Abstract
A Reflectance Absorptance Transmittance (RAT) meter, developed for routine measurements of Abtλ nm on leaves, was used to measure absorptance of algal films mounted on glass fibre filter disks for use in PAM (Pulse Amplitude Modulation) fluorometry. Absorptance readings (Abtλ nm) are essential to quantitate the photosynthetic electron transport rate from PAM data and are also helpful if algal disks are used in an IRGA (InfraRed Gas Analyzer). Measurements were made on Synechococcus R-2 (Cyanophyceae), Acaryochloris marina (Chlorobacteria), Chlorella vulgaris (Chlorophyceae), Chaetoceros sp. (Bacillariophyceae) and Isochrysis sp. (Haptophyta) in blue (445 nm), green (525 nm) and red (625 nm) light. Plots of Abtλ vs. Chl a (mg Chl a m-2) or Chl d for Acaryochloris (mg Chl d m-2) of the algae followed exponential saturation curves (Abt%λ = Abt%λ, ∞ (1-e-k×Chl)) usually reaching asymptotes in the blue light used by blue-diode PAM machines. Synechococcus, Chlorella, Chaetoceros and Isochrysis Abt%445nm saturates ≈ 50-100 mg Chl a m-2 (Synechococcus: Abt%445 nm, max = 77.3±0.94, ±95% conf. limits, Chlorella: Abt%445 nm, max = 77.0±2.06, Chaetoceros: Abt%445 nm, max = 80.6±1.87; Isochrysis: 83.2±8.06 mg Chl a m-2); Acaryochloris above about 100 mg Chl d m-2. Asymptotic loading of cells was in general easily achieved in blue light in the case of Synechococcus, Chlorella and Acaryochloris but not in green, red or “white” light. It was difficult to achieve asymptotic loading for Chaetoceros and Isochrysis due to mucilaginous blockage of the filter disks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
We describe the application of a Reflectance-Absorptance-Transmittance (RAT) meter designed by Aquation Pty Ltd, Australia to experimentally measure the absorptance of unicellular algae and photosynthetic bacteria mounted onto glass fibre disks (Ritchie and Runcie 2013, 2014). The RAT meter uses a Red-Green-Blue (RGB) diode as a light source to provide blue (445 nm), green (525 nm) or red (625 nm) light sources similar to those wavelengths used by blue and red-diode based Pulse Amplitude Modulation (PAM) machines. We show that the RAT meter provides a convenient method for estimating absorptance in the laboratory and field for algal and photosynthetic bacterial films compared with Taylor-absorption sphere based methods. The non-destructive method enables measurement of films of photosynthetic organisms on a glass fibre disc if the same algal disk is used for PAM measurements. Glass fibre disks impregnated with algae are also a convenient method of estimating algal respiration and photosynthesis using IRGA (InfraRed Gas Analysers) where the absorptance of the experimental material is also an important consideration.
Fluorescence-based techniques for measuring photosynthesis such as those used by PAM machines, Photosynthetic Efficiency Analyzers (PEA) (Hansatech Instruments, Kings Lynn, Norfolk, England, UK, http://www.hansatech-instruments.com/) and Fast-Repetition Rate Fluorometry (FRRf) equipment (Oxborough et al. 2012)
are all very useful for estimating photosynthetic electron transport rate (ETR). They offer the great advantage of speed of measurement and hence very large amounts of data can be collected in short periods of time. But PAM, PEA and FRRf methods make particular demands on adequate measurements of absorbed irradiance because not only is irradiance a required input for ETR calculations but photosynthesis is calculated from fluorescence light output from absorbed light. Photosynthetic rate as ETR is calculated from the product of the fluorescence-based calculation of the quantum yield of photochemical energy conversion (Genty et al. 1989; Krause and Weis 1991; Schreiber et al. 1995; Figueroa et al. 2003), a photosystem II/I allocation factor (for oxygenic photosynthetic organisms the (assumed) allocation factor is 0.5; Melis 1989), the incident irradiance (E) and the (photosynthetic) absorptance (Abtλ nm) of the incident light (Ritchie and Runcie 2014). Hence, knowing the amount of light absorbed by the photosynthetic organism (oxygenic or anoxygenic photosynthesis) (Absorptance, Abtλ nm) is essential to calculate the photosynthetic rate using PEA, FRRf or PAM machines. Absorptance values are also essential for calculations of photosynthetic efficiency where photosynthesis is measured using oxygen, 14CO2 or CO2 monitoring methods. Some photosynthetic organisms have non-photosynthetic absorbing pigments: these may sometimes need to be taken into account, particularly for “white” light absorptance determinations in apparatus using a “white” light source.
