Abstract
Global food crisis due to climate change, pandemic COVID-19 outbreak, and Russia-Ukraine conflict leads to catastrophic consequences; almost 10 percent of the world’s population go to bed hungry daily. Narrative solution for green agriculture with high vegetation and crop yield is mandatory; novel nanomaterials can improve plant immunity and restrain plant diseases. Iron is fundamental nutrient element; it plays vital role in enzyme activity and RNA synthesis; furthermore it is involved in photosynthesis electron-transfer chains. This study reports on the facile synthesis of colloidal ferric oxide nanoparticles as novel nano-fertilizer to promote vegetation and to suppress Fusarium wilt disease in tomato plant. Disease index, protection percent, photosynthetic pigments, and metabolic indicators of resistance in plant as response to induction of systemic resistance (SR) were recorded. Results illustrated that Fe2O3 NPs had antifungal activity against F. oxysporum. Fe2O3 NPs (at 20 µg/mL) was the best treatment and reduced percent disease indexes by 15.62 and gave highly protection against disease by 82.15% relative to untreated infected plants. Fe2O3 NPs treatments in either (non-infected or infected) plants showed improvements in photosynthetic pigments, osmolytes, and antioxidant enzymes activity. The beneficial effects of the synthesized Fe2O3 NPs were extended to increase not only photosynthetic pigments, osmolytes contents but also the activities of peroxidase (POD), polyphenol oxidase (PPO), catalase (CAT) and superoxide dismutase (SOD), enzymes of the healthy and infected tomato plants in comparison with control. For, peroxidase and polyphenol oxidase activities it was found that, application of Fe2O3 NPs (10 µg/mL) on challenged plants offered the best treatments which increased the activities of POD by (34.4%) and PPO by (31.24%). On the other hand, application of Fe2O3 NPs (20 µg/mL) on challenged plants offered the best treatments which increased the activities of CAT by (30.9%), and SOD by (31.33%).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
COVID-19 pandemic, climate change, and Russia-Ukraine conflict contributed to the surge of food cost. According to world food program, there are 44 million people in 38 countries at the ‘emergency’ phase of food insecurity [1, 2]. Novel approaches for green agriculture with enhanced plant immunity, vegetative growth, and crop productivity are highly appreciated [3]. Wilt disease, caused by the soil pathogen Fusarium oxysporum, has a negative impact on plant growth, metabolic properties, and crop yield. Consequently crop quality and quantity could be decreased significantly [4,5,6,7,8]. It should be considered that; some fungal species can create a harmful impact on crops and plants. Fusarium wilt disease is a systemic disease, as the fungus spreads inside the infected plant. It is difficult to combat Fusarium wilt disease chemically [9]. This disease is very dangerous, especially in areas where the hot weather prevails during the planting season [10]. In addition, plant malnutrition is one of the most serious problems that threaten agricultural wealth; as it causes huge losses in agricultural production, reduction in the product quality, as well as the secretion of toxins that cause poisoning and multiple serious diseases affecting humans and animals that feed on this product [11]. The plant may be exposed to a series of oxidative explosions in the cells, and the enzymes do not perform important chemical transformations to protect it from oxidative explosions, causing cell death and the susceptibility to infection with pathogens may increase [12].
Tomato is one of the most important vegetable crop in Egypt and it’s grown all year round in Egypt. However, production faces some problems in summer season due to high temperature and insect born viruses diseases prevailing in this time period [13].
Fertilizers are defined as natural or synthetic materials that provide the plant with nutrients necessary for its growth, development, and crop production. Depending on their source, fertilizers are classified into two main categories, including organic (natural) and chemical (synthetic) fertilizers [14]. Researchers found that plants treated with nano fertilizers and natural bio-stimulants, tends to have more activities of antioxidant enzymes [15]. Nanotechnology could play an important role in agriculture. The potential uses and benefits of nanotechnology are enormous and can be exploited to improve production and resistance to plant diseases [16]. Nanotechnology enables plants to exploit water, pesticides, and fertilizers more efficiently [17]. Nanotechnology (polymer/inorganic nanocomposites) may bring potential benefits via improving plant immunity, disease resistance, and securing high crop yields [18], drug release [19], wastewater treatment [20], controlled release of a third-generation EGFR inhibitor [21], and long-term release of favipiravir [22].
Iron compounds can act as catalyst for photosynthesis process; furthermore they are involved in enzyme activity and RNA synthesis [23]. Consequently Fe2O3 NPs can facilitate intracellular chemical changes and can act as catalysts [24].
Stable colloidal Fe2O3 NPs were developed via hydrothermal processing, and the developed Fe2O3 NPs demonstrated stable colloidal particles. Ferric oxide colloid was employed as nano-fertilizer for tomato plant. The main goal of this study was the improvement of tomato plant resistance against Fusarium wilt by Fe2O3 NPs and the assessment of the ISR indicators of treated tomato plants. Fe2O3 NPs treatments in either (non-infected or infected) plants demonstrated improvements in photosynthetic pigments, osmolytes, and antioxidant enzymes activity. The beneficial effects of Fe2O3 NPs were extended to increase not only photosynthetic pigments, osmolytes contents but also the activities of peroxidase (POD), polyphenol oxidase (PPO), catalase (CAT) and superoxide dismutase (SOD) enzymes of the healthy and infected tomato plants in comparison with control.
2 Materials and Methods
2.1 Materials and Instrumentation
Colloidal ferric oxide particles were fabricated via green synthesis technology (Hydrothermal synthesis); this was accomplished via direct conversion of ferric nitrate to ferric oxide. Further details about hydrothermal synthesis of colloidal ferric oxide particles was reported in the following references [25, 26]. The adopted fluids for hydrothermal synthesis are sub-critical or super-critical fluids (ScF) as shown in Supplementary Fig. S1 [27]. Supercritical water (ScW) requires extreme conditions (Tc 374.2 °C, Pc 220.5 bar); Supplementary Fig. S2 demonstrates the phase diagram of water.
At standard conditions Kw has the value of 1 × 10−14 mol/l [28, 29]. As water approaches its critical point, its dissociation constant increases to about three orders of magnitude; therefore it becomes a suitable solvent for ionic compounds and free radical processing. However, Kw decreases dramatically over the critical point [30]. Supplementary Fig. S3 demonstrates the changes in dielectric constant, density, and ionic product of water with temperature at 24 MPa.
The enhanced OH− level at the critical point can be exploited for NPs synthesis. This can be achieved through hydrolysis of metal salt (Eq. 1) immediately followed by a dehydration step (Eq. 2) [29].
A schematic for continuous hydrothermal synthesis is demonstrated in Supplementary Fig. S4. In this technique the super-critical water (ScW) flow was instantly mixed with cold metal salt. Nanoparticles are formed at the interface of the two fluids inside the reactor (R).
