Abstract
Cultivating crops often presents numerous challenges, including resource loss such as water, fertilizers, and pesticides, as well as the spread and escalation of infections. Nanotechnology offers promising solutions to enhance plant immunity and resolve agricultural issues. In this study, in order to prevent Fusarium-wilt disease in eggplants, we concentrated on the simple manufacture of colloidal ferric oxide nanoparticles (Fe2O3 NPs) as a promising nanofertilizers. To evaluate the effectiveness of systemic resistance (SR) development, we evaluated markers of metabolic resistance, photosynthetic pigments, plant protection, and disease index (DI). Positively, Fe2O3 NPs exhibit significant antifungal activity against Fusarium oxysporum. However, when applied at a concentration of 20 µg/mL, Fe2O3 NPs proved to be the most effective treatment, reducing the percent disease index (PDI) from 82.5% in infected control plants to 22.5%. Similar results were observed with a concentration of 10 µg/mL Fe2O3 NPs. In both healthy and diseaseed plants, Fe2O3 NP treatments also showed beneficial effects on the activity of antioxidant enzymes, osmolytes, and photosynthetic pigments. Notably, compared to untreated Fusarium-infected plants, the application of Fe2O3 NPs at a concentration of 20 µg/mL significantly increased the levels of osmolyte, comprising soluble sugar, proline, and soluble protein, by 32.88%, 47.09%, and 31.34%, respectively. Furthermore, in both healthy and diseased eggplants, Fe2O3 NPs at a concentration of 20 µg/mL increased the levels of photosynthetic pigments, osmolytes, peroxidase, polyphenol oxidase, catalase, and superoxide dismutase enzymes. Overall, our research findings indicates that Fe2O3 NPs can successfully decreased the harmful effects that F. oxysporum causes to infected eggplants. With their promising therapeutic potential, these nanoparticles provide a secure and effective substitute for chemical fungicides in the management of Fusarium wilt disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to climate change, the agricultural industry is currently dealing with a number of difficulties, such as the growing demand for food and agricultural goods and the declining amount of arable land available which seems as a research gaps. In order to attain both agricultural and economic stability, it is imperative that agricultural growth be promoted [1]. Within this framework, nanotechnology is essential for creating novel approaches to tackle a range of agricultural issues which confirms the significance of our research [2].
Pesticides and fertilisers are important elements that have a big impact on the productivity and growth of crops like eggplants. On the other hand, conventional chemical pesticides and fertilisers can harm the environment and make sustainable agriculture more difficult [3]. Moreover, the agricultural economy is financially impacted by the exorbitant expense of buying chemical fertilisers in bulk. Therefore, exploring alternatives to traditional chemical fertilizers, such as nano-fertilizers, is highly recommended [4].
To enhance agricultural productivity, it is crucial to improve soil properties through the application of therapeutic nutrients and the implementation of safe and effective strategies that enhance plant resistance and growth [5, 6]. The use of therapeutic nutrients to boost plant immunity and development plays a vital role in achieving this goal and improving agricultural productivity [7].
The risk presented by soil pathogens, notably in the case of the fusarial wilt disease brought on by Fusarium oxysporum, is one of the major concerns in agricultureThis pathogen significantly reduces crop yields, posing a threat to food security and leading to a decline in both the quantity and quality of crops [8]. Fungal wilt, a disease that affects the plant’s vascular system, poses a significant challenge in terms of chemical treatment [9]. This disease becomes particularly dangerous during hot planting seasons [10]. Furthermore, the combination of malnourished crops, diseases, and the impact of climate change exacerbates the problem by reducing crop quality and triggering toxin release [11,12,13].
Due to its delicious fruits, the seasonal plant known as Solanum melongena is grown all over the world, including in Egypt, where it is very important economically [14]. Plant nutrition and fertilisation are essential agricultural practises. Both organic and artificial fertilisers and nutrients are essential for giving plants the nourishment they need for strong development and higher yields. Depending on their source and degree of purity, fertilisers can be generically classified as organic (natural) or chemical (synthetic) [15]. Researchers are becoming more interested in using bio-fertilizers and therapeutic nutrients as a result of the finding that plants treated with natural biostimulants and nano-fertilizers show significant physiological immunity [16]. Nanotechnology plays a crucial role in the field of agriculture, offering a wide range of applications and benefits. It can be utilized as therapeutic nutrition to enhance crop yield and improve plant resistance to diseases [17]. Through nanotechnology, plants can optimize their water, insecticide, and fertilizer usage more efficiently [4, 18].
Nano-fertilizers serve as an efficient alternative to traditional fertilizers in fertilization programs. They offer several advantages, including increased agricultural yields, disease reduction, and enhanced plant immunity, while requiring smaller quantities and exhibiting greater environmental stability [19]. Iron compounds, in addition to acting as catalysts in the photosynthesis process, also play a role in RNA production and enzyme function.
According to the literature review, metal oxide NPs, such as ZnO NPs were found to be effective at reducing a variety of abiotic stresses when applied to plants. The efficacy of just one treatment of Bacillus fortis IAGS 223 and ZnO NPs was assessed for reducing Cd (75 mg kg−1)-induced phytotoxicity in Cucumis melo plants. However, Mubeen et al. [20] demonstrate that calcium nanoparticles coated with benzenedicarboxylic acid serve as a novel strategy to reduce coupled stress of DDT and cadmium in Brassica alboglabra by modifying bioaccumulation, antioxidative machinery, and osmoregulators.
Additionally, Ahmad et al. [21] demonstrates that Si and Fe NPs, when used in combination, have a significant ability to reduce Cd-induced phytotoxicity by reducing Cd absorption and enhancing plant development characteristics. Finally, the effect of MgO NP in the reduction of lead-induced stress in the Daucus carota is explained by Faiz et al. [22].