Absorptance (Abtλ nm) is defined as the amount of irradiance absorbed by a translucent object and is usually expressed as a percentage (Abt%λ nm) with the wavelength or spectral range of the light source specified. Absorptance is calculated as Abt%λ nm = 100 – T%λ nm – R%λ nm where, T%λ nm is the percentage transmittance through the object at a given wavelength and R%λ nm is the percentage reflected irradiance.
Many PAM studies use a standard default absorptance value (AbtF) of 0.84 (or Abt%F = 84) derived from a study using a Taylor Sphere by Björkman and Demmig (1987) for PPFD (400 – 700 nm) irradiance. It is the default value often incorporated into the software of popular PAM machines. Estimates of ETR based on AbtF = 0.84 are most properly designated relative ETR (rETR) to reflect the fact that they are based on an experimentally measured fluorescence yield (Y or ΦPSII) (Genty et al. 1989; Schreiber et al. 1995) and PPFD (400 – 700 nm) irradiance (mol photons m-2 s-1) but not on an experimentally determined absorptance value (Abt%λ nm).
When using a PAM machine, the default absorptance value of 0.84 (AbtF or Abt400-700 nm ≈ 0.84) is not appropriate for unicellular algal films when using a blue or red diode light source (Ritchie and Runcie 2014) nor for photosynthetic bacteria using a blue-light source (Ritchie and Runcie 2013). The standard absorptance value (AbtF) is derived from measurements of absorptance of leaves, not algal films, and the value from Björkman and Demmig (1987) is an overall mean calculated for the whole PPFD range (400-700 nm) not blue or red light sources. Calculation of ETR from data obtained using a blue-diode PAM requires absorptance values for incident blue light (Abt%445nm) and a red-diode based PAM machine requires absorptance values for red light. For a PAM machine using a “white” actinic light source (solar temperature ≈ 5778 K) absorptance values for incident “white” light (PPFD400-700 nm) are needed. An estimate of Abt%400-700 nm requires either absorptance measurements under incident blue, green and red light or using a “white” light source such as an RGB diode with currents applied to the red, green and blue channels to provide an approximate “white” light (Ritchie and Runcie 2014). Some studies have used a Taylor sphere fitted with a quartz halogen 6000 K light source (comparable to sunlight) to estimate Abt%400-700 nm for macroalgae and aquatic macrophytes (Frost-Christensen and Sand-Jensen 1992; Mercado et al. 1996; Cebrian et al. 1999; Longstaff et al. 2002; Figueroa et al. 2003; Runcie and Durako 2004) or other setups (Figueroa et al. 2009). Schultz (1996) used natural sunlight as the light source for his Taylor sphere. Absorptance measurements are needed for attempts to calculate quantum efficiency of photosynthesis (Ritchie 2010).