Ferric oxide NPs were developed via instant mixing of superheated water stream at 350 °C, and 240 bar (Flow A, 20 ml/min), with metal salt precursor (0.05 M ferric nitrate solution) at 25 °C, 240 bar (Flow B, 10 ml/min). Ferric oxide NPs were fabricated in a sustainable manner at the interface of the two streams inside the reactor (R) (Supplementary Fig. S4).
It is widely accepted that mono-dispersed particles were formed as nucleation and subsequent particle growth are the same for all particles. Further details about hydrothermal processing of Fe2O3 NPs can be found in the following references [25, 26, 31,32,33].
Surface morphology of colloidal ferric oxide nano-fertilizers was investigated with SEM, JEOL JSM-5600 LV, Japan. Quantification of deposited iron was conducted suing EDAX detector (JEOL JSM-5600 LV, Japan). Crystalline structure of colloidal ferric oxide nano-fertilizers was investigated with XRD (Shimadzu XRD-6000, Japan). Dynamic light scattering (DLS-PSS-NICOMP 380-ZLS particles sized system St. Barbara, California, USA) measurements were conducted to determine the average size distribution of the synthesized nano-fertilizers. In addition, high-resolution transmission electron microscope (HR-TEM, JEM2100, Jeol, Japan) was used as a fundamental tool for investigating the shape, appearance and the average particle size of the prepared nano-fertilizers. Drop coating NPs samples produced HRTEM examinations onto carbon-coated TEM grids after drying by incubation at 37.0 ± 2 °C in an incubator.
2.2 In Vitro Assessment of Antifungal Activity
Agar well diffusion method was applied to study the antifungal activity of the synthesized colloidal ferric oxide nano-fertilizers (Fe2O3 NPs) according to Parveen, et al. [34], with a few modifications. Fungal inoculum was extent systematically on the sterilized solidified potato dextrose agar (PDA) medium. At the same time, five discs 5 mm diameter were loaded with 50 µl of different concentrations of Fe2O3 NPs with triplicates The plates are kept for 2 h at the fridge to permit diffusion. The culture plates were incubated at 25 °C for 7 days, and the zones of inhibition (ZOI) were observed and measured.
2.3 In Vivo Assessment Efficacy of Fe2O3 NPs on Tomato Plant
2.3.1 Source of F. oxysporum f. sp. Lycopersici
F. oxysporum f. sp. Lycopersici RCMB008001 was obtained from Regional Center for mycology et al. Al-Azhar University, then was confirmed by pathogenicity test according to Hibar et al. [35]. The inoculum of the pathogenic fungus F. oxysporum was prepared according to Buttner et al. [36].
2.3.2 Experimental Design
Four-week-old tomato seedlings (Solanum lycopersicum 023) were obtained from Agricultural Research Center (ARC), Giza, Egypt. Uniform seedlings were transplanted into plastic pots (30 in diameter) containing a mixture of sand and clay (1: 3 W/W), total 5 kg, in a plastic pot. After the transplant, the seedlings left for 5 days before any treatments with normal irrigation. Afterwards, the inoculum of F. oxysporum (pathogen) (106) was applied. Two concentrations (10 µg/mL and 20 µg/mL) from colloidal ferric oxide nano-fertilizers (Fe2O3 NPs) were applied for 3 times (1 time each week; in the period before and after flowering). This experiment was carried out in the garden of Plant and Microbiology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt. The pots were arranged in a completely randomized design with five replicates as follows treatments; 1: control healthy, 2: control infected, 3: healthy treated with Fe2O3 NPs (10 µg/mL), 4: Infected treated with Fe2O3 NPs (10 µg/mL), 5: infected treated with Fe2O3 NPs (20 µg/mL), and 6: infected treated with Fe2O3 NPs (20 µg/mL). Sixty days old plants have been carefully uprooted and analyzed for the different parameters described below.
2.3.3 Disease Symptoms and Disease Index
Disease symptoms were assessed 60 days old and the disease index and protection percent were evaluated according to Farrag et al. [37]. Percent Disease Index (PDI) was calculated using the five-grade scale according to the following formula:
where n1-n4 is the number of plants in the indicated classes and nt is the total number of plants tested.
Additionally, Percent Protection (P %) was calculated using the following formula:
where A is PDI in infected control plants and B is PDI in infected treated plants.
2.3.4 Morphological and Biochemical Resistance Indicators in Tomato Plant
The plant samples were collected for different morphological growth traits (shoot high, root length and number of leaves). Photosynthetic pigments were assayed according to Vernon et al. [38]. The soluble carbohydrate content of the dried shoot was calculated as the method mentioned by Iigoyen et al. [39]. Total protein was determined according to Lowry et al. [40]. Total shoot phenol content was assayed as described by Diaz et al. [41], and enzyme activity were determined by the advanced publications [42,43,44], and [45].
3 Results and Discussion
3.1 Characterization of Colloidal Ferric Oxide Nano-Fertilizers
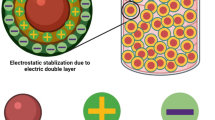
α-Fe2O3 was manufactured using hydrothermal synthesis technique. The synthesized colloidal particles demonstrated deep red color (Fig. 1a), and the particles did not flocculate with time. Stabilization mechanism was correlated to electrostatic stabilization with nitrate ions (Fig. 1b). The colloidal particles demonstrated Zeta potential value of +38.5 mV (Fig. 2). Zeta potential confirmed the electrostatic stabilization of colloidal ferric oxide particles.
HRTEM micrographs demonstrated mono-dispersed Fe2O3 NPs possesses a spherical shape of 5 nm average particle size (Fig. 3a). HRTEM micrographs confirmed high quality mono-dispersed particles with uniform particle size. On the other hand, particle size distribution was calculated by DLS, and the result indicated that the average Fe2O3 NPs particle size distribution was found to be 7.5 nm by 100% as displayed in Fig. 3b.
It was noted that, the particle size distribution estimated from DLS analysis was more than the average particle size determination by HRTEM images. The reasons are defined as the DLS method estimated the hydrodynamic radius which founded around the synthesized colloidal Fe2O3 NPs and enclosed by the water particles regarding the large sizes of the capped Fe2O3 NPs [46].
XRD diffractogram showed high-degree hematite (α-Fe2O3). XRD system was conducted to study the crystal composition and state of the incorporated Fe2O3 NPs (Fig. 4). The conducted XRD models agree to the specific α-Fe2O3 original (JCPDS No. 33-0664). The unique peaks looked at the next 2θ arranges ≈ 24.12°, 33.58°, 35.35°, 40.78°, 49.59°, 54.22°, 57.41°, 62.55°, and 65.62° corresponding to (012, 104, 110, 113, 024, 116, 018, 214, and 300) planes, respectively and showing its standard cubic spinel composition [47]. There are no unknown crystalline phases and impurities in the Fe2O3 NPs.
