015) Nanoparticles for the treatment of wounds. Curr Pharm Des 21(29):4329–4341
El-Aassar M et al (2020) Wound healing of nanofiber comprising Polygalacturonic/Hyaluronic acid embedded silver nanoparticles: in-vitro and in-vivo studies. Carbohyd Polym 238:116175
Zhang K et al (2020) Delayed application of silver nanoparticles reveals the role of early inflammation in burn wound healing. Sci Rep 10(1):1–12
Chhabra H et al (2016) A nano zinc oxide doped electrospun scaffold improves wound healing in a rodent model. RSC Adv 6(2):1428–1439
Zangeneh MM et al (2019) Preparation, characterization, and evaluation of cytotoxicity, antioxidant, cutaneous wound healing, antibacterial, and antifungal effects of gold nanoparticles using the aqueous extract of Falcaria vulgaris leaves. Appl Organomet Chem 33(11):e5216
Boomi P et al (2020) Phyto-engineered gold nanoparticles (AuNPs) with potential antibacterial, antioxidant, and wound healing activities under in vitro and in vivo conditions. Int J Nanomed 15:7553
Vedhanayagam M, Unni Nair B, Sreeram KJ (2018) Collagen-ZnO scaffolds for wound healing applications: role of dendrimer functionalization and nanoparticle morphology. ACS Applied Bio Mater. 1(6):1942–1958
Lau P et al (2017) Influence of gold nanoparticles on wound healing treatment in rat model: photobiomodulation therapy. Lasers Surg Med 49(4):380–386
Leu J-G et al (2012) The effects of gold nanoparticles in wound healing with antioxidant epigallocatechin gallate and α-lipoic acid. Nanomedicine. 8(5):767–775
Mahmoud NN et al (2019) Gold nanoparticles loaded into polymeric hydrogel for wound healing in rats: effect of nanoparticles’ shape and surface modification. Int J Pharm 565:174–186
Aly UF et al (2019) Formulation and evaluation of simvastatin polymeric nanoparticles loaded in hydrogel for optimum wound healing purpose. Drug Des Dev Ther 13:1567
Singla R et al (2017) In situ functionalized nanobiocomposites dressings of bamboo cellulose nanocrystals and silver nanoparticles for accelerated wound healing. Carbohyd Polym 155:152–162
Singla R et al (2017) In vivo diabetic wound healing potential of nanobiocomposites containing bamboo cellulose nanocrystals impregnated with silver nanoparticles. Int J Biol Macromol 105:45–55
Rath G et al (2016) Collagen nanofiber containing silver nanoparticles for improved wound-healing applications. J Drug Target 24(6):520–529
Wang C-Y et al (2020) Advances in Antimicrobial Organic and Inorganic Nanocompounds in Biomedicine. Advanced Therapeutics 3(8):2000024
Mehdi M et al (2021) Green synthesis and incorporation of Sericin silver nanoclusters into electrospun ultrafine cellulose acetate fibers for anti-bacterial applications. Polymers 13(9):1411
Zhao M et al (2022) AgNPs/nGOx/Apra nanocomposites for synergistic antimicrobial therapy and scarless skin recovery. J Materials Chem B 10(9):1393–1402
Rashki S et al (2021) Chitosan-based nanoparticles against bacterial infections. Carbohydr Polym 251:117108
Piras AM et al (2015) Chitosan nanoparticles loaded with the antimicrobial peptide temporin B exert a long-term antibacterial activity in vitro against clinical isolates of Staphylococcus epidermidis. Front Microbiol 6:372
Holzinger M, Le Goff A, Cosnier S (2014) Nanomaterials for biosensing applications: a review. Front Chem 2:63
Gao P et al (2021) Synergistic integration of metal nanoclusters and biomolecules as hybrid systems for therapeutic applications. Acta Pharmaceutica Sinica B 11(5):1175–1199
Sanhai WR et al (2008) Seven challenges for nanomedicine. Nat Nanotechnol 3(5):242–244
Fard JK, Jafari S, Eghbal MA (2015) A review of molecular mechanisms involved in toxicity of nanoparticles. Advanced Pharm Bull 5(4):447
Soenen SJ, De Cuyper M (2009) Assessing cytotoxicity of (iron oxide-based) nanoparticles: an overview of different methods exemplified with cationic magnetoliposomes. Contrast Media Mol Imaging 4(5):207–219
Buchman JT et al (2019) Understanding nanoparticle toxicity mechanisms to inform redesign strategies to reduce environmental impact. Acc Chem Res 52(6):1632–1642