This article focuses on the use of environmentally friendly and innovative colloidal Fe2O3 NPs, prepared without the use of solvents, synthetic materials, or any harmful substances. These NPs, produced in the form of a chemically balanced emulsion, are easily absorbed by plants and leave no environmental residues. They enhance plant resistance against fusarial wilt disease, improve photosynthetic pigments, and support immune responses. Colloidal Fe2O3 NPs are considered therapeutic nutrients due to their safety, chemical balance, and low cost, therefore, is it required to run the present investigation.
Materials and Methods
Synthesis of Colloidal Ferric Oxide NPs
Hydrothermal processing is a novel green synthesis technique that was used to produce ferric oxide. Ferric nitrate was directly converted to ferric oxide to produce ferric oxide particles [23, 24]. For the synthesis of nanoparticles, super-critical fluids (ScF) were used [25]. Super-critical water (ScW) needs extreme conditions (Tc 374.2 °C, Pc 220.5 bar). Additionally, 1 × 10−14 mol/L is the water dissociation constant (Kw) [26, 27]. ScW is a suitable solvent for processing free radicals because, as it approaches the critical point, the water dissociation constant increases by around three orders of magnitude [28]. The properties of water also alter as it reaches 24 MPa in temperature. For the generation of NP, the elevated (OH) ion concentrations at the critical point might be used. Nanoparticles can be produced (Eq. 2) by hydrolyzing metal salts (Eq. 1) and then drying them [26].
In the hydrothermal processing method, salt and supercritical water (ScW) are mixed with cold metal. Inside the reactor, where the two fluids converge (R), nanoparticles (NPs) are produced. It is widely recognized that monodispersed particles are generated, as the evolution of each particle after nucleation is identical. For more detailed information on the production of Fe2O3 NPs, please refer to the provided references [23, 24, 29,30,31].
Characterization of Colloidal Ferric Oxide NPs
The colloidal ferric oxide nanoparticles’ surface shape and external appearance were investigated with a scanning electron microscope (SEM), more precisely the Japanese JEOL JSM-5600 LV type. To analyse the elemental composition of the deposited iron, an EDAX detector (also from JEOL JSM-5600 LV) was used. An investigation into the crystalline structure of the colloidal ferric oxide nanoparticles (CFONPs) was conducted by X-ray diffraction (XRD) examination, utilising the Japanese Shimadzu XRD-6000 machinery.
The DLS-PSS-NICOMP 380-ZLS particles sized system from St. Barbara, California, USA was used for a dynamic light scattering (DLS) investigation to ascertain the average particle size distribution of the generated ferric oxide nanoparticles. 200 µL of nanoparticle samples were moved to a short-term, tiny cuvette. After letting the samples settle for 2.0 min at 25.0 ± 2 °C, five measurements were made. The dimensions and morphology of the synthesised ferric oxide nanoparticles were examined using a high-resolution transmission electron microscope (HR-TEM). In particular, the Japan-made JEM2100 model from Jeol was employed. Drop-coated nanoparticle samples from the HRTEM were applied to carbon-coated TEM grids for the HR-TEM investigations. The samples were then allowed to dry in an incubator at 37.0 ± 2 °C.
In Vitro Assessment of Antifungal Activity
The antifungal capability of the colloidal nano-fertilizers (Fe2O3 NPs) was investigated using the agar well diffusion technique, as reported by the recent results [32] in their study. On potato dextrose agar (PDA) medium that had been sterilised and solidified, the fungal inoculum was systematically grown. Three distinct doses of Fe2O3 NPs in 50 µL were applied to duplicate 5 mm diameter discs with care at the same time. The plates were chilled for two hours in order to promote the diffusion of bioactive secondary metabolites. After being incubated at 25 °C for 7 days, the culture plates were inspected in order to identify and quantify the inhibitory zones.
In Vivo Assessment Efficacy of Fe2O3 NPs on Eggplant
Source of Fusarium oxysporum f. sp. Lycopersici
The strain F. oxysporum f. sp. Lycopersici RCMB 008001 was supplied by Al-Azhar University’s Regional Centre for Mycology. The pathogenicity test then gave confirmation, according to Hibar et al. [33]. The inoculum of the pathogenic fungus Fusarium oxysporum was established, according to Buttner et al. [34].
Experimental Design
We bought identical, symptom-free four-week-old aubergine (Solanum elongena) seedlings from the Agricultural Research Centre in Giza, Egypt. The seedlings were placed in 30 × 30 cm plastic pots containing 5 kg of soil, a mixture of sand and clay (1:3 W/W), inside plastic greenhouses located inside the Department of Botany and Microbiology’s garden at Al-Azhar University in Cairo, Egypt. The seedlings were left to stand for five days prior to being treated in any way. After that, a pathogenic fungal inoculum (10 mL/mL Fusarium oxysporum) was injected into the soil (106).
One week following the fungal infection, a dosage of 10 µg/mL and 20 µg/mL of Fe2O3 NPs was administered three times, once per week (during the time leading up to and including flowering). Ten replicates of each of the following were used: one healthy control, two infected controls, three healthy pots treated with Fe2O3 NPs (10 µg/mL), four infected pots treated with Fe2O3 NPs (10 µg/ml), five infected pots treated with Fe2O3 NPs (20 µg/mL), and six infected pots treated with Fe2O3 NPs (20 µg/mL). Sixty-day-old plants were meticulously pulled up and evaluated according to the several standards outlined below.
Disease Symptoms and Disease Index
According to Farrag et al. [5], illness symptoms were noticed and noted 15 days after infection, and after 60 days, the disease index and the protection ratio were assessed. Note that the following procedure was used to compute the PDI using a five grade system:
where nt is the total number of plants examined and n1–n4 is the number of plants in the relevant classes. Additionally, the following formula was used to determine Percent Protection (P%):
where PDI in infected treated plants is represented by B and PDI in infected control plants by A.