This matches with the unique composition of the complete α-Fe2O3 crystal with a rhombohedral centered hexagonal building (R3c space system) [48,49,50,51,52]. The most important diffraction peak near 35.35° implies that (110) facets remain the dominant α-Fe2O3 crystal construction with 5.95 nm crystal size according to Williamson-Hall (W–H) equation [53].
The surface morphology, purity, and the elemental composition of the prepared α-Fe2O3 NPs were studied, as shown in Fig. 5. SEM analysis showed that the prepared α-Fe2O3 NPs had a semi-spherical structure, with a uniform distribution as displayed in Fig. 5a.
EDX analysis revealed the high purity of the prepared α-Fe2O3 NPs, as indicated by the presence of atoms characteristic to each component of it (Fe and O atoms) and the absence of foreign atoms that may appear as impurity, Also, carbon atom (C) was corresponded to the holder which used for the SEM imaging process as illustrated in Fig. 5b.
SEM micrographs of dry Fe2O3 NPs confirmed the vast tendency of NPs to diminish their number and surface area, with dramatic decrease in NPs surface area and reactivity [54, 55]. There is great advantages of using NPs in colloidal state.
3.2 In Vitro Antifungal Potential
Iron oxide nanoparticles showed significant anti-mycotic activity against rot fungal pathogens [56]. Therefore, Fe2O3 NPs were synthesized in this study to control F. oxysporum. The antifungal activity of Fe2O3 NPs was assessed using the well diffusion method [57]; as shown in Supplementary Fig. S5. Results illustrated that Fe2O3 NPs had antifungal activity against F. oxysporum.
There are a relationship between the crystal structure (XRD) of the synthesized Fe2O3 NPs and their anti-fungal properties [58, 59]; the massive surface area and massive reactivity (high interfacial surface area, and small crystal size and structure), therefore increase reactivity of the synthesized nanocomposite against fungal cells [60, 61].
The small size and high surface area increasing the possibility for more interaction with the fungal cells (surface charge will interact more effectively with the fungus) through the charge attraction and diffusion across the fungal cell wall and therefore increasing the antifungal potential [62, 63].
3.3 Control of Wilt Disease Caused by F. oxysporum by Fe2O3 NPs (In Vivo)
Disease severity was the first guide to govern systemic resistance in treated plants by Fe2O3 NPs. Application of Fe2O3 NPs at concentrations (20 µg/mL and 10 µg/mL) were applicable in decreasing disease index (Table 1). Fe2O3 NPs (at 20 µg/mL) was active more than 10 µg/mL treatment that reduced percent disease indexes by 15.62 and gave highly protection by 82.15% compared to infected plants that similar to recent studies [64, 65].
Our results similar to Ashraf et al. [66], which reported that Fe3O4 NPs significantly reduced the disease severity in tomato plants infected with F. oxysporum by an average of 47.8% resulting in increased plant growth variables at exposure to 10 µg/mL of iron oxide NPs. Plants stimulate a toxic oxidative-burst by accumulative iron concentrations to reduce pathogen virulence; roots mutualistic interactions also encounter phytodiseases via iron uptake as well as antagonism for iron achievement produces a systemic resistance that signal mechanisms in roots for iron-uptake [66].
3.4 Morphological Indicators
Morphological features (shoot length, root length, and number of leaves) were significantly decreased due to Fusarium wilt infection. The reduction of all growth parameters showed dangerous losses in plant. In this respect, the drop in growth may be associated with different reasons; among them Fusarium enters through the roots of the plant and proliferates in the vascular tissues leading to breakdown of the water economy of the infected plants [67].
The results indicated that foliar application with Fe2O3 NPs colloidal solution was great enhancement of growth parameters similar to the literature that discussed NPs to enhance the growth of different crop plants [68].
Concerning the effect of foliar application; Fe2O3 NPs colloidal solution as foliar spray method on the tested plants (Fig. 6), it was noticed that application with (Fe2O3 NPs 20 µg/mL, and 10 µg/mL) improved shoot length (by 76.07%, and 70.45%), root length (84.75, and 53.81%) and number of leaves (43.09, and 25.86%), respectively versus infected plants.
Healthy tomato plants treated with Fe2O3 NPs solutions as foliar spray method (Fe2O3 NPs 20 µg/mL and 10 µg/mL) showed an improvement in all morphological aspects shoot length by (20.30% and 17.38%), root length (28.61, and 3.53%) and number of leaves (23.30 and 20.39%), respectively when compared to control.
Iron deficiency leads to failure in the production of chlorophyll and yellowing of areas between the veins of the leaves (commonly referred to as iron chlorosis), which leads to dwarfism and a sharp decrease in vegetative growth characteristics. In severe deficiency, leaves become almost pale white due to loss of chlorophyll. Complete leaf fall can occur and buds can die [69].
Interestingly, iron is a micronutrient that plays a vital role in chlorophyll synthesis, carbohydrate production, cell respiration, the chemical reduction of nitrate and sulfate, and in nitrogen assimilation. Stimulating systemic resistance against nutritional deficiency symptoms and pathogens [70].
The current study showed that the concentration of 20 ppm (20 µg/mL) was better than the concentration of 10 ppm (10 µg/mL), as it led to an improvement in vegetative growth characteristics, which means an improvement in chlorophyll and cell respiration.
Results of the present study are similar to the results of other studies in which Fe3O4 NPs-treated rocket seedlings showed increased shoot elongation after seed germination and have a positive impact on rocket seed germination. Moreover, it is proved that NPs aggregate to root pores and reduce the root hydraulic conductivity by inhibiting the water uptake [71]. It is possible that, the absence of the necessary amount of water causing the elongation in the cells of root [72].
3.5 Photosynthetic Pigments
The contents of chlorophyll a and b were significantly decreased in infected plants by 18.95% and 18.44%, respectively as shown in Fig. 7. The decline in chlorophyll may be due to the generation of reactive oxygen species (ROS) causing damage to chlorophyll, which means plants were failed to capture the light and so photosynthesis will decrease or stopped [73].
Additionally, this decrease may be due to chlorophyll deprivation, decreased chlorophyll production and permanency of thylakoid membrane [74]. The present results indicate that, the effects of two concentrations of Fe2O3 NPs (20, and 10 µg/mL) as foliar spray method, on photosynthetic pigments of tomato plants were investigated. In contrast, there were positive effects of all treatments on plant metabolism. These positive effects may be due to the iron which is a vital nutrient for plants, and its function to take and provide electrons and plays essential functions in the electron-transport chains of photosynthesis and respiration [75, 76].
Foliar fertilization could maintain good plant nutritional status, and iron in particular can be applied to foliage in different chemical forms, including chelates and inorganic iron salts [77].