Wang E et al (2017) Silver nanoparticle induced toxicity to human sperm by increasing ROS (reactive oxygen species) production and DNA damage. Environ Toxicol Pharmacol 52:193–199
Yu Z et al (2020) Reactive oxygen species-related nanoparticle toxicity in the biomedical field. Nanoscale Res Lett 15:1–14
Chen G et al (2016) Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem Rev 116(5):2826–2885
Shvedova AA et al (2012) Mechanisms of carbon nanotube-induced toxicity: focus on oxidative stress. Toxicol Appl Pharmacol 261(2):121–133
Batchelor-McAuley C, Tschulik K, Neumann C, Laborda E, Compton R (2014) Why are silver nanoparticles more toxic than bulk silver? Towards understanding the dissolution and toxicity of silver nanoparticles. Int J Electrochem 9(3):1132–1138
Lehman SE et al (2016) Silica nanoparticle-generated ROS as a predictor of cellular toxicity: mechanistic insights and safety by design. Environ Sci Nano 3(1):56–66
Vallyathan V, Shi X (1997) The role of oxygen free radicals in occupational and environmental lung diseases. Environ Health Perspect 105(suppl 1):165–177
Abdal Dayem A et al (2017) The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int J Mol Sci 18(1):120
Sadhukhan P, Sil PC (2020) The regulation of intracellular redox homeostasis in cancer progression and its therapy. Pathology. Elsevier, pp 105–114
Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87(1):315–424
Young I, Woodside J (2001) Antioxidants in health and disease. J Clin Pathol 54(3):176–186
Kanti Das T, Wati MR, Fatima-Shad K (2015) Oxidative stress gated by Fenton and Haber Weiss reactions and its association with Alzheimer’s disease. Arch Neurosci 2(2):e60038
Gupta P, Lakes A, Dziubla T (2016) A free radical primer. Oxidative Stress and Biomaterials. Elsevier, pp 1–33
Kehrer JP (2000) The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 149(1):43–50
Halliwell B, Gutteridge JM (1992) Biologically relevant metal ion-dependent hydroxyl radical generation An update. FEBS Lett 307(1):108–112
Collin F (2019) Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int J Mol Sci 20(10):2407
Saptarshi SR, Duschl A, Lopata AL (2013) Interaction of nanoparticles with proteins: relation to bio-reactivity of the nanoparticle. J Nanobiotechnol 11(1):1–12
Gunawan C et al (2014) Nanoparticle–protein corona complexes govern the biological fates and functions of nanoparticles. J Mater Chem B 2(15):2060–2083
Bai X et al (2021) In vivo protein corona formation: characterizations, effects on engineered nanoparticles’ biobehaviors, and applications. Front Bioengineering Biotechnol 9:263
Kihara S et al (2020) Structure of soft and hard protein corona around polystyrene nanoplastics—particle size and protein types. Biointerphases 15(5):051002
Chang X-L, Yang S-T, Xing G (2014) Molecular toxicity of nanomaterials. J Biomed Nanotechnol 10(10):2828–2851
Dhawan A, Sharma V (2010) Toxicity assessment of nanomaterials: methods and challenges. Anal Bioanal Chem 398(2):589–605
Yao JJ et al (2017) Local cellular responses to titanium dioxide from orthopedic implants. BioResearch open access 6(1):94–103
He C et al (2010) Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 31(13):3657–3666
Bai X et al (2017) Toward a systematic exploration of nano-bio interactions. Toxicol Appl Pharmacol 323:66–73
Cheng L-C et al (2013) Nano–bio effects: interaction of nanomaterials with cells. Nanoscale 5(9):3547–3569
Huo S et al (2014) Ultrasmall gold nanoparticles as carriers for nucleus-based gene therapy due to size-dependent nuclear entry. ACS Nano 8(6):5852–5862
Kou L, Bhutia YD, Yao Q, He Z, Sun J and Ganapathy V (2018) Transporter-guided delivery of nanoparticles to improve drug permeation across cellular barriers and drug exposure to selective cell types. Front Pharmacol 9:27
Mitchell MJ et al (2021) Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discovery 20(2):101–124
Zhu M et al (2012) Physicochemical properties determine nanomaterial cellular uptake, transport, and fate. Acc Chem Res 46(3):622–631