Biochemical Resistance Indicators in Plant
The pigments used in photosynthesis were measured, according to Vernon et al. [35]. The amount of soluble carbohydrates in the dried shoot was determined using the procedure outlined by Irigoyen et al. [36]. The total protein was calculated using the methodology of Lowry et al. [37]. Proline content in the dry shoot was evaluated in accordance with Pinior et al. [38], enzyme activity was calculated in accordance with the following references [39,40,41,42], and According to Diaz and Martin’s [43] instructions, the overall shoot amount of phenol was measured.
Statistical analysis
Duncan’s multiple ranges and the least significant difference (LSD), which are calculated by specialized software (SPSS version 15), are used to statistically examine the data.
Results and Discussion
Characterization of Colloidal Nano-Fertilizers
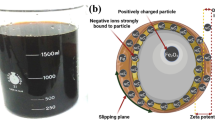
The synthetic Fe2O3 NPs were produced by hydrothermal synthesis. The lab-made colloidal particles had a deep crimson colour. The particles did not flocculate during this period. It was discovered that the stabilisation mechanism was electrostatic stabilisation using nitrate ions (Fig. 1). The colloidal particles’ Zeta potential was + 38.5 mV (Fig. S1). The electrostatic stability of colloidal ferric oxide particles was supported by zeta potential.
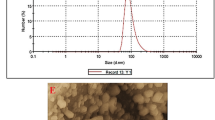
Spherical monodispersed Fe2O3 NPs with an average particle size of 5 ± 1.0 nm are visible in HRTEM micrographs (Fig. 2a). The homogeneously sized, superior monodispersed particles were confirmed by HRTEM micrographs which confirms their appearance as spherical or rounded particles. Particle size distribution analysis using DLS revealed that the average Fe2O3 NPs particle size distribution was 7.5 nm by 100%, as indicated in Fig. 2b. It was observed that the particle size distribution computed by DLS analysis was larger than the average particle size ascertained by HRTEM images. The hydrodynamic radius surrounded by the water particles and formed around the produced colloidal Fe2O3 NPs was measured using the DLS technique for the large sizes of the capped Fe2O3 NPs [44].
The diffractogram of the hematite (Fe2O3) sample showed a high degree of organisation. An X-ray diffraction (XRD) system was used to examine the crystal structure and incorporation status of the Fe2O3 NPs (Fig. 3). The acquired XRD patterns matched the recognised Fe2O3 standard (JCPDS No. 33-0664) exactly. The typical cubic spinel composition of Fe2O3 was indicated by distinct peaks found at 24.12°, 33.58°, 35.35°, 40.78°, 49.59°, 54.22°, 57.41°, and 65.62° [45]. There were no unknown crystalline phases or impurities found in the Fe2O3 NPs.
The crystal structure of the synthesized Fe2O3 NPs was found to have a hexagonal arrangement with a rhombohedral center, which corresponds to the overall composition of Fe2O3 (R3c space system) [46,47,48,49,50]. The most prominent diffraction peak observed at around 35.35° suggests that the (110) facets are the predominant crystal structure in Fe2O3, with a crystal size of 5.95 nm as determined by the Williamson–Hall (W–H) equation [51].
The produced Fe2O3 NPs’ exterior shape, purity, and elemental composition were investigated, as shown in Fig. 4. As illustrated in Fig. 4a, SEM examination demonstrated that the generated Fe2O3 NPs possessed a semi-spherical shape and a uniform distribution.
The created Fe2O3 NPs were extremely pure, as demonstrated by the EDX evaluation, which also revealed that the carbon atom (C) corresponded to the container utilized for the SEM imaging operation, as shown in Fig. 4b. Conversely, in SEM micrographs, dry Fe2O3 NPs exhibited a noticeable tendency to decrease in quantity and surface area. One feature of aggregates is a notable reduction in the surface area of NPs [52, 53].
In Vitro Antifungal Potential
According to a study conducted by Koka et al. [54], Fe2O3 NPs have shown significant anti-mycotic efficacy against rot fungal infections. Building on these discoveries, the current work seeks to determine whether Fe2O3 NPs can be used to control the fungal pathogen F. oxysporum. It was determined that Fe2O3 NPs had antifungal activity by applying the diffusion method as explained by El-Batal et al. [55]. Nonetheless, the study’s findings show that F. oxysporum showed resistance to Fe2O3 NPs’ antifungal effects.
Control of Wilt Disease Caused by F. oxysporum Using Ferric Oxide NPs
A decrease in disease severity indices and an increase in the percentage of protection against the pathogen (Fe2O3 NPs) are two important markers of resistance in treated plants. The severity of F. oxysporum wilt disease in eggplant, which reached 82.5%, is shown in Table 1. This finding is consistent with other research [56, 57] that reported on the high virulence of Fusarium oxysporum.
On the other hand, as Table 1 shows, applying Fe3O4 NPs as a fertilizer to sick plants reduced the degree of severity of the disease and improved protection from Fusarium wilt. Comparing the untreated infected plants (82.50 PDI%), the most effective treatment, Fe2O3 NPs, significantly reduced the percent disease indexes to 22.5% and provided highly efficient protection against Fusarium-wilt disease, with a protection rate of 72.70%. The second-best treatment, 10 µg/mL, resulted in a PDI reduction to 32.5% and provided protection by 60.60%.
These results are in line with research by Ashraf et al. [58], which found that applying 10 µg/mL of Fe3O4 NPs to infected plants improved plant growth and lessened the severity of Fusarium wilt disease. The work of Chakraborty et al. [59], which emphasises the critical role of iron in the process of photoconversion, can be used to explain our results. Ferredoxin and cytochrome are two important proteins that are involved in the reactions of this process and require iron as a component. Iron is also essential for the synthesis of chlorophyll and is important for respiration.