Results in Fig. 7, indicated that, the contents of carotenoids were significantly increased in tomato plants in response to Fusarium infection. Moreover, the obtained results illustrated that the infected plants treated with Fe2O3 NPs showed a significant decreasing in carotenoid content compared with control infected. It was noticed that application of Fe2O3 NPs on healthy and infected plants, showed increase in the carotenoid contents. This increase might be attributed to enhanced stomatal conductance, transpiration rate and/or cell size and number [78].
It has been recognized that penetration by foliar-applied iron compounds can occur via cuticle cracks and imperfections and through stomata, leaf hairs, and other specialized epidermal cells. The importance of stomatal versus circular leaf absorption, particularly with regard to aqueous solutions, is a subject of much concern [79].
Iron is a vital nutrient for plants, and take and provide electrons and plays essential functions in the electron-transport chains of photosynthesis and respiration [80, 81]. A main portion of iron is confined in chloroplasts where photosynthetic process happens as indicated in Fig. 8. Foliar fertilization could maintain good plant nutritional status, and iron in particular can be applied to foliage in different chemical forms, including chelates and inorganic Fe salts [82].
3.6 Biochemical Resistance Indicators in Tomato Plants
Fusarium infected tomato plants showed decreases in contents of soluble carbohydrate, soluble protein by 34.46%, 6.27%, respectively (Fig. 9). In this work, there is a positive correlation between the reduction in osmolytes contents (soluble carbohydrate and soluble protein) and a reduction in photosynthetic pigments and growth of tomato plants in response to Fusarium infection. Soluble sugars are involved in the responses to a number of stresses, and act as nutrient and metabolite signaling molecules that activate specific or hormonal-crosstalk transduction pathways, resulting in important modifications of gene expression [83].
On the other hand, foliar application with Fe2O3 NPs enhanced contents of soluble carbohydrate and soluble protein in shoots of Fusarium infected tomato plants when compared to infected plants. The synthesized Fe2O3 NPs (20 µg/mL) recorded increase in contents of soluble carbohydrate and soluble protein by (43.32%, and 104.27%), respectively compared to infected plants. Healthy tomato plants treated with Fe2O3 NPs (20 µg/mL, and 10 µg/mL) showed an improvement in soluble carbohydrate by (20.37%, and 70.98%), and soluble protein (95.32%, and 110.41%), respectively when compared to control plants.
In the present work, application of Fe2O3 NPs solutions as foliar spray method showed increase in the total soluble protein contents in comparison with untreated plants. The continuous accumulations of newly-induced proteins may help in the localization of pathogen infection; the reverse is not true, since the presence of a non-significant amount of induced proteins is a necessary condition to the observed systemic infection. These induced proteins have been defined as pathogenesis related proteins, they implicated in plant defense because of their anti-pathogenic activities [84].
3.7 Phenols Contents in Tomato Plant Leaves
Fusarium infected tomato plants exhibited significant increases in the contents of total phenols by 32.25% when compared to control (Fig. 10). In this study, fungal infection increased the contents of total phenols in shoots of tomato plants in accordance with other investigators [73, 85, 86].
Phenolic compounds and ascorbic acid support antioxidant roles by scavenging the free radicals, reducing their reactivity to the membrane components [87]. Moreover, phenolic compounds are also able to stabilize cell membranes by reducing membrane fluidity, which results in reduced mobility of free radicals across membranes, thus limiting membrane peroxidation [88]. Application of Fe2O3 NPs colloid solutions with different concentration (Fe2O3 NPs 20 µg/mL, and 10 µg/mL) resulted in a significant increase in the content of total phenols by 12.19% and 31.70%, respectively when compared with infected plants (Fig. 10). Under normal conditions, Fe2O3 NPs solutions-treated tomato plants (20 µg/mL, and 10 µg/mL) showed an improvement in total phenol content by (41.93%, and 64.51%), respectively comparing to healthy plants. These results accordance with [89]; they reported that iron nanoparticles improved phenol contents of plants.
3.8 Oxidative Enzymes Activity
To obtain more clear indication on some defense-responsible enzymes, mean activities of peroxidase (POD), polyphenol oxidase (PPO), catalase (CAT), and superoxide dismutase (SOD), of the healthy and infected tomato plants were determined in this study.
POD, PPO, CAT, and SOD activities were greater in the infected plants as well as plants treated with Fe2O3 NPs solutions with different concentration (20 µg/mL, and 10 µg/mL). For more, antioxidant enzyme activities provide a large number of defensive enzymes associated with biotic stress [73, 90, 91]. For, peroxidase (POD) and polyphenol oxidase (PPO) activities it was found that, application of Fe2O3 NPs (10 µg/mL, and 20 µg/mL) on challenged plants increased the activities of POD by (34.43% and 10.37%) and PPO (41.23% and 13.95%), respectively when compared with only infected plants (Fig. 11).
For catalase (CAT), and superoxide dismutase (SOD) activities it was found that, application of Fe2O3 NPs (20 µg/mL and 10 µg/mL) on challenged plants were increased the activities of CAT by (30.98% and 26.05%) and SOD by (31.33% and 28.69%), respectively compared with infected plants (Fig. 11). Our results showed that antioxidant enzymes activity increased significantly in plants exposed to Fusarium infection. The plant showed different strategies to cope with infection as they increase the activity of certain antioxidant enzymes to keep ROS at the lower level in the cell [92]. Nanoparticles can activate anti-stress activities in plants [93].
To eliminate ROS and reduce the toxic effects of stress, plants are equipped with enzymatic antioxidant systems including SOD and POD [94]. According to our results, Fe2O3 NPs induced higher oxidative stress and higher activity of antioxidant enzymes than untreated plants, which is consistent with literature results [95, 96]. Because iron is involved in enzyme activity and RNA synthesis [97], and due to the high reactivity of the NPs, the synthesized Fe2O3 NPs can facilitate intracellular chemical changes and can act as catalysts [98, 99]. As in our results, Fe2O3 NPs in Cucurbita pepo had a positive effect on plant growth and increased activity of antioxidant enzymes [100].
The significant differences between the healthy treated (Fe2O3 NPs) tomato plant, and the infected one was investigated (Fig. 12). The effect of Fusarium on the plant is represented in Fig. 12a. Symptoms of wilting and a severe decrease in the vegetative total appear; which is reflected on the photosynthesis and all physiological processes. Figure 12b demonstrated an infected plant; that was treated with the fertilizer compound (Fe2O3 NPs). It is obvious that noticeable improvement in the morphological characteristics, and another infected plant that was not treated showed signs of wilting.