Average absorptance values of about 0.95 for 445 nm (blue) and about 0.89 for red light sources (625 nm) are found in a range of mature leaves of vascular plants (Ritchie and Runcie 2014). Some seagrasses have absorptance properties very like terrestrial vascular plant leaves (Posidonia, Runcie and Durako 2004) but Ritchie and Runcie (2014) point out that in the case of some xerophytes, seagrasses such as Halophila and freshwater aquatic angiosperms absorptances may range to as low as 0.30 for mature leaves. The low absorptances of some seagrasses are attributed to their photosynthetic epidermal cells and lack of palisade mesophyll. This explanation would also account for the low absorptances generally found in the few macrophytic algae where absorptances have been measured (several species, Frost-Christensen and Sand-Jensen 1992; Mercado et al. 1996; Figueroa et al. 2009; epiphytes on the seagrass Posidonia oceanica, Cebrian et al. 1999; Ulva, Longstaff et al. 2002; Ulva and Porphyra, Figueroa et al. 2003; Ulva, Beach et al. 2006). Absorptances of macroalgae can vary enormously even for the same species or genus or season. Beach et al. (2006) noted that despite Ulva species being algae with very favourable flat sheet morphology for absorptance studies, various researchers have found absorptances ranging from 19 to 93%. To complicate matters, Figueroa et al. (2009) noted strong seasonal variation in absorptance of Ulva. Such absorptance ranges would change the calculated ETR by a factor of nearly 5, depending on which absorptance factor was used. Attempts to correlate photosynthetic ETR with photosynthetic measurements using other methods such as 14C or oxygen electrodes often do not tally very well. Some of this may be due to situations where absorptances were not measured experimentally (e.g. studies on Ulva, Beer et al. 2000; Longstaff et al. 2002; Carr and Björk 2003; Beach et al. 2006) compared to studies where absorptances of Ulva were measured (Figueroa et al. 2009).
The usual method for measuring the amount of light absorbed by a plant is to use a Taylor or Ulbricht Integrating sphere attached to a spectroradiometer or spectrophotometer. Integrating sphere methods have previously been limited by the prohibitive costs and the cumbersome nature of the experimental setup, particularly for field work (Runcie and Durako 2004; Ritchie and Runcie 2014) but spectrophotometers with Integrating Spheres (Taylor Sphere attachments) are now more readily available (Ritchie and Sma-Air 2020a, b). Little information is available on absorptances of non-vascular terrestrial plants and other photosynthetic organisms such as algal films of Chlorella, Trebouxia, Trentepohlia or Chlorococcum mounted on glass fibre disks (Ritchie and Runcie 2013; Ritchie 2014; Chandaravithoon et al. 2018, 2020; Ritchie and Heemboo 2021 Ritchie and Sma-Air 2022), macrophytic algae (Frost-Christensen and Sand-Jensen 1992; Mercado et al. 1996; Cebrian et al. 1999; Longstaff et al. 2002; Figueroa et al. 2003, 2009), lichens (Solhaug et al. 2010; Ritchie 2014), corals etc.: Beer et al. 1998, 2000; Enríquez et al. 2005; Stambler and Dubinsky 2005; Rodríguez-Román et al. 2006; Hennige et al. 2009). Most PAM-based estimates of ETR on such organisms are actually rETR measurements because experimentally determined absorptances were unavailable.
The present study focuses on the absorptances of algae-impregnated filter disks. However, the absorptance characteristics of photosynthetic mats or surfaces are of great interest physiologically, ecologically and from an evolutionary point of view for example microbial mats, encrusting algae, corals, stromatolites and cushions of bryophytes (Larkum et al. 2018; Hennige et al. 2009; Ritchie 2013, 2014; Ritchie and Heemboo 2021; Ritchie and Sma-Air 2022). Absorptance of algal mats also have biotechnological significance where it may be important to know how much light is actually absorbed.
Materials and methods
Culturing the Cells: The cyanobacterium Synechococcus PCC7942 was originally obtained from the Pasteur Culture Collection (PCC). Acaryochloris marina (MBIC11017) (Miyashita & Chihara) was a gift from A.W.D. Larkum (UTS-Sydney, NSW, Australia). The green alga Chlorella vulgaris Beyerinck (Beijerinck), the diatom Chaetoceros sp. and Isochrysis sp. (Prymnesiales, Haptophyta) were from the Phuket Marine Biological Centre, Laem Panwa, Phuket 83000. Synechococcus and Chlorella grew well in BG-11 medium (Allen 1973) and Chlorella also grew well in seawater supplemented with nitrate, phosphate and trace elements as for BG-11. No added vitamins were needed. Chaetoceros, Isochrysis and Acaryochloris were grown in seawater supplemented with BG-11 trace elements, 100 mmol m-3 sodium silicate, 200 mmol m-3 KH2PO4 and 1 mol m-3 sodium nitrate. f/2 vitamins were added as described by McLachlan (1973).