Zhao Y et al (2011) Interaction of mesoporous silica nanoparticles with human red blood cell membranes: size and surface effects. ACS Nano 5(2):1366–1375
Powers KW et al (2007) Characterization of the size, shape, and state of dispersion of nanoparticles for toxicological studies. Nanotoxicology 1(1):42–51
Borm PJ et al (2006) The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol 3(1):11
Renwick L, Donaldson K, Clouter A (2001) Impairment of alveolar macrophage phagocytosis by ultrafine particles. Toxicol Appl Pharmacol 172(2):119–127
Oberdörster G, Ferin J, Lehnert BE (1994) Correlation between particle size, in vivo particle persistence, and lung injury. Environ Health Perspect 102(suppl 5):173–179
Liang X et al (2017) Related topic: safety evaluation of nanomaterials. Skin Permeation and Disposition of Therapeutic and Cosmeceutical Compounds. Springer, pp 313–322
Forest V et al (2017) Impact of cerium oxide nanoparticles shape on their in vitro cellular toxicity. Toxicol In Vitro 38:136–141
Zhao X et al (2013) Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent. Arch Toxicol 87(6):1037–1052
Samei M, Sarrafzadeh MH, Faramarzi MA (2019) The impact of morphology and size of zinc oxide nanoparticles on its toxicity to the freshwater microalga, Raphidocelis subcapitata. Environ Sci Pollut Res Int 26(3):2409–2420
Karakoti A, Hench L, Seal S (2006) The potential toxicity of nanomaterials—the role of surfaces. Jom 58(7):77–82
Cui W et al (2012) Effects of aggregation and the surface properties of gold nanoparticles on cytotoxicity and cell growth. Nanomedicine. 8(1):46–53
Albanese A, Chan WC (2011) Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano 5(7):5478–5489
Tripathy N et al (2014) Effect of ZnO nanoparticles aggregation on the toxicity in RAW 264.7 murine macrophage. J Hazardous Mater. 270:110–117
Uboldi C et al (2016) Role of the crystalline form of titanium dioxide nanoparticles: rutile, and not anatase, induces toxic effects in Balb/3 T3 mouse fibroblasts. Toxicol In Vitro 31:137–145
Gurr J-R et al (2005) Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 213(1–2):66–73
Li H et al (2019) Evaluation of biomimetically synthesized mesoporous silica nanoparticles as drug carriers: structure, wettability, degradation, biocompatibility and brain distribution. Mater Sci Eng, C 94:453–464
Nguyen KC, et al (2013) Comparison of toxicity of uncoated and coated silver nanoparticles. in Journal of Physics: Conference Series. IOP Publishing.
Müller J et al (2017) Coating nanoparticles with tunable surfactants facilitates control over the protein corona. Biomaterials 115:1–8
Schaeublin NM et al (2011) Surface charge of gold nanoparticles mediates mechanism of toxicity. Nanoscale 3(2):410–420
El Badawy AM et al (2011) Surface charge-dependent toxicity of silver nanoparticles. Environ Sci Technol 45(1):283–287
Kim IY et al (2015) Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomedicine. 11(6):1407–1416
Kwon Y-M et al (2009) Dose-dependent cytotoxicity of clinically relevant cobalt nanoparticles and ions on macrophages in vitro. Biomed Mater 4(2):025018
Gandamalla D, Lingabathula H, Yellu N (2019) Nano titanium exposure induces dose-and size-dependent cytotoxicity on human epithelial lung and colon cells. Drug Chem Toxicol 42(1):24–34
Grass G, Rensing C, Solioz M (2011) Metallic copper as an antimicrobial surface. Appl Environ Microbiol 77(5):1541–1547
Palza H (2015) Antimicrobial polymers with metal nanoparticles. Int J Mol Sci 16(1):2099–2116
Vlastou E, Gazouli M, Ploussi A, Platoni K, Efstathopoulos EP (2017) Nanoparticles: nanotoxicity aspects. In Journal of Physics: Conference Series, vol. 931, no. 1. IOP Publishing, p 012020
Huang K-S et al (2016) Recent advances in antimicrobial polymers: a mini-review. Int J Mol Sci 17(9):1578
Yang H et al (2009) Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol 29(1):69–78
Zhang H-Z et al (2010) Preparation of folate-modified pullulan acetate nanoparticles for tumor-targeted drug delivery. Drug Delivery 17(1):48–57