Iron is necessary for the process of converting energy from other substances, such as carbohydrates, into adenosine triphosphate (ATP). Enzymes like catalase and peroxidases, which are vital for shielding cells from oxidation and may enhance resistance to disease, require iron as a component [60]. Plants have evolved a defence mechanism that allows them to trigger oxidative bursts, which lead to the accumulation of iron concentrations and the production of toxic free radicals. This system aids in lowering the danger of infections [58]. The role that iron plays in these processes emphasises the importance of iron in plant physiology and disease defence mechanisms.
Photosynthetic Pigments
The marked reduction in photosynthetic pigment levels indicated the presence of a pathological infestation. Chlorophyll a, b, and carotenoids were significantly reduced in infected plants compared to healthy control plants by 56.80%, 49.89%, and 38.09%, respectively (Fig. 5). This severe deficiency in photosynthetic pigments can be attributed to the oxidative burst within the cells and the accumulation of reactive oxygen species (ROS). These ROS can cause damage to chlorophyll, resulting in the plant’s failure to effectively capture light, leading to a decrease or cessation of photosynthesis [57, 61]. The decrease in photosynthetic pigments serves as an important indicator of the detrimental effects of the fungal infection on the plant’s ability to carry out efficient photosynthesis, which is essential for its growth and development. The results show that two colloidal Fe2O3 NP concentrations’ effects on both healthy and sick plant photosynthetic pigments were investigated. However, relative to control infected plants, plants with infection and subjected to colloidal Fe2O3 NPs (10 and 20 µg/mL) shown a considerable increase. The most efficient way to increase the amounts of chlorophyll a, b, and carotenoid in infected plants by 91.58%, 30.8%, and 182.0%, respectively, was to apply Fe2O3 NPs (20 µg/mL).
The outcomes also demonstrated that healthy plants exposed to colloidal Fe2O3 NPs exhibited a considerable rise in chlorophyll a, b, and carotenoid levels in comparison to control healthy plants. This increase could be explained by increased cell size and/or number, stomatal conductance, and/or transpiration rate [62, 63].
This increase might be approved to enhance stomatal conductance, transpiration rate, and/or cell size and number [64]. It may also be because fertilization has been reported to cause plant defense reactions [65, 66], furthermore, it may activate NADPH oxidase, resulting in the formation of H2O2 or antioxidant action. Colloidal Fe2O3 NPs could therefore initiate ROS scavenging mechanisms in plants [62]. It’s interesting to note that iron is a micronutrient that is essentially important for the production of chlorophyll, cell respiration, chemical nitrate and sulfate reduction, nitrogen assimilation, and promotion of systemic resistance to infections and symptoms of malnutrition [63, 67].
As seen in Fig. 6, the chloroplasts, which are where the photosynthetic activity takes place, include a core amount of iron. Foliar fertilization is regarded as a beneficial nutrient that keeps plants healthy. Chelates and inorganic iron salts are two chemical forms of iron fertilization [68].
Biochemical Resistance Indicators in Eggplant Seedlings
Figure 7 illustrates how the soluble carbohydrate and protein levels of fusarium-infected plants decreased by 51.5% and 45.0%, respectively. The results indicated that infection with F. oxysporum was destructive as it caused the failure of light capture and photosynthesis, which consequently reduced soluble carbohydrates and soluble protein in eggplant seedlings, similar to previous studies [57, 69].
Carbohydrates play a crucial role in the plant’s response to various stresses, acting as signaling molecules that activate specific pathways for nutrient and metabolite signaling. This activation leads to significant alterations in gene expression, often involving hormonal pathways [70]. Interestingly, the results of the study revealed that foliar spraying of Fe2O3 NPs stimulated the formation of carbohydrates and proteins in both healthy and infected plants, compared to untreated plants. In the case of infected plants, the highest increase in soluble carbohydrate and soluble protein levels was recorded with the application of Fe2O3 NPs at a concentration of 20 µg/mL, resulting in a respective increase of 32.88% and 47.09% (Fig. 7).
The application of Fe2O3 NPs increased the production of glycolysis-related enzymes, leading to an increase in the levels of soluble sugars and soluble proteins [71]. Conversely, the presence of induced pathogenicity-related proteins serves as strong evidence for the induction of systemic plant immunity. Protein accumulation in plants plays a crucial role in localizing infection by pathogens [72, 73].
According to the study’s findings, infected plants had greater levels of total phenols and free proline than healthy control plants did. Figure 8 shows the considerable rise in free proline and total phenol levels in fusarium-infected plants of 29.33% and 22.67%, respectively. As a defence against free radicals that might damage photosynthetic pigments and interfere with photosynthesis, plants accumulate free proline [74, 75]. This rise in free proline levels in reaction to Fusarium infection may be the plant’s attempt to lessen the pathogen’s oxidative stress. Similarly, the elevation in total phenols in infected plants indicates their involvement in the plant’s defense response against the fungal infection. Phenolic compounds have been known to possess antimicrobial properties and can contribute to the reinforcement of the plant’s immune system [72, 73]. Overall, the increase in free proline and total phenols in infected plants signifies their role in the plant’s defense against Fusarium infection and highlights the plant’s activation of various mechanisms to combat the stress caused by the pathogen. In plants treated with Fe2O3 NPs, including both healthy and infected plants, the content of free proline and total phenols was elevated compared to absolute control plants. This increase suggests an enhancement in plant resistance against disease, as free proline and phenols play a direct role in protecting the plant from oxidative damage and scavenging free radicals [62].
In particular, the application of Fe2O3 NPs at a concentration of 20 µg/mL resulted in the highest recorded increase in the levels of free proline and total phenols in infected plants treated with Fe2O3 NPs. In comparison to plants that were infected, this treatment produced increases of 31.34% and 30.1%, respectively (Fig. 8). Proline’s function in osmoregulation and scavenging reactive oxygen species (ROS) is responsible for the increase in proline content [76]. In challenging circumstances, proline accumulation helps preserve cellular homeostasis and acts as a defence against oxidative stress.