4 Conclusion
Stable colloidal ferric oxide particles of 5 nm particle size were efficiently developed by hydrothermal processing. The most important diffraction peak near 35.35° implies that (110) facets remain the dominant α-Fe2O3 crystal construction with 5.95 nm crystal size according to Williamson-Hall (W-H) equation. SEM analysis showed that the prepared α-Fe2O3 NPs had a semi-spherical structure, with a uniform distribution. The developed particles were employed as nano-fertilizer for tomato plant against Fusarium wilt disease. Application of colloidal Fe2O3 NPs was applicable in decreasing disease index compared to the infected control. Fe2O3 NPs (at 20 µg/mL) was the best treatment and reduced percent disease indexes by 15.62 and gave highly protection against disease by 82.15%, and came next Fe2O3 NPs (10 µg/mL) which reduced percent disease indexes by 25 and have highly protection against disease by 71.42%, related to untreated infected plants. The present results indicate that, the effects of two concentrations of Fe2O3 NPs (20 µg/mL, and 10 µg/mL) on photosynthetic pigments of tomato plants (healthy & infected) were investigated. We did not observe any photosynthesis inhibition in tomato leaves. The infected plants treated with Fe2O3 NPs, showed the most potent effect in terms of the length of shoots and roots and the number of leaves per plant. Additionally, tomato plants which treated with Fe2O3 NPs (20, and 10 µg/mL) showed a significant increase in the content of chlorophyll a and b and carotenoids, total carbohydrates, total soluble proteins, the total phenols ,and antioxidant enzymes activity (POD, PPO, CAT and SOD) compared to the non-treated infected tomato plant. According to the colloidal stability (Zeta results), small size (HRTEM), purity (EDX), promising in vivo and in vitro results, and high activity in low concentration (20 µg/mL), the bioavailability of the synthesized green nano-fertilizer may be applied in large scale. Additionally, there are some factors must take into consideration for bioavailability of the synthesized green nano-fertilizer such as the stability of nanocomposite in the field conditions, Temp., the acidic or alkaline pH, and the presence of some non-pathogenic microbes in the soil such as PGPR.
Data Availability
The datasets supporting the conclusions of this article are included within the article and its additional files.
References
W.F. Programme, World Food Programme at a Glance. Accessed 16 Apr 2022
T.O. Oyekale, A. Oyekale, Determinants of food insecurity during COVID-19 pandemic in Nigeria: a random effects ordered probit approach. Acta Univ. Danub. Œcon. 17(6), 1 (2021)
D. Bhardwaj, M.W. Ansari, R.K. Sahoo, N. Tuteja, Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 13(1), 1–10 (2014)
K. Abada, K.E. Eid, A protocol suggested for management of cantaloupe downy mildew. Am. J. Life Sci. 2(6–2), 1–10 (2014)
A.M. Abdelaziz, S.S. Salem, A. Khalil, D.A. El-Wakil, H.M. Fouda, A.H. Hashem, Potential of biosynthesized zinc oxide nanoparticles to control Fusarium wilt disease in eggplant (Solanum melongena) and promote plant growth. Biometals 35(3), 601–616 (2022)
A.M. Abdelaziz, S. Dacrory, A.H. Hashem, M.S. Attia, M. Hasanin, H.M. Fouda, S. Kamel, H. ElSaied, Protective role of zinc oxide nanoparticles based hydrogel against wilt disease of pepper plant. Biocatal. Agric. Biotechnol. 35, 102083 (2021)
A.M. Aldinary, A.M. Abdelaziz, A.A. Farrag, M.S. Attia, Biocontrol of tomato Fusarium wilt disease by a new Moringa endophytic Aspergillus isolates. Mater. Today (2021). https://doi.org/10.1016/j.matpr.2021.03.423
A. Abd Alhakim, A. Hashem, A.M. Abdelaziz, M.S. Attia, Impact of plant growth promoting fungi on biochemical defense performance of tomato under Fusarial infection. Egypt. J. Chem. (2022). https://doi.org/10.21608/ejchem.2022.124008.5532
A. Jain, S. Sarsaiya, Q. Wu, Y. Lu, J. Shi, A review of plant leaf fungal diseases and its environment speciation. Bioengineered 10(1), 409–424 (2019)
J. Gressel, A. Hanafi, G. Head, W. Marasas, A.B. Obilana, J. Ochanda, T. Souissi, G. Tzotzos, Major heretofore intractable biotic constraints to African food security that may be amenable to novel biotechnological solutions. Crop Prot. 23(8), 661–689 (2004)
D.S. Powlson, P.J. Gregory, W.R. Whalley, J.N. Quinton, D.W. Hopkins, A.P. Whitmore, P.R. Hirsch, K.W. Goulding, Soil management in relation to sustainable agriculture and ecosystem services. Food Policy 36, S72–S87 (2011)
M.-H. Chi, S.-Y. Park, S. Kim, Y.-H. Lee, A novel pathogenicity gene is required in the rice blast fungus to suppress the basal defenses of the host. PLoS Pathog. 5(4), e1000401 (2009)
S.A. Naik, S. Hongal, M. Harshavardhan, K. Chandan, A.J. Kumar, M.C. Kyriacou, Y. Rouphael, P. Kumar, Productive characteristics and fruit quality traits of cherry tomato hybrids as modulated by grafting on different Solanum spp. rootstocks under Ralstonia solanacearum infested greenhouse soil. Agronomy 11(7), 1311 (2021)
H.-L. Xu, Effects of a microbial inoculant and organic fertilizers on the growth, photosynthesis and yield of sweet corn. J. Crop. Prod. 3(1), 183–214 (2001)
S.K. Yadav, S. Lal, S. Yadav, J. Laxman, B. Verma, M. Sushma, R. Choudhary, P. Singh, S. Singh, V. Sharma, Use of nanotechnology in agri-food sectors and apprehensions: an overview. Seed Res. 47(2), 99–149 (2019)
M. Rai, A. Ingle, Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 94(2), 287–293 (2012)
I.O. Adisa, V.L.R. Pullagurala, J.R. Peralta-Videa, C.O. Dimkpa, W.H. Elmer, J.L. Gardea-Torresdey, J.C. White, Recent advances in nano-enabled fertilizers and pesticides: a critical review of mechanisms of action. Environ. Sci. 6(7), 2002–2030 (2019)