Lu F et al (2009) Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small 5(12):1408–1413
Chan JM et al (2010) Polymeric nanoparticles for drug delivery. Cancer Nanotechnology. Springer, pp 163–175
Cho K et al (2008) Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res 14(5):1310–1316
Prabhu RH, Patravale VB, Joshi MD (2015) Polymeric nanoparticles for targeted treatment in oncology: current insights. Int J Nanomed 10:1001
Alibolandi M et al (2015) In vitro and in vivo evaluation of therapy targeting epithelial-cell adhesion-molecule aptamers for non-small cell lung cancer. J Control Release 209:88–100
Grabowski N et al (2015) Surface coating mediates the toxicity of polymeric nanoparticles towards human-like macrophages. Int J Pharm 482(1–2):75–83
Zielińska A, Carreiró F, Oliveira AM, Neves A, Pires B, Venkatesh DN, Durazzo A, Lucarini M, Eder P, Silva AM, Santini A, Souto EB (2020) Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules 25(16):3731
Silva AM, Alvarado HL, Abrego G, Martins-Gomes C, Garduño-Ramirez ML, García ML, Calpena AC, Souto EB (2019) In vitro cytotoxicity of Oleanolic/Ursolic acids-loaded in plga nanoparticles in different cell lines. Pharmaceutics 11(8):362
Yu T, Malugin A, Ghandehari H (2011) Impact of silica nanoparticle design on cellular toxicity and hemolytic activity. ACS Nano 5(7):5717–5728
Tsai C-P et al (2009) Monoclonal antibody-functionalized mesoporous silica nanoparticles (MSN) for selective targeting breast cancer cells. J Mater Chem 19(32):5737–5743
Pasqua L et al (2009) Recent development, applications, and perspectives of mesoporous silica particles in medicine and biotechnology. Curr Med Chem 16(23):3054–3063
Hnizdo E, Vallyathan V (2003) Chronic obstructive pulmonary disease due to occupational exposure to silica dust: a review of epidemiological and pathological evidence. Occup Environ Med 60(4):237–243
Hao N, Li L, Tang F (2017) Roles of particle size, shape and surface chemistry of mesoporous silica nanomaterials on biological systems. Int Mater Rev 62(2):57–77
Manzano M, Vallet-Regí M (2020) Mesoporous silica nanoparticles for drug delivery. Adv Func Mater 30(2):1902634
Alyassin Y et al (2020) Application of mesoporous silica nanoparticles as drug delivery carriers for chemotherapeutic agents. Drug Discovery Today 25(8):1513–1520
Docter D et al (2014) The protein corona protects against size-and dose-dependent toxicity of amorphous silica nanoparticles. Beilstein J Nanotechnol 5(1):1380–1392
Patel KD, Singh RK, Kim H-W (2019) Carbon-based nanomaterials as an emerging platform for theranostics. Mater Horiz 6(3):434–469
Crisan L et al (2020) Carbon-based nanomaterials as scaffolds in bone regeneration. Part Sci Technol 38(8):912–921
Sajjadi M, Nasrollahzadeh M, Jaleh B, Soufi GJ, Iravani S (2021) Carbon-based nanomaterials for targeted cancer nanotherapy: recent trends and future prospects. J Drug Target 29(7):716–741
Mahor A et al (2021) Carbon-based nanomaterials for delivery of biologicals and therapeutics: A cutting-edge technology. C. 7(1):19
Serrano-Aroca Á, et al (2021) Carbon-based nanomaterials: promising antiviral agents to combat COVID-19 in the microbial-resistant era. ACS nano,
Cui D et al (2005) Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol Lett 155(1):73–85
Khashan KS et al (2020) Anticancer activity and toxicity of carbon nanoparticles produced by pulsed laser ablation of graphite in water. Advances in Natural Sciences: Nanoscience and Nanotechnology 11(3):035010
Gholipourmalekabadi M et al (2017) Targeted drug delivery based on gold nanoparticle derivatives. Curr Pharm Des 23(20):2918–2929
Panahi Y et al (2017) Preparation, surface properties, and therapeutic applications of gold nanoparticles in biomedicine. Drug research 11(02):77–87
Huang X et al (2008) Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci 23(3):217
Takeuchi I et al (2017) Biodistribution and excretion of colloidal gold nanoparticles after intravenous injection: effects of particle size. Bio-Med Mater Eng 28(3):315–323