Conversely, plants use the accumulation of phenolic compounds as an adaptive strategy to fight disease [77, 78]. Because of their antimicrobial qualities, phenols support the immune system of the plant. These results are in line with a number of earlier investigations that found that infected plants had significantly higher levels of proline and phenols than healthy plants [57, 62, 79, 80]. The fact that Fe2O3 NPs treatment causes an increase in free proline and total phenols provides additional evidence for these compounds’ function in strengthening plant resistance to disease.
Oxidative Enzymes Activity
This study measured the enzymatic activity of antioxidant enzymes to assess the induction of systemic resistance in eggplants. One of the most significant ways that plants respond to pathological stresses is by increasing the activity of antioxidant enzymes, which scavenge reactive oxygen species (ROS) and shield cells from oxidative stress. Peroxidase (POD), an antioxidant enzyme, is involved in the process of turning hydrogen peroxide (H2O2) into water [81].
The findings displayed in Figs. 9 and 10 demonstrated a considerable rise in the activity levels of antioxidant enzymes (POD, PPO, CAT, and SOD) when contrasting Fusarium-infected plants to healthy plants. Plants use this increase in enzymatic activity as a defence mechanism to offset the effects of free radicals that the Fusarium infection causes to cells. These results are in line with those of a number of earlier studies, such as References [57, 79, 82].
The application of Fe2O3 NPs at a concentration of 20 µg/mL was found to be the most effective treatment for infected plants, as it led to significantly higher enzymatic activities (POD, PPO, CAT, and SOD) in comparison to untreated infected plants. At a concentration of 10 µg/mL, Fe2O3 NPs proved to be an effective next treatment. According to these results, Fe2O3 NPs may strengthen the enzymatic defence mechanism of infected plants, strengthening their resistance to Fusarium wilt. When plants were challenged with Fe2O3 NPs at a concentration of 20 µg/mL, their POD and PPO activities were higher than those of infected plants. PPO activity was measured at 1.36347 and 1.308 for Fe2O3 NPs (10 µg/mL) and Fe2O3 NPs (20 µg/mL) treatments, respectively, while POD activity was measured at 0.9706 and 0.9314. The POD and PPO activities of infected plants, on the other hand, were lower, with recorded values of 0.7391 and 1.0382, respectively (Fig. 9).
In a similar vein, compared to infected plants, challenged plants showed higher CAT and SOD activity after receiving Fe2O3 NPs (20 µg/mL). For the Fe2O3 NPs (20 µg/mL) treatment, the CAT activity was 1.4933 and the SOD activity was 0.218. CAT and SOD activities, on the other hand, were lower in infected plants; measured values were 1.137 and 0.1553, respectively (Fig. 10). To lower ROS, also and diminish the negative impacts of stress, plants utilize antioxidant enzymes notably SOD and POD. It has been demonstrated that applying nanoparticles, like Fe2O3 NPs, can enhance these plant anti-stress reactions [83, 84].
Our findings support those of Wang et al. [85], who found that when plants are exposed to Fe2O3 NPs, they cause greater oxidative stress and boost antioxidant enzyme activity. This is explained by iron’s role in RNA synthesis and enzyme activity [86].
Metal nanoparticles can act as catalysts and effective agents, and they can facilitate intracellular chemical changes because of their high reactivity [87]. Fe2O3 NPs may play a role in strengthening the plant’s defence mechanisms against oxidative stress brought on by the fungal infection, as evidenced by the observed increase in antioxidant enzyme activities in response to their treatment. Similar to what we found, Fe2O3 NPs enhanced the activity of antioxidant enzymes and positively impacted plant growth in Cucurbita pepo [88]. According to Tripathi et al. [89], iron is necessary for the cell oxide reduction reaction and functions as a co-factor for several antioxidant enzymes, including CAT, SOD, and POD. It also scavenges reactive oxygen species (ROS). The impact of ferric oxide nano-fertilizers on eggplants is finally demonstrated in Fig. 11, along with a comparison to the positive control (healthy eggplant) and negative control (Fusarium-infected eggplant without nano-treatment).
Works performed by Akram et al. [90], and Anjum et al. [91], provide the scientific grounds of plant resistance against Fusarium fungal pathogen.
In order to achieve a comprehensive understanding of the generated defense mechanisms in tomato plants caused by B. subtilis IAGS174 against Fusarium wilt disease, Akram et al. [90] undertook biochemical, histological, cytochemical, and molecular studies. The re-modulation of lignin production in tomato plants according to the effect of a bacterial inducer is the study’s most startling discovery.
The goal of Akram et al. [90] is to provide a thorough analysis of the metabolomics alterations that occur in tomato plants during disease stress and to identify key pathways and features that this plant develops in order to successfully defend itself against Fusarium wilt assault. In order to study alterations in plant histology and metabolomics reprogramming, tomato plants had contact with F. oxysporum within both suitable and unsuitable interactions. This knowledge will be helpful in plant breeding programs for resistance.