Synechococcus, Acaryochloris, Chlorella, Chaetoceros and Isochrysis were grown in 250 and 500 mL conical flasks, shaken and stirred daily. Cultures of all five phototrophic microbes were kept on shelves fitted with overhead fluorescent lights (Panasonic 36 W daylight, colour temperature 6500 K: TIS 956-2533) in continuous light at about 27 oC. The light intensity in the culture room was approximately 100-150 μmol photons m-2 s-1 (PPFD 400 – 700 nm), measured using a Li-Cor photon flux meter Model LI-189 (Li-Cor Corp, USA).
Chemicals
DMSO (dimethyl sulfoxide, (CH3)2SO) was from WINNEX (Thailand) Co. Ltd, Thailand. Acetone (CH3)2CO 99.5 AR/ACS was from LOBA Chemie PVT. LTD, India. 90% acetone and 100% DMSO were neutralised with magnesium carbonate.
Preparation of Cells for Experiments: Synechococcus, Acaryochloris, Chlorella, Chaetoceros and Isochrysis cultures were filtered onto Whatman GF/C glass fibre disks (Whatman International, U.K.) using a Millipore apparatus designed for 25 mm filters as described by Ritchie and Runcie (2013). The inside diameter of the Millipore filtration apparatus was 16.2 mm and so the disks of microbial cells adhering to the glass-fibre filter had a surface area of 206.12 × 10-6 m2. The Synechococcus, Acaryochloris, Chlorella, Chaetoceros and Isochrysis-impregnated disks provided highly reproducible material for experiments. The disks were not allowed to dry out and were placed in a darkened Petri dish with a layer of filter paper moistened with seawater or BG-11 medium as appropriate before making absorptance measurements. Excessive delays in measurements were avoided. Glass fibre filters eventually block if overloaded with cells. Full loading where no more cells could be loaded onto the glass fibre filters varied from one species to another: ≈ 200 mg Chl a m-2 for Synechococcus, Acaryochloris and Chlorella but < 100 mg Chl a m-2 for Chaetoceros and Isochrysis because of the mucilaginous nature of the cells.
Scanning dual beam spectrophotometry
A standard dual beam scanning spectrophotometer was used for routine chlorophyll determinations (Shimadzu UV-1601, UV-Visible Spectrophotometer, Shimadzu Corporation, Japan, Software: UV-Probe 2.21, Shimadzu Corporation, Japan). Routine chlorophyll determinations were made in MgCO3-neutralised 90% acetone or DMSO (Jeffrey and Humphrey 1975; Ritchie 2006; Ritchie et al. 2021, 2022) using quartz cuvettes. Chlorella is a recalcitrant alga and heating at 55 oC was usually required to effectively extract chlorophylls. Fewer problems were encountered with the other algae. DMSO is a far better quantitative extractant than acetone. Following our recent practice 850 nm was used as the zero wavelength rather than 750 nm. Routine scans from 850 to 600 nm were used for data acquisition and exported as EXCEL.csv files.
Integrating sphere spectrometer
A UV Vis Spectrophotometer Model: AE-s90-2D, Serial Number: AT161006, A & E Lab (UK) Co. Ltd fitted with a 60 mm (2 1/2-inch) integrating sphere was used as described in detail in Ritchie and Sma-Air (2020a, b) to measure the optical properties of cell suspensions. The spectrophotometer was run using UV-VIS Analyst version 5.43, prom Version 0.000, Copyright 2013 Macroeasy Technologies Ltd, License number UV 20081012-001-8828-FFF.
