Abstract
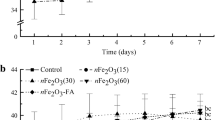
There is an urgent need to address comprehensive biosafety issues associated with the use of Fe2O3 nanoparticles (IONPs). The present study was performed to investigate the effect of 6-nm IONPs and citrate-coated IONPs (IONPs-Cit) on photosynthetic characteristics and root elongation during germination of Glycine max (L) Merr. Plant physiological performance was assessed after foliar and soil IONPs fertilization. No adverse impacts at any growth stage of the soybeans were observed after application of IONPs. The Fe2O3 nanoparticles produced a significant positive effect on root elongation, particularly when compared to the bulk counterpart (IOBKs) suspensions of concentrations greater than 500 mg L−1. Furthermore, IONPs-Cit significantly enhanced photosynthetic parameters when sprayed foliarly at the eight-trifoliate leaf stage (P < 0.05). The increases in photosynthetic rates following spraying were attributed to increases in stomatal opening rather than increased CO2 uptake activity at the chloroplast level. We observed more pronounced positive effects of IONPs via foliar application than by soil treatment. This study concluded that IONPs coated with citric acid at IONPs to citrate molar ratio of 1:3 can markedly improve the effectiveness of insoluble iron oxide for Fe foliar fertilization.


Similar content being viewed by others
Abbreviations
- A n :
-
CO2 assimilation rate
- g s :
-
Stomatal conductance
- C i :
-
Intercellular CO2 concentration
- T r :
-
Transpiration rate
- IONPs:
-
Fe2O3 nanoparticles
- IONPs-Cit:
-
Citrate-coated IONPs
- IOBKs:
-
Bulk Fe2O3
- IOBKs-Cit:
-
Citrate-coated IOBKs
References
Abadia J, Fernandez AA, Morales F, Sanz M, Abadia A (2002) Correction of iron chlorosis by foliar sprays. Acta Hort 594:115–121
Alidoust D, Suzuki S, Matsumura S, Yoshida M (2012) Chemical speciation of heavy metals in the fractionated rhizosphere soils of sunflower cultivated in a humic Andosol. Commun Soil Sci Plant Anal 43(17):2314–2322
Ankamwar B, Lai TC, Huang JH, Liu RS, Hsiao M, Chen CH, Hwu YK (2010) Biocompatibility of Fe3O4 nanoparticles evaluated by in vitro cytotoxicity assays using normal, glia and breast cancer cells. Nanotechnology 21:75102
Auffan M, Decome L, Rose J, Orsiere T, De Meo M, Briois V, Chaneac C, Olivi L, Berge-Lefranc JL, Botta A, Wiesner MR, Bottero JY (2006) In vitro interactions between DMSA-coated maghemite nanoparticles and human fibroblasts: a physicochemical and cyto-genotoxical study. Environ Sci Technol 40(14):4367–4373
Barrena E, Casals E, Colon J, Font X, Sanchez A, Puntes V (2009) Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 75:850–857
Dehner CA, Barton L, Maurice PA, Dubois JL (2010) Size-dependent bioavailability of hematite (α-Fe2O3) nanoparticles to a common aerobic bacterium. Environ Sci Technol 45:977–983
Eichert T, Burkhardt J, Goldbach HE (2002) Some factors controlling stomatal uptake. Acta Hort 594:85–90
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33:317–345
Fernandez V, Ebert G (2005) Foliar iron fertilization: a critical review. J Plant Nut 28:2113–2124
Fernandez V, Ebert G, Winkelmann G (2005) The use of microbial siderophores for foliar iron application studies. Plant Soil 272:245–252
Fernandez V, Del Río V, Abadía J, Abadía A (2006) Foliar iron fertilization of peach (Prunus persica (L.) Batsch): effects of iron compounds, surfactants and other adjuvants. Plant Soil 289:239–252
Fox TR, Comerford NB, McFee WW (1990) Phosphorus and aluminium from a spodic horizon mediated by organic acids. Soil Sci Soc Am J 54:1763–1767
Ghodak G, Seo YD, Lee DS (2011) Hazardous phytotoxic nature of cobalt and zinc oxide nanoparticles assessed using Allium cepa. J Hazard Mater 186:952–955
Gobran GR and Clegg S (1996) A conceptual model for nutrient availability in the mineral soil-root system: Can J Soil Sci 76:125–131