P. Pramanik, P. Krishnan, A. Maity, N. Mridha, A. Mukherjee, V. Rai, Application of nanotechnology in agriculture, in Environmental Nanotechnology, vol. 4, (Springer, Cham, 2020), pp. 317–348
L. Xu, Z. Chu, H. Wang, L. Cai, Z. Tu, H. Liu, C. Zhu, H. Shi, D. Pan, J. Pan, Electrostatically assembled multilayered films of biopolymer enhanced nanocapsules for on-demand drug release. ACS Appl. Bio Mater. 2(8), 3429–3438 (2019)
M. Abd Elkodous, G.S. El-Sayyad, M.A. Maksoud, R. Kumar, K. Maegawa, G. Kawamura, W.K. Tan, A. Matsuda, Nanocomposite matrix conjugated with carbon nanomaterials for photocatalytic wastewater treatment. J. Hazard. Mater. 410, 124657 (2021)
L. Xu, H. Wang, Z. Chu, L. Cai, H. Shi, C. Zhu, D. Pan, J. Pan, X. Fei, Y. Lei, Temperature-responsive multilayer films of micelle-based composites for controlled release of a third-generation EGFR inhibitor. ACS Appl. Polym. Mater. 2(2), 741–750 (2020)
L. Xu, X. Zhang, Z. Chu, H. Wang, Y. Li, X. Shen, L. Cai, H. Shi, C. Zhu, J. Pan, Temperature-responsive multilayer films based on block copolymer-coated silica nanoparticles for long-term release of favipiravir. ACS Appl. Nano Mater. 4(12), 14014–14025 (2021)
R. Lill, S.-A. Freibert, Mechanisms of mitochondrial iron-sulfur protein biogenesis. Annu. Rev. Biochem. 89, 471–499 (2020)
B. Wang, J.-J. Yin, X. Zhou, I. Kurash, Z. Chai, Y. Zhao, W. Feng, Physicochemical origin for free radical generation of iron oxide nanoparticles in biomicroenvironment: catalytic activities mediated by surface chemical states. J. Phys. Chem. C 117(1), 383–392 (2013)
S. Elbasuney, M. Yehia, A. Hamed, S. Ismael, M. Mokhtar, E. Elsaka, M. Gobara, A. Saleh, G.S. El-Sayyad, Ferric oxide colloid: novel nanocatalyst for heterocyclic nitramines. J. Mater. Sci. 32(4), 4185–4195 (2021)
S. Elbasuney, A. Hamed, M. Yehia, S. Ismael, A. Saleh, M. Gobara, M. Mokhtar, G.S. El-Sayyad, Colloidal nanothermite particles: advanced nanocatalyst and energy dense material for ammonium perchlorates. J. Electron. Mater. 50(11), 6128–6134 (2021)
P. Savage, S. Gopalan, T. Mizan, C. Martino, Reactions at supercritical conditions: applications and fundamentals. AIChE J. 41(7), 1723–1778 (1995)
R. Perry, D. Green, Perry’s chemical engineer’s handbook, 6th edn. (Graw-Hill Inc., Singapore, 1984)
T. Adschiri, Y. Hakuta, K. Arai, Hydrothermal synthesis of metal oxide fine particles at supercritical conditions. Ind. Eng. Chem. Res. 39(12), 4901–4907 (2000)
P.E. Savage, Organic chemical reactions in supercritical water. Chem. Rev. 99(2), 603–621 (1999)
S. Elbasuney, G.S. El-Sayyad, M. Yehia, S.K. Abdel Aal, Facile synthesis of RGO-Fe2O3 nanocomposite: a novel catalyzing agent for composite propellants. J. Mater. Sci. 31(23), 20805–20815 (2020)
S. Elbasuney, M. Yehia, A. Hamed, M. Mokhtar, M. Gobara, A. Saleh, E. Elsaka, G.S. El-Sayyad, Synergistic catalytic effect of thermite nanoparticles on HMX thermal decomposition. J. Inorg. Organomet. Polym Mater. 31(6), 2293–2305 (2021)
S. Elbasuney, G.S. El-Sayyad, S. Ismael, M. Yehia, Colloid thermite nanostructure: a novel high energy density material for enhanced explosive performance. J. Inorg. Organomet. Polym Mater. 31(2), 559–565 (2021)
S. Parveen, A.H. Wani, M.A. Shah, H.S. Devi, M.Y. Bhat, J.A. Koka, Preparation, characterization and antifungal activity of iron oxide nanoparticles. Microb. Pathog. 115, 287–292 (2018)
K. Hibar, V. Edel-Herman, C. Steinberg, N. Gautheron, M. Daami-Remadi, C. Alabouvette, M. El Mahjoub, Genetic diversity of Fusarium oxysporum populations isolated from tomato plants in Tunisia. J. Phytopathol. 155(3), 136–142 (2007)
G. Büttner, B. Pfähler, B. Märländer, Greenhouse and field techniques for testing sugar beet for resistance to Rhizoctonia root and crown rot. Plant Breed. 123(2), 158–166 (2004)
A. Farrag, M. Attia, A. Younis, A. Abd Elaziz, Potential impacts of elicitors to improve tomato plant disease resistance. Al Azhar Bull. Sci. 9, 311–321 (2017)
L.P. Vernon, B. Ke, Photochemistry of chlorophyll in vivo, in The Chlorophylls. (Elsevier, Amsterdam, 1966), pp. 569–607
J. Irigoyen, D. Einerich, M. Sánchez-Díaz, Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol. Plant. 84(1), 55–60 (1992)
O.H. Lowry, N.J. Rosebrough, A.L. Farr, R.J. Randall, Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951)
D.H. Diaz, G.C. Martin, Peach seed dormancy in relation to endogenous inhibitors and applied growth substances. Amer. Soc. Hort. Sci. J. 651–654 (1972)
H. Bergmeyer, Determination with glucose oxidase and peroxidase. Methods Enzym. Anal. 3, 1205–1215 (1974)
A. Matta, A. Dimond, Symptoms of Fusarium wilt in relation to quantity of fungus and enzyme activity in tomato stems. Phytopathology 53(5), 574 (1963)
S. Marklund, G. Marklund, Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 47(3), 469–474 (1974)
H. Aebi, [13] Catalase in vitro, in Methods in enzymology. (Elsevier, Amsterdam, 1984), pp. 121–126
A. Baraka, S. Dickson, M. Gobara, G.S. El-Sayyad, M. Zorainy, M.I. Awaad, H. Hatem, M.M. Kotb, A. Tawfic, Synthesis of silver nanoparticles using natural pigments extracted from Alfalfa leaves and its use for antimicrobial activity. Chem. Pap. 71(11), 2271–2281 (2017)
V.C. Karade, S.B. Parit, V.V. Dawkar, R.S. Devan, R.J. Choudhary, V.V. Kedge, N.V. Pawar, J.H. Kim, A.D. Chougale, A green approach for the synthesis of α-Fe2O3 nanoparticles from Gardenia resinifera plant and it’s in vitro hyperthermia application. Heliyon 5(7), e02044 (2019)