Absorptance measurements using RAT
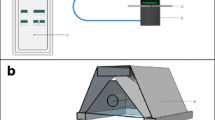
A RGB-diode based leaf absorptance RAT meter was designed by Aquation Pty Ltd, Umina Beach, Australia for the measurement of absorptance of leaves at the same light wavelengths as used by PAM fluorometers (Ritchie and Runcie 2014). The RAT was fitted with a three-colour “RGB” LED as the light source [SML-LX1610RGBW/A diode light source (Lumex Inc., 290 E. Helen Rd, Palatine, IL 60067-6976, USA)] (Carreres-Prieto et al. 2020). The bandwidths of the RGB diode used in the RAT meter were blue, 445±15 nm bandwidth; green, 525±15 nm bandwidth and red, 625±15 nm bandwidth. Intensities of each colour can be individually adjusted by changing the current to each diode. We have found that the RAT was not only useful for measuring the absorptance properties of leaves and thalli of lichens and macroalgae algae for which it was designed (Ritchie and Runcie 2014) but was also particularly well suited for absorptance readings on algal films mounted upon glass fibre disks (Cebrian et al. 1999; Ritchie 2013; Ritchie and Runcie 2013). The RAT measures transmittance of this light through a specimen to obtain T% and also measures reflectance (R %) using a diode set at 45o to the light beam in an arrangement based upon Schultz (1996) (see Fig. 1). Absorptance is calculated as Abt%λ = 100-T%λ-R% λ (Runcie and Durako 2004). The meter is calibrated using a black and a white (0 and 100% reflectance respectively) standard card for the particular light source being used (red, green, blue or a RGB combination of sources giving “white” light) following the factory calibration instructions. Some difficulties were encountered in the case of Acaryochloris because it absorbs 625 nm light so poorly (Fig. 2) for low loadings of the alga on the glass fibre disks, giving zero apparent absorptance for loadings up to about 30 mg Chl d m-2 if the standard white polyester card was used. Satisfactory results on Acaryochloris were obtained if a blank glass fibre filter disk was used as a zero rather than the factory supplied white plastic card (see Supplementary Figure). A blank glass fibre disk was also found to be much more satisfactory as a zero for Isochrysis than the factory-supplied white card.
Basic arrangement of RGB Light Emitting Diode (RGB-LED) light source and transmittance and reflection detecting photodiodes for measuring absorptance of cells mounted on a filter disc. LEDs are used as the irradiance source. An RGB diode can provide 445 nm (blue), 525 nm (green) or 625 nm (red) light sources. Adapted from Schultz (1996) and Ritchie and Runcie (2014). The working distance is about 2.5 cm between the light source and the specimen
Integrating sphere absorbances of cells suspensions of Synechococcus, Acaryochloris, Chlorella, Chaetoceros and Isochrysis standardised onto the blue absorbance peak after zeroing on 850 nm. The bandwidths of the RGB diode used in the RAT meter were blue, 445±15 nm bandwidth; green, 525±15 nm bandwidth and red, 625±15 nm bandwidth
Commercial “white Light” diodes are typically configured to have a very high level of blue light and very little green and red light compared to sunlight and so can be misleading for photosynthetic work: in the present study an RGB adjustable for Blue, Green and Red light was used set at blue 50%/green 100%/red 100% to better represent sunlight (Larkum et al. 2018; Carreres-Prieto et al. 2020). Calibration steps involved firstly measuring 100% transmittance with no sample, 0% reflectance with the black card and 100% reflectance with the white standard. The RAT needs to be re-calibrated for each coloured light source.
Saturating absorptance vs. chlorophyll curves were fitted using a simple exponential saturating model. Curves were determined for blue, green and red light and for “white” light. Curves could be determined easily for the algae in the present study with Chl a as their primary photosynthetic pigment as previously described for a selection of vascular plants and lichen (Ritchie and Runcie 2014). More caution was needed to make satisfactory measurements of the chlorobacterium, A. marina, because of the Chl d + a pigmentation of the organism (Supplementary Figure).
Statistics
Unless otherwise stated all values quoted are means ± 95% confidence limits with the number of data points quoted in brackets. Using least squares methods and error bars of the fitted parameters Abt%λ, ∞ and exponential constant k were determined and the asymptotic errors determined by matrix inversion using EXCEL. Zar (2014) was used as the standard statistical reference text.