Conclusion
With an average size of 5 nm, the hydrothermal method effectively created stable colloidal Fe2O3 NPs. The most prominent diffraction peak at 35.35° indicated that the dominant crystal structure of Fe2O3 NPs still had (110) facets and a crystal size of 5.95 nm, as per the Williamson-Hall (W-H) equation. The Fe2O3 NPs’ semi-spherical shape and uniform distribution were verified by SEM analysis. To stop the wilt disease, these Fe2O3 NPs were then given to the eggplants as a kind of nanofertilizer. At a concentration of 20 µg/mL, Fe2O3 NPs demonstrated the highest efficacy among the various treatments, reducing the percent disease index by 22.5% and offering strong disease protection by 72.7%. Infected plants had considerably lower amounts of chlorophyll a, b, and carotenoids than healthy control plants, with differences of 56.80%, 49.89%, and 38.09%, respectively. The most successful method, however, was to treat the infected plants with Fe2O3 NPs at a concentration of 20 µg/mL, which resulted in increases in the levels of carotenoids, chlorophyll a, and b by 91.58%, 30.8%, and 182.0%, respectively. Additionally, when compared to untreated Fusarium-infected plants, Fe2O3 NPs (20 µg/mL) showed the highest documented increase in the levels of osmolytes (soluble sugar, proline, and soluble protein), with increases of 32.88%, 47.09%, and 31.34%, respectively. When compared to untreated infected plants, Fe2O3 NPs at a concentration of 20 µg/mL showed the greatest enhancement in activities of POD, PPO, CAT, and SOD in terms of enzymatic activities, which followed by Fe2O3 at a concentration of 10 µg/mL.
Data Availability
The datasets supporting the conclusions of this article are included within the article and its additional files.
References
M. Mumtaz, J. A. P. de Oliveira, and S. H. Ali (2019). Climate Change Impacts and Adaptation in Agricultural Sector: The Case of Local Responses in Punjab, Pakistan, Climate Change and Agriculture. IntechOpen London, UK.
A. H. Hashem, A. M. Abdelaziz, A. A. Askar, H. M. Fouda, A. M. Khalil, K. A. Abd-Elsalam, and M. M. Khaleil (2021). J. Fungi 7, 195.
Y. Shang, M. K. Hasan, G. J. Ahammed, M. Li, H. Yin, and J. Zhou (2019). Molecules 24, 2558.
I. O. Adisa, V. L. R. Pullagurala, J. R. Peralta-Videa, C. O. Dimkpa, W. H. Elmer, J. L. Gardea-Torresdey, and J. C. White (2019). Environ. Sci. 6, 2002.
A. Farrag, M. Attia, A. Younis, and A. Abd Elaziz (2017). Al Azhar Bull. Sci. 9, 311.
A. M. Khalil, A. F. Ahmed, E. E. Mahmoud, and A. M. Abdelaziz (2015). Afr. J. Mycol. Biotechnol. 20, 23.
D. Bhardwaj, M. W. Ansari, R. K. Sahoo, and N. Tuteja (2014). Microbial Cell Factories 13, 1.
K. Abada and K. E. Eid (2014). Am. J. Life Sci. 2, 1.
A. Jain, S. Sarsaiya, Q. Wu, Y. Lu, and J. Shi (2019). Bioengineered 10, 409.
J. Gressel, A. Hanafi, G. Head, W. Marasas, A. B. Obilana, J. Ochanda, T. Souissi, and G. Tzotzos (2004). Crop Protect. 23, 661.
A. A. Alrashidi, H. A. S. Alhaithloul, M. H. Soliman, M. S. Attia, S. M. Elsayed, A. M. Sadek, and M. A. Fakhr (2022). Notulae Botanicae Horti Agrobotanici Cluj-Napoca 50, 12614.
A. Shah, M. Nazari, M. Antar, L. A. Msimbira, J. Naamala, D. Lyu, M. Rabileh, J. Zajonc, and D. L. Smith (2021). Front. Sustain. Food Syst. 5.
A. A. Shah, S. Aslam, M. Akbar, A. Ahmad, W. U. Khan, N. A. Yasin, B. Ali, M. Rizwan, and S. Ali (2021). Plant Physiol. Biochem. 158, 1.
H. A. S. Alhaithloul, M. S. Attia, and M. A. Abdein (2019). Int. J. Bot. Stud 4, 55.
H.-L. Xu (2001). J. Crop Prod. 3, 183.
S. K. Yadav, S. Lal, S. Yadav, J. Laxman, B. Verma, M. Sushma, R. Choudhary, P. Singh, S. Singh, and V. Sharma (2019). Seed Res 47, 99.
M. Rai and A. Ingle (2012). Appl. Microbiol. Biotechnol. 94, 287.
W. Weisany, S. Samadi, N.A.-R. Tahir, J. Amini, and S. Hossaini (2022). Physiol. Mol. Plant Pathol. 122.
P. Pramanik, P. Krishnan, A. Maity, N. Mridha, A. Mukherjee, and V. Rai, Application of Nanotechnology in Agriculture, Environmental Nanotechnology, vol. 4. (Springer, New York, 2020), pp. 317-348.
S. Mubeen, I. Shahzadi, W. Akram, W. Saeed, N. Y. Ahmad, A. Ahmad, A. A. Shah, M. H. Siddiqui, and S. Alamri (2022). Front. Plant Sci. 13.
A. Ahmad, N. A. Yasin, W. U. Khan, W. Akram, R. Wang, A. A. Shah, M. Akba, A. Ali, and T. Wu (2021). Plant Physiol. Biochem. 166, 874.
S. Faiz, N. A. Yasin, W. U. Khan, A. A. Shah, W. Akram, A. Ahmad, A. Ali, N. H. Naveed, and L. Riaz (2022). Int. J. Phytoremediat. 24, 364.
S. Elbasuney, A. Hamed, M. Yehia, S. Ismael, A. Saleh, M. Gobara, M. Mokhtar, and G. S. El-Sayyad (2021). J. Electron. Mater. 50, 6128.
S. Elbasuney, M. Yehia, A. Hamed, S. Ismael, M. Mokhtar, E. Elsaka, M. Gobara, A. Saleh, and G. S. El-Sayyad (2021). J. Mater. Sci. 32, 4185.