Tsai C-Y et al (2004) A biological strategy for fabrication of Au/EGFP nanoparticle conjugates retaining bioactivity. Nano Lett 4(7):1209–1212
Thomas M, Klibanov AM (2003) Conjugation to gold nanoparticles enhances polyethylenimine’s transfer of plasmid DNA into mammalian cells. Proc Natl Acad Sci 100(16):9138–9143
Zhang X-D et al (2012) Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials 33(27):6408–6419
Lewinski N, Colvin V, Drezek R (2008) Cytotoxicity of nanoparticles. Small 4(1):26–49
Mironava T et al (2010) Gold nanoparticles cellular toxicity and recovery: effect of size, concentration and exposure time. Nanotoxicology 4(1):120–137
Pan Y et al (2007) Size-dependent cytotoxicity of gold nanoparticles. Small 3(11):1941–1949
Schmid G (2008) The relevance of shape and size of Au55 clusters. Chem Soc Rev 37(9):1909–1930
Woźniak A et al (2017) Size and shape-dependent cytotoxicity profile of gold nanoparticles for biomedical applications. J Mater Sci - Mater Med 28(6):92
Li X et al (2018) The systematic evaluation of size-dependent toxicity and multi-time biodistribution of gold nanoparticles. Colloids Surf, B 167:260–266
Enea M et al (2021) Cellular uptake and toxicity of gold nanoparticles on two distinct hepatic cell models. Toxicol In Vitro 70:105046
Cox A et al (2017) Reprint of: silver and titanium dioxide nanoparticle toxicity in plants: a review of current research. Plant Physiol Biochem 110:33–49
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27(1):76–83
Locatelli E et al (2014) Targeted delivery of silver nanoparticles and alisertib: in vitro and in vivo synergistic effect against glioblastoma. Nanomedicine 9(6):839–849
Rafique M et al (2017) A review on green synthesis of silver nanoparticles and their applications. Artif Cells Nanomed Biotechnol 45(7):1272–1291
Park MV et al (2011) The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 32(36):9810–9817
Teodoro JS et al (2011) Assessment of the toxicity of silver nanoparticles in vitro: a mitochondrial perspective. Toxicol In Vitro 25(3):664–670
Feng QL et al (2000) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 52(4):662–668
Li L et al (2021) Silver nanoparticles and silver ions cause inflammatory response through induction of cell necrosis and the release of mitochondria in vivo and in vitro. Cell Biol Toxicol 37(2):177–191
Kumari M et al (2017) Tailoring shape and size of biogenic silver nanoparticles to enhance antimicrobial efficacy against MDR bacteria. Microb Pathog 105:346–355
Makama S et al (2017) Effects of systematic variation in size and surface coating of silver nanoparticles on their in vitro toxicity to macrophage RAW 264.7 cells. Toxicol Sci. 162(1):79–88
Skandalis N et al (2017) The effect of silver nanoparticles size, produced using plant extract from Arbutus unedo, on their antibacterial efficacy. Nanomaterials 7(7):178
Andreoli C, Prota V, De Angelis I, Facchini E, Zijno A, Meccia E, Barletta B, Butteroni C, Corinti S, Chatgilialoglu C, Krokidis MG, Masi A, Condello M, Meschini S, Di Felice G, Barone F (2021) A harmonized and standardized in vitro approach produces reliable results on silver nanoparticles toxicity in different cell lines. J Appl Toxicol 41(12):1980–1997
Coviello V, Forrer D, Amendola V (2022) Recent developments in plasmonic alloy nanoparticles: synthesis, modelling, properties and applications. Chemphyschem 23(21):e202200136
Qiu GY, Ng SP, Wu CML (2018) Bimetallic Au-Ag alloy nanoislands for highly sensitive localized surface plasmon resonance biosensing. Sensors Actuators B-Chem 265:459–467
Funding
Part of the research reported in this paper was supported by the National Institute of Dental & Craniofacial Research of the National Institutes of Health under award numbers R15DE027533, R56 DE029191, and 3R15DE027533-01A1W1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abbasi, R., Shineh, G., Mobaraki, M. et al. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: a review. J Nanopart Res 25, 43 (2023). https://doi.org/10.1007/s11051-023-05690-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05690-w