Results
Figure 2 shows in vivo integrating sphere scans on Synechococcus, Acaryochloris, Chlorella, Chaetoceros and Isochrysis standardised onto the blue absorbance peaks for Chl a in the cases of Synechococcus, Chlorella and Chaetoceros and Isochrysis (436, 434, 439 and 439 nm respectively) and the Chl d peak for Acaryochloris (455 nm). The in vivo scans are based cell suspensions with the following chlorophyll contents based on 90% acetone extracts (μg mL-1, n = 4): Synechococcus, Chl a 1.534±0.0927; Acaryochloris, Chl d 1.947±0.198, Chl a 0.1089± 0.0125; Chlorella, Chl a 2.485±0.1592, Chl b 0.8036±0.0446; Chaetoceros Chl a 4.238±0.157, Chl c1c2 1.146±0.0367; Isochrysis, Chl a 4.02 ±0.0317, Chl c2 1.554 ± 0.0303. In vivo pigment peaks: for Synechococcus the phycocyanin peak is at 628 nm and the red Chl a peak at 677 nm, for Acaryochloris the phycocyanin and Chl a red peaks were not conspicuous under the conditions under which it was grown, the Chl d peak was at 709 nm, for Chlorella the Chl b “knee” is apparent at about 650 nm and the Chl a red peak was at 677 nm, Chaetoceros and Isochrysis both had Chl c1c2 and Chl c2 peaks at about 630 nm and a Chl a peak at 675 nm and a very marked carotenoid peak at 458 nm almost as high as the Chl a peak.
The amount of cells that can be loaded onto a glass fibre filter disk is finite because eventually the filter medium becomes clogged if overloaded. The upper cell loading limit for Synechococcus, Acaryochloris and Chlorella was about 250 mg Chl a m-2 for a GFA glass fibre disk (Figs. 3, 4 and 5). The total number of data points was n = 26 (Synechococcus) and n = 28 (Chlorella). Data were fitted to an exponential saturation curve (Abt%445 nm = Abt%445 nm, ∞ (1 – e-k×Chl a) (Chl d in the case of Acaryochloris) using least squares methods as described by Ritchie and Runcie (2013). Hence, the ½-loading point for the cells on a chlorophyll (a or d) basis was Ln (2)/k (Table 1). The exponential saturation model was generally an excellent fit to the experimental data, with significant r-values between 0.9574 and 0.9989. In the case of Synechococcus (Fig. 3, Table 1), asymptotes were reached in the case of blue, red and “white” light but had not reached asymptotic absorptances in the case of green light even at the maximum practical loading of the alga onto the glass fibre disk (≈ 200-250 mg Chl a m-2). This is consistent with the poor absorbance of Synechococcus in green light (≈ 500 – 600 nm) shown in Fig. 2. For Synechococcus the asymptotic Abt%445 nm, ∞ for disks (n = 26) were 77.3 ± 0.944; Abt%525nm, ∞ = 74.8 ± 1.82; Abt%625 nm, ∞ = 77.8 ± 0.950; Abt%white, ∞ = 75.9 ± 0.799.
Absorptance (Abt%λ nm) of Synechococcus cells mounted on GFA Glass fibre disks in blue, green, red and “white” light. Data were fitted to exponential saturation curves (Abt%λ nm = Abt%λ nm, ∞ (1 – e-k×Chl a) using least squares methods for blue (445 nm), green (525 nm) red (625 nm) light and “white” light. The fitted parameters are shown in Table 1. For Synechococcus all 4 absorptance curves reached an asymptote within the data range. The mean Abt%445 nm for disks loaded with > 100 mg Chl a m-2 was 76.8±1.26 (n = 12); Abt%525 nm = 68.71±2.23 (12); Abt%625 nm = 76.9±1.06 (n = 12), “white” Abt% = 74.0±1.43 (n = 12)
Absorptance (Abt% λ nm) of Acaryochloris cells mounted on GFA Glass fibre disks in blue, green and red light fitted to exponential saturation curves as described for Synechococcus. The fitted parameters are shown in Table 1. Acaryochloris absorbed poorly at 525 and 625 nm and the red peak of Chl d is mainly outside the PPFD range and so absorbance in “white” light is rather low. The mean Abt%445 nm for disks loaded with >≈100 mg Chl d m-2 was 80.4 ± 2.94 (n = 16)