Grignon C, Sentenac H (1991) pH and ionic conditions in the apoplast. Annu Rev Plant Physiol Plant Mol Biol 42:103–128
He S, Feng Y, Ren H, Zhang Y, Gu N, Lin X (2011) The impact of iron oxide magnetic nanoparticles on the soil bacterial community. J Soil Sediment 11:1408–1417
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195
Hsu HH, Ashmead HD (1984) Effect of urea and ammonium nitrate on the uptake of iron through leaves. J Plant Nutr 7:291–299
Inamullah and Isoda A (2005) Adaptive changes in soybean and cotton to water stress I. Transpiration changes in relation to stomatal area and stomatal conductance. Plant Prod Sci 8:16–26
Kallay N, Matijevic E (1985) Adsorption at solid/solution interfaces; I. Interpretation of surface complexation of oxalic and citric acids with hematite. Langmuir 1:195–201
Karlsson HL, Cronholm P, Gustafsson J, Moller L (2008) Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol 21(9):1726–1732
Khodakovskaya M, Dervishi E, Mahmood M, Xu Y, Li Z, Watanabe F, Biris AS (2009) Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ASC nano 3(10):3221–3227
Kotsmar C, Yoon KY, Yu H, Ryoo SY, Barth J, Shao S, Prodanovic M, Milner TE, Bryant SL, Huh C, Johnston KP (2010) Stable citrate-coated iron oxide superparamagnetic nanoclusters at high salinity. Ind Eng Chem Res 49:12435–12443
Kraemer SM (2004) Iron oxide dissolution and solubility in the presence of siderophores. Aquat Sci 66:3–18
Lei Z, Fashui H, Shipeng L, Liu C (2005) Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res 104:83–91
Long SP, Shawanal XG, Naidu SL, Ort DR (2006) Can improvement in photosynthesis increase crop yields. Plant Cell Environ 29:315–330
Marschner H, Romheld V, Horst WJ, Martin P (1986) Root-induced changes in the rhizosphere: importance for the mineral nutritional of plants. Z. Pflanzenernaehr Bodenk 149:441–456
Schonherr J, Huber R (1977) Plant cuticles are polyelectrolytes with isoelectric points around three. Plant Physiol 59:145–150
Seeger EM, Baun A, Kästner M, Trapp S (2009) Insignificant acute toxicity of TiO2 nanoparticles to willow trees. J Soil Sediment 9:46–53
Silva AMN, Kong X, Parkin MC, Cammack R, Hider RC (2009) Iron (III) citrate speciation in aqueous solution. Dalton Trans 28(40):8616–8625
Singh N, Jenkins GJS, Asadi R, Doak SH (2010) Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev 1:5358–5373
Stroh A, Zimmer C, Gutzeit C, Jakstadt M, Marschinke F, Jung T (2004) Iron oxide particles for molecular magnetic resonance imaging cause transient oxidative stress in rat macrophages. Free Radic Biol Med 36:976–984
US Environmental Protection Agency (1996) Ecological effects test guidelines (OPPTS 580.4200): Seed germination/root elongation toxicity test
Wang YX, Hussain SM, Krestin GP (2001) Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol 11:2319–2331
Zhang Y, Kallay N, Matijevic E (1985) Interactions of metal hydrous oxides with chelating agents. VII. Hematite-oxalic and citric acid systems. Langmuir 1:201–206
Zhu H, Han J, Xiao JQ, Jin Y (2008) Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J Environ Monit 10:713–717
Acknowledgments
The authors wish to thank Prof. Kazuyuki Inubushi of Chiba University for allowing the use of soil testing apparatus. We would also like to thank all three reviewers for their insightful comments, which contributed greatly to the manuscript overall quality and completeness.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Ferrarese-Filho.
Rights and permissions
About this article
Cite this article
Alidoust, D., Isoda, A. Effect of γFe2O3 nanoparticles on photosynthetic characteristic of soybean (Glycine max (L.) Merr.): foliar spray versus soil amendment. Acta Physiol Plant 35, 3365–3375 (2013). https://doi.org/10.1007/s11738-013-1369-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-013-1369-8