P. Savage, S. Gopalan, T. Mizan, and C. Martino (1995). Am. Inst. Chem. Eng. J. 41, 1723.
T. Adschiri, Y. Hakuta, and K. Arai (2000). Ind. Eng. Chem. Res. 39, 4901.
R. Perry and D. Green, Perry’s Chemical Engineer’s Handbook (Graw-Hill Inc., Singapore, 1984).
P. E. Savage (1999). Chem. Rev. 99, 603.
S. Elbasuney, G. S. El-Sayyad, M. Yehia, and S. K. Abdel Aal (2020). J. Mater. Sci. 31, 20805.
S. Elbasuney, G. S. El-Sayyad, S. Ismael, and M. Yehia (2021). J. Inorg. Organomet. Polym. Mater. 31, 559.
S. Elbasuney, M. Yehia, A. Hamed, M. Mokhtar, M. Gobara, A. Saleh, E. Elsaka, and G. S. El-Sayyad (2021). J. Inorg. Organomet. Polym. Mater. 31, 2293.
S. Parveen, A. H. Wani, M. A. Shah, H. S. Devi, M. Y. Bhat, and J. A. Koka (2018). Microbial Pathogen. 115, 287.
K. Hibar, V. Edel-Herman, C. Steinberg, N. Gautheron, M. Daami-Remadi, C. Alabouvette, and M. El Mahjoub (2007). J. Phytopathol. 155, 136.
G. Büttner, B. Pfähler, and B. Märländer (2004). Plant Breed. 123, 158.
L. P. Vernon and B. Ke, Photochemistry of Chlorophyll In Vivo, The Chlorophylls. (Elsevier, Amsterdam, 1966), pp. 569-607.
J. Irigoyen, D. Einerich, and M. Sánchez-Díaz (1992). Physiologia Plantarum 84, 55.
O. H. Lowry, N. J. Rosebrough, A. L. Farr, and R. J. Randall (1951). J. Biol. Chem. 193, 265.
A. Pinior, G. Grunewaldt-Stöcker, H. von Alten, and R. J. Strasser (2005). Mycorrhiza 15, 596.
H. Aebi, Catalase in Vitro. Methods in Enzymology. (Elsevier, Amsterdam, 1984), pp. 121-126.
H. Bergmeyer (1974). Determination with glucose oxidase and peroxidase. Methods of enzymatic analysis, 1205-1215.
S. Marklund and G. Marklund (1974). Eur. J. Biochem. 47, 469.
A. Matta and A. Dimond (1963). Phytopathology 53, 574.
D. H. Diaz and G. C. Martin (1972). J. Am. Soc. Hortic. Sci. 97, 651.
A. Baraka, S. Dickson, M. Gobara, G. S. El-Sayyad, M. Zorainy, M. I. Awaad, H. Hatem, M. M. Kotb, and A. Tawfic (2017). Chem. Pap. 71, 2271.
V. C. Karade, S. B. Parit, V. V. Dawkar, R. S. Devan, R. J. Choudhary, V. V. Kedge, N. V. Pawar, J. H. Kim, and A. D. Chougale (2019). Heliyon 5.
D. E. Fouad, C. Zhang, H. El-Didamony, L. Yingnan, T. D. Mekuria, and A. H. Shah (2019). Results Phys. 12, 1253.
T. Liang, X. Guo, B. Yuan, S. Kong, H. Huang, D. Fu, F. Zhang, J. Xu, and X. Li (2019). Ceram. Int.
M. Sharma (2017). Lab Manual, 1.
M. Tadic, M. Panjan, B. V. Tadic, J. Lazovic, V. Damnjanovic, M. Kopani, and L. Kopanja (2019). J. Electr. Eng. 70, 71.
Q. Z. Zeng, S. Y. Ma, W. X. Jin, H. M. Yang, H. Chen, Q. Ge, and L. Ma (2017). J. Alloys Compds. 705, 427.
A. H. Ashour, A. I. El-Batal, M. I. A. A. Maksoud, G. S. El-Sayyad, S. Labib, E. Abdeltwab, and M. M. El-Okr (2018). Particuology 40, 141.
S. Elbasuney, A. Elsaidy, M. Kassem, H. Tantawy, R. Sadek, and A. Fahd (2018). J. Inorg. Organomet. Polym. Mater. 28, 2231.
S. Elbasuney (2014). Powder Technol. 268, 158.
J. A. Koka, A. H. Wani, and M. Y. Bhat (2019). J. Drug Deliv. Ther. 9, 173.
A. I. El-Batal, G. S. El-Sayyad, F. M. Mosallam, and R. M. Fathy (2020). J. Clust. Sci. 31, 79.
N. Akter, M. R. Islam, M. B. Hossain, M. N. Islam, S. R. Chowdhury, S. Hoque, R. H. Nitol, and R. Tasnin (2021). Am. J. Plant Sci. 12, 1155.
M. S. Attia, H. A. El-Naggar, M. M. Abdel-Daim, and G. S. El-Sayyad (2021). Environ. Sci. Pollut. Res. 28, 35854.
H. Ashraf, T. Anjum, S. Riaz, T. Batool, S. Naseem, and I. S. Ahmad (2021). Sustainable Synthesis of Microwave Assisted IONPs by Using Spinacia Oleracea: Enhances Resistance Against Fungal Wilt Infection by Inducing ROS and Modulating Defense System in Tomato Plants.
J. Liu, S. Chakraborty, P. Hosseinzadeh, Y. Yu, S. Tian, I. Petrik, A. Bhagi, and Y. Lu (2014). Chem. Rev. 114, 4366.
M. B. Chicaiza Lema (2020). Study of metalloproteins involved in nitrogen fixation in sugarcane, Universidad de Investigación de Tecnología Experimental Yachay.