Absorptance (Abt% λ nm) of Chlorella cells mounted on GFA Glass fibre disks in blue, green and red light. Data were fitted to exponential saturation curves. The fitted parameters are shown in Table 1. For Chlorella only the absorptance vs. Chl a curve for blue light reached an asymptote within the experimental range: the mean Abt445 nm for disks loaded with > 100 mg Chl a m-2 was 76.5±1.97 (n = 12) and the asymptotic values was Abt%445 nm, ∞ = 77.0±2.06 (n = 28) for blue light
Figure 4 shows absorptance (Abt% λ nm) of Acaryochloris cells mounted on GFA Glass fibre disks in blue, green and red light fitted to exponential saturation curves as described for Synechococcus. The fitted parameters are shown in Table 1. Acaryochloris absorbs strongly at 445 nm (blue), less strongly at 525 nm (green) and very poorly at 625 nm (red) (Fig. 2). This is because the alga contains predominantly Chl d and the red peak of Chl d is mainly outside the PPFD range and so absorbance in “white” light is hence also rather low (Fig. 2) but the cell suspension absorbs very strongly in the range 350 to 500 nm. The mean Abt%445 nm for disks loaded with >≈100 mg Chl d m-2 was 80.4±2.94 (16). More satisfactory results were obtained for Acaryochloris at 625 nm if the RGB Rat was zeroed on a blank glass fibre disk (see Supplementary Figure). Whether or not the RAT was zeroed using the factor y-suppled white card or blank glass fibre disk did not seem to make a difference for blue, green and “white” light because the alga absorbs strongly in blue and green light (Fig. 2). A range of replication numbers for the different light sources used appears in Table 1 for Acaryochloris because a new set of absorptances in red (625 nm) light were needed using a blank glass fibre filter as the white blank rather than the factory supplied white card (see Supplementary Figure).
As in the case of the cyanobacterium, Synechococcus, the green alga Chlorella has very poor absorbance of light in the range 500 to 600 nm (Fig. 2). Absorptance vs. [Chl a (mg m-2)] for Chlorella (Fig. 5, Table 1) again followed simple exponential saturation curves but the asymptotic maximum absorptance was not reached within the range of possible loading of the algal cells on the glass fibre disk (≈ 100-250 mg m-2 Chl a) for green, red or “white” light but it was in the case of blue light: Abt%445 nm, 110-250 mg Chl a = 76.5±0.197 (n = 12), Abt%445 nm, ∞ = 77.0±2.06 (n = 28).
For Chaetoceros, the upper cell loading limit was about 122 mg Chl a m-2 for a GFA glass fibre disk (Fig. 6, Table 1) and less than 100 mg Chl a m-2 for Isochrysis (Table 1, Fig. 7). Absorptance vs. [Chl a (mg m-2)] again followed simple exponential saturation curves but the rate at which the asymptotic maximum absorptance was reached was much faster in blue light, slightly slower in the case of green and “white” light and slowest in the case of red light. The Abt%445 nm for disks loaded with > 60 mg Chl a m-2 reached a saturation point for Chaetoceros (Fig. 6) and so a maximum standard Abt%445nm, >50 mg Chl a of 78.7±1.77 (n = 18) could be calculated as a standard absorptance for use with a blue-diode PAM machine for use where discs were loaded with a film of Chaetoceros cells with more than 60 mg m-2 Chl a. Blue light reached a saturation point (83.2±8.06, n = 33) in the case of Isochrysis (Fig. 7) only at near the highest cell loading (≈100 mg m-2 Chl a). In red light (625 nm) the absorptance vs. [Chl a (mg m-2)] curve did not even nearly reach an asymptote and so individual absorptances of glass fibre discs impregnated with Chlorella, Chaetoceros or Isochrysis would need to be measured if photosynthesis was to be measured using a red-diode or “white” light PAM mode (Figs. 5, 6 and 7).