D.E. Fouad, C. Zhang, H. El-Didamony, L. Yingnan, T.D. Mekuria, A.H. Shah, Improved size, morphology and crystallinity of hematite (α-Fe2O3) nanoparticles synthesized via the precipitation route using ferric sulfate precursor. Results Phys. 12, 1253–1261 (2019)
M. Sharma, α-Fe2O3 Preparation by Sol-Gel Method, Lab Manual, pp. 1–16 (2017)
T. Liang, X. Guo, B. Yuan, S. Kong, H. Huang, D. Fu, F. Zhang, J. Xu, X. Li, Design of functionalized α-Fe2O3 (III) films with long-term anti-wetting properties. Ceram. Int. 46(5), 6129–6135 (2019)
M. Tadic, M. Panjan, B.V. Tadic, J. Lazovic, V. Damnjanovic, M. Kopani, L. Kopanja, Magnetic properties of hematite (α-Fe2O3) nanoparticles synthesized by sol-gel synthesis method: the influence of particle size and particle size distribution. J. Electr. Eng. 70(7), 71–76 (2019)
Q.Z. Zeng, S.Y. Ma, W.X. Jin, H.M. Yang, H. Chen, Q. Ge, L. Ma, Hydrothermal synthesis of monodisperse α-Fe2O3 hollow microspheroids and their high gas-sensing properties. J. Alloy. Compd. 705, 427–437 (2017)
A.H. Ashour, A.I. El-Batal, M.I.A.A. Maksoud, G.S. El-Sayyad, S. Labib, E. Abdeltwab, M.M. El-Okr, Antimicrobial activity of metal-substituted cobalt ferrite nanoparticles synthesized by sol–gel technique. Particuology 40, 141–151 (2018)
S. Elbasuney, A. Elsaidy, M. Kassem, H. Tantawy, R. Sadek, A. Fahd, M. Gobara, Super-thermite (Al/Fe2O3) fluorocarbon nanocomposite with stimulated infrared thermal signature via extended primary combustion zones for effective countermeasures of infrared seekers. J. Inorg. Organomet. Polym. Mater. 28(6), 2231–2240 (2018)
S. Elbasuney, Dispersion characteristics of dry and colloidal nano-titania into epoxy resin. Powder Technol. 268, 158–164 (2014)
J.A. Koka, A.H. Wani, M.Y. Bhat, Evaluation of antifungal activity of magnesium oxide (MgO) and Iron oxide (FeO) nanoparticles on rot causing fungi. J. Drug Deliv. Ther. 9(2-s), 173–178 (2019)
A.I. El-Batal, G.S. El-Sayyad, F.M. Mosallam, R.M. Fathy, Penicillium chrysogenum-mediated mycogenic synthesis of copper oxide nanoparticles using gamma rays for in vitro antimicrobial activity against some plant pathogens. J. Clust. Sci. 31(1), 79–90 (2020)
M. Akbar, U. Haroon, M. Ali, K. Tahir, H.J. Chaudhary, M.F.H. Munis, Mycosynthesized Fe2O3 nanoparticles diminish brown rot of apple whilst maintaining composition and pertinent organoleptic properties. J. Appl. Microbiol. 132(5), 3735–3745 (2022)
G.S. El-Sayyad, H.S. El-Bastawisy, M. Gobara, A.I. El-Batal, Gentamicin-assisted mycogenic selenium nanoparticles synthesized under gamma irradiation for robust reluctance of resistant urinary tract infection-causing pathogens. Biol. Trace Elem. Res. 195(1), 323–342 (2020)
B.L. da Silva, M.P. Abuçafy, E.B. Manaia, J.A.O. Junior, B.G. Chiari-Andréo, R.C.R. Pietro, L.A. Chiavacci, Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: an overview. Int. J. Nanomed. 14, 9395 (2019)
F. Ahmed, C. Awada, S.A. Ansari, A. Aljaafari, A. Alshoaibi, Photocatalytic inactivation of Escherichia coli under UV light irradiation using large surface area anatase TiO2 quantum dots. R. Soc. Open Sci. 6(12), 191444 (2019)
L. Yien, N.M. Zin, A. Sarwar, H. Katas, Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012, 1–9 (2012)
S. Sharmin, M.M. Rahaman, C. Sarkar, O. Atolani, M.T. Islam, O.S. Adeyemi, Nanoparticles as antimicrobial and antiviral agents: a literature-based perspective study. Heliyon 7(3), e06456 (2021)
N. Akter, M.R. Islam, M.B. Hossain, M.N. Islam, S.R. Chowdhury, S. Hoque, R.H. Nitol, R. Tasnin, Management of wilt complex of eggplant (Solanum melongena L.) caused by Fusarium oxysporum Ralstonia solanacearum and Meloidogyne spp. Am. J. Plant Sci. 12(7), 1155–1171 (2021)
M.S. Attia, H.A. El-Naggar, M.M. Abdel-Daim, G.S. El-Sayyad, The potential impact of Octopus cyanea extracts to improve eggplant resistance against Fusarium-wilt disease: in vivo and in vitro studies. Environ. Sci. Pollut. Res. 28(27), 35854–35869 (2021)
H. Ashraf, T. Anjum, S. Riaz, T. Batool, S. Naseem, I.S. Ahmad, Sustainable synthesis of microwave assisted IONPs by using spinacia oleracea: enhances resistance against fungal wilt infection by inducing ROS and modulating defense system in tomato plants. J. Nanobiotechnol. 20, 8 (2022). https://doi.org/10.1186/s12951-021-01204-9
S. Sundaramoorthy, P. Balabaskar, Evaluation of combined efficacy of Pseudomonas fluorescens and Bacillus subtilis in managing tomato wilt caused by Fusarium oxysporum f. sp. Lycopersici (Fol). Plant Pathol. J. 12(4), 154–161 (2013)
I. Plaksenkova, M. Jermaļonoka, L. Bankovska, I. Gavarāne, V. Gerbreders, E. Sledevskis, J. Sniķeris, I. Kokina, Effects of Fe3O4 nanoparticle stress on the growth and development of rocket Eruca sativa. J. Nanomater. 2019, 1–10 (2019)
K. Glozer, L. Ferguson, Pomegranate production in Afghanistan. UCDAVIS Coll Agric. Environ. Sci. 1, 32 (2008)
R. Hell, U.W. Stephan, Iron uptake, trafficking and homeostasis in plants. Planta 216(4), 541–551 (2003)
N. Zuverza-Mena, D. Martínez-Fernández, W. Du, J.A. Hernandez-Viezcas, N. Bonilla-Bird, M.L. López-Moreno, M. Komárek, J.R. Peralta-Videa, J.L. Gardea-Torresdey, Exposure of engineered nanomaterials to plants: insights into the physiological and biochemical responses-a review. Plant Physiol. Biochem. 110, 236–264 (2017)
Y. Ma, M. Rajkumar, J. Vicente, H. Freitas, Inoculation of Ni-resistant plant growth promoting bacterium Psychrobacter sp. strain SRS8 for the improvement of nickel phytoextraction by energy crops. Int. J. Phytoremediation 13(2), 126–139 (2010)