M. I. Ghani, A. Ali, M. J. Atif, M. Ali, B. Amin, M. Anees, and Z. Cheng (2019). Agronomy 9, 89.
S. Elbasuney, G. S. El-Sayyad, M. S. Attia, and A. M. Abdelaziz (2022). J. Inorg. Organomet. Polym. Mater. 32, 4270.
M. Ghorbanpour, A. Movahedi, M. Hatami, K. Kariman, F. Bovand, and M. Shahid (2021). Photosynthetica 59, 570.
P. Ferus, M. Barta, and J. Konôpková (2019). Trees 33, 1179.
B. H. Ownley, K. D. Gwinn, and F. E. Vega (2010). BioControl 55, 113.
J. Poveda, P. Abril-Urias, and C. Escobar (2020). Front. Microbiol. 11, 992.
D. Alidoust and A. Isoda (2013). Acta Physiologiae Plantarum 35, 3365.
J. Abadía, A. Álvarez-Fernández, F. Morales, M. Sanz, and A. Abadía (2001). Int. Symp. Foliar Nutr. Perennial Fruit Plants 594, 115.
L. Mei, L. Hua, X.-L. Su, T. Ying, W.-K. Huang, M. Jie, and X.-L. Jiang (2019). J. Integr. Agric. 18, 607.
I. Couée, C. Sulmon, G. Gouesbet, and A. El Amrani (2006). J. Exp. Bot. 57, 449.
K. Kráľová and J. Jampílek, Metal-and Metalloid-Based Nanofertilizers and Nanopesticides for Advanced Agriculture. Inorganic Nanopesticides and Nanofertilizers: A View from the Mechanisms of Action to Field Applications. (Springer, Cham, 2022), p. 295.
F. Golshani, B. A. Fakheri, E. Behshad, and R. M. Vashvaei (2015). PRs proteins and their mechanism in plants, Biological forum. Research Trend, p. 477.
R. Russo, A. Sicilia, M. Caruso, C. Arlotta, S. Di Silvestro, F. G. Gmitter Jr., E. Nicolosi, and A. R. Lo Piero (2021). Int. J. Mol. Sci. 22, 882.
A. M. Abdelaziz, S. Dacrory, A. H. Hashem, M. S. Attia, M. Hasanin, H. M. Fouda, S. Kamel, and H. ElSaied (2021). Biocatal. Agric Biotechnol. 35.
S. Lisar, R. Motafakkerazad, M. M. Hossain, and I. Rahman (2012). Water Stress 25, 33.
K. Nahar, M. Hasanuzzaman, M. Alam, A. Rahman, J.-A. Mahmud, T. Suzuki, and M. Fujita (2017). Protoplasma 254, 445.
F. Daayf, A. El Hadrami, A. F. El-Bebany, M. A. Henriquez, Z. Yao, H. Derksen, I. El Hadrami, and L. R. Adam (2012). Recent Adv. Polyphenol Res. 3, 191.
V. Lattanzio, P. A. Kroon, S. Quideau, and D. Treutter (2009). Recent Adv. Polyphenol Res. 1, 1.
M. S. Attia, A. M. Abdelaziz, A. A. Al-Askar, A. A. Arishi, A. M. Abdelhakim, and A. H. Hashem (2022). J. Fungi 8, 775.
M. Mikulic-Petkovsek, V. Schmitzer, J. Jakopic, V. Cunja, R. Veberic, A. Munda, and F. Stampar (2013). Physiol. Mol. Plant Pathol. 84, 138.
S. S. Gill and N. Tuteja (2010). Plant Physiol. Biochem. 48, 909.
J. Kollmen and D. Strieth (2022). Life 12, 223.
A. Konate, X. He, Y.-K. Rui, and Z.-Y. Zhang (2017). Magnetite (Fe3O4) nanoparticles alleviate growth inhibition and oxidative stress caused by heavy metals in young seedlings of cucumber (Cucumis Sativus L.), ITM Web of Conferences. EDP Sciences, p. 03034.
N. Taran, L. Batsmanova, M. Kovalenko, and A. Okanenko (2016). Nanoscale Res. Lett. 11, 1.
H. Wang, X. Kou, Z. Pei, J. Q. Xiao, X. Shan, and B. Xing (2011). Nanotoxicology 5, 30.
S. K. Dhoke, P. Mahajan, R. Kamble, and A. Khanna (2013). Nanotechnol. Dev. 3.
Z. Asadi-Kavan, R. A. Khavari-Nejad, A. Iranbakhsh, and F. Najafi (2020). J. Plant Interact. 15, 166.
N. Pariona, A. I. Martinez, H. Hdz-García, L. A. Cruz, and A. Hernandez-Valdes (2017). Saudi. J. Biol. Sci. 24, 1547.
D. K. Tripathi, S. Singh, S. Gaur, S. Singh, V. Yadav, S. Liu, V. P. Singh, S. Sharma, P. Srivastava, and S. M. Prasad (2018). Front. Environ. Sci. 5, 86.
W. Akram, A. Ahmad, N. A. Yasin, T. Anjum, B. Ali, S. Fatima, S. Ahmed, M. J. Simirgiotis, and G. Li (2021). J. Plant Interact. 16, 411.
T. Anjum, W. Akram, S. Shafique, S. Shafique, and A. Ahmad (2017). Int. J. Agric. Biol. 19, 1073.
Acknowledgements
None.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No fund.
Author information
Authors and Affiliations
Contributions
SE suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. GSE suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. AMA suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. MSA suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. MMT and SHR wrote & edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Approval
Not applicable.
Informed Consent
Not applicable.
Research Involving Human Participation and/or Animals
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elbasuney, S., El-Sayyad, G.S., Abdelaziz, A.M. et al. Stable Colloidal Iron Oxide Nanoparticles: A New Green Nanofertilizer and Therapeutic Nutrient for Eggplant Immune Response Against Fusarium Wilt Disease. J Clust Sci 35, 983–997 (2024). https://doi.org/10.1007/s10876-023-02527-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-023-02527-3