M.S. Attia, G.S. El-Sayyad, M. Abd Elkodous, A.I. El-Batal, The effective antagonistic potential of plant growth-promoting rhizobacteria against Alternaria solani-causing early blight disease in tomato plant. Sci. Hortic. 266, 109289 (2020)
C.E. Benedetti, P. Arruda, Altering the expression of the chlorophyllase gene ATHCOR1 in transgenic Arabidopsis caused changes in the chlorophyll-to-chlorophyllide ratio. Plant Physiol. 128(4), 1255–1263 (2002)
G.C. Vanlerberghe, Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int. J. Mol. Sci. 14(4), 6805–6847 (2013)
G.R. Rout, S. Sahoo, Role of iron in plant growth and metabolism. Rev. Agric. Sci. 3, 1–24 (2015)
J. Abadía, A.-F. López-Millán, A. Rombolà, A. Abadía, Organic acids and Fe deficiency: a review. Plant Soil 241(1), 75–86 (2002)
M.A. Khan, Halophyte seed germination: success and pitfalls, in International symposium on optimum resource utilization in salt affected ecosystems in arid and semi arid regions. (Desert Research Centre, Reno, 2002), pp. 346–358
V. Fernández, G. Ebert, Foliar iron fertilization: a critical review. J. Plant Nutr. 28(12), 2113–2124 (2005)
D. Alidoust, A. Isoda, Effect of γFe2O3 nanoparticles on photosynthetic characteristic of soybean (Glycine max (L.) Merr.): foliar spray versus soil amendment. Acta Physiol. Plant. 35(12), 3365–3375 (2013)
M. Ghorbanpour, A. Movahedi, M. Hatami, K. Kariman, F. Bovand, M. Shahid, Insights into nanoparticle-induced changes in plant photosynthesis. Photosynthetica 59(4), 570–586 (2021)
J. Abadía, A. Álvarez-Fernández, F. Morales, M. Sanz, A. Abadía, Correction of iron chlorosis by foliar sprays. Int. Symp. Foliar Nutr. Perenn. Fruit Plants 594, 115–121 (2001)
I. Couée, C. Sulmon, G. Gouesbet, A. El Amrani, Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 57(3), 449–459 (2006)
L. Van Loon, P. Bakker, C. Pieterse, Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 36(1), 453–483 (1998)
M.S. Attia, A.M. Younis, A.F. Ahmed, A. Elaziz, Comprehensive management for wilt disease caused by Fusarium oxysporum in tomato plant. Int. J. Innov. Sci. Eng. Technol. 4(12), 2348–7968 (2016)
A.H. Hashem, A.M. Abdelaziz, A.A. Askar, H.M. Fouda, A. Khalil, K.A. Abd-Elsalam, M.M. Khaleil, Bacillus megaterium-mediated synthesis of selenium nanoparticles and their antifungal activity against Rhizoctonia solani in Faba bean plants. J. Fungi 7(3), 195 (2021)
A. Michalak, Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 15(4), 523–530 (2006)
K. Kubalt, The role of phenolic compounds in plant resistance. Biotechnol. Food Sci. 80(2), 97–108 (2016)
N. Ibrahem, H. Latif, M.M. Saifeif, A. Mogazy, Impact of different crystal sizes of nano-iron oxide as fertilizer on wheat plants photosynthetic pigments content. Egypt. J. Chem. 64(8), 4635–4639 (2021)
M. Kordrostami, B. Rabiei, H.H. Kumleh, Biochemical, physiological and molecular evaluation of rice cultivars differing in salt tolerance at the seedling stage. Physiol. Mol. Biol. Plants 23(3), 529–544 (2017)
A.A.H.A. Latef, M.F.A. Alhmad, M. Kordrostami, A.-B.A.-E. Abo-Baker, A. Zakir, Inoculation with Azospirillum lipoferum or Azotobacter chroococcum reinforces maize growth by improving physiological activities under saline conditions. J. Plant Growth Regul. 39(3), 1293–1306 (2020)
S.S. Gill, N. Tuteja, Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48(12), 909–930 (2010)
H.F. Alharby, E.M. Metwali, M.P. Fuller, A.Y. Aldhebiani, Impact of application of zinc oxide nanoparticles on callus induction, plant regeneration, element content and antioxidant enzyme activity in tomato (Solanum lycopersicum Mill.) under salt stress. Arch. Biol. Sci. 68(4), 723–735 (2016)
A. Konate, X. He, Z. Zhang, Y. Ma, P. Zhang, G.M. Alugongo, Y. Rui, Magnetic (Fe3O4) nanoparticles reduce heavy metals uptake and mitigate their toxicity in wheat seedling. Sustainability 9(5), 790 (2017)
J. Hu, H. Guo, J. Li, Y. Wang, L. Xiao, B. Xing, Interaction of γ-Fe2 O3 nanoparticles with Citrus maxima leaves and the corresponding physiological effects via foliar application. J. nanobiotechnol. 15(1), 1–12 (2017)
A. Soltani, K.R. Kahkhaie, S.M. Haftcheshmeh, A.A. Jalali Nezhad, M.M. Akbar Boojar, The comparative study of the effects of Fe2O3 and TiO2 micro-and nanoparticles on oxidative states of lung and bone marrow tissues and colony stimulating factor secretion. J. Cell. Biochem. 120(5), 7573–7580 (2019)
S.K. Dhoke, P. Mahajan, R. Kamble, A. Khanna, Effect of nanoparticles suspension on the growth of mung (Vigna radiata) seedlings by foliar spray method. Nanotechnol. Dev. 3(1), e1–e1 (2013)
D. Lin, B. Xing, Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ. Pollut. 150(2), 243–250 (2007)
Z. Asadi-Kavan, R.A. Khavari-Nejad, A. Iranbakhsh, F. Najafi, Cooperative effects of iron oxide nanoparticle (α-Fe2O3) and citrate on germination and oxidative system of evening primrose (Oenthera biennis L.). J. Plant Interact. 15(1), 166–179 (2019)
N. Pariona, A.I. Martinez, H. Hdz-García, L.A. Cruz, A. Hernandez-Valdes, Effects of hematite and ferrihydrite nanoparticles on germination and growth of maize seedlings. Saudi J. Biol. Sci. 24(7), 1547–1554 (2017)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable.
Informed Consent
Not applicable.
Research Involving Human Participation and/or Animals
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elbasuney, S., El-Sayyad, G.S., Attia, M.S. et al. Ferric Oxide Colloid: Towards Green Nano-Fertilizer for Tomato Plant with Enhanced Vegetative Growth and Immune Response Against Fusarium Wilt Disease. J Inorg Organomet Polym 32, 4270–4283 (2022). https://doi.org/10.1007/s10904-022-02442-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02442-6