Absorptance (Abt% λ nm) of Chaetoceros cells mounted on GFA Glass fibre disks in blue, green, red and “white” light. Data were fitted to exponential saturation curves (Table 1). The green and “white” curves are very similar because of the very high carotenoid content (Fig. 2) but the algal disks absorbed very poorly in red light giving an unsatisfactory asymptotic value. For Chaetoceros only the absorptance vs. Chl a curve for blue light reached an asymptote within the experimental range: the mean Abt445 nm for disks loaded with 50 to 122 mg Chl a m-2 was 78.7±1.77 (n = 18) and the asymptotic value, Abt%445 nm, ∞ = 80.6±1.87 (n = 28)
Absorptance (Abt% λ nm) of Isochrysis cells mounted on GFA Glass fibre disks in blue, green, red and “white” light. A blank glass fibre disk was used as a blank for all measurements of Isochrysis. Data were fitted to exponential saturation curves (Table 1). As in the case for Acaryochloris the factory-supplied white card was unsatisfactory for red-diode measurements of Isochrysis and so a blank glass fibre disk was used. Within the cell-loading range that could be used (up to ≈ 100 mg m-2) absorptances did not quite reach asymptotes except in the case of blue and “white” light light (Table 1)
Discussion
The absorbance curves shown in Fig. 2 using an integrating sphere are similar to those already published (Larkum et al. 2018; Ritchie and Sma-Air 2020b) and show that Synechococcus, Acaryochloris, Chlorella and the Chl c organisms Chaetoceros (Chl a + c1c2) and Isochrysis (Chl a + c2) all strongly absorb blue light due the blue absorption maxima of Chl a and d. Acaryochloris with its unique Chl d-based photosynthesis was once thought to be a very unusual organism but it is now known to be widespread rather than merely a photosynthetic curiosity (Larkum et al. 2012). Generally blue light absorptance saturates at a lower irradiance than green, red or “white” light (Figs. 3, 4, 5, 6 and 7, Table 1). Saturating absorptances of algal films are often considerably different to the standard default value of Abt%F = 84 (Björkman and Demmig 1987) and are limited by the loading characteristics of cells onto filter disks.
The colour-temperature of the sun is about 5778 K and so produces relatively little blue light compared to green and red light (Ritchie et al. 2017; Larkum et al. 2018). It is also important that solar energy rapidly attenuates in water with photosynthetically useful red light disappearing in a few meters of water and then green light, such that at depths of greater than about 20 metres only blue light remains (Ritchie et al. 2017; Larkum et al. 2018). In the open ocean, surface inhibition of photosynthesis is well documented but at the depths where photosynthesis is optimum in oceanic water (≈20 to 40 m) only blue light is actually available for photosynthesis. In aquatic environments the importance of blue light for photosynthesis is perhaps given less attention than it deserves, all the organisms used in this study are very good at absorbing photosynthetically usable blue light in particular diatoms and Isochrysis (Figs. 2, 6 and 7, Table 1). Ability to use wavelengths >550 nm found in the solar spectrum is of more importance to terrestrial and shallow water photosynthesis (Frost-Christensen and Sand-Jensen 1992; Larkum et al. 2018) and Acaryochloris can use far red (>690 nm) for photosynthesis in habitats where blue and 680 nm light is largely filtered out by Chl a-based photosynthetic organisms living above it (Larkum et al. 2012).
Figures 3, 4, 5, 6 and 7 show that the RGB-RAT in blue light (445 nm) gives plausible absorptance values (Abt%445 nm) on photosynthetic organisms filtered onto glass fibre disks. The shapes of the absorptance vs. concentration of chlorophyll curves vary considerably from one organism to another. This has important implications on choosing what absorptance data is used for other studies. For example, blue light absorptance data would be appropriate for PAM studies using all five organisms if you were using a blue diode-type PAM machine that used blue light as both the measuring and actinic light source. Red light absorptance data would be appropriate if one wished to measure ETR of the oxygenic photosynthetic organisms with Chl a as their primary photosynthetic pigment using a PAM based on red diode technology. The red light (625 nm) of the RGB diode used in the RAT meter may be satisfactory for measuring absorptance of Chl a (Synechococcus, Chlorella, Chaetoceros and Isochrysis, all with a red-peak at about 676 nm) but not for the red peak absorptance of Acaryochloris (709 nm, Chl d, Figs. 2 and 4). Absorbance in “white” light and in 630 nm red light is heavily weighted by the phycocyanin present in Synechococcus (Figs. 2 and 3). Chaetoceros and Isochrysis heavily absorb in blue and green light and hence in “white” light (Figs. 2, 6 and 7) but failed to reach asymptotic absorptance in red light (Figs. 6 and 7, Table 1) on the range of disk loadings used in this study. Higher loadings clogged the filter disks. Mucilaginous cells such as many chromophytes (Chl c-containing organisms) present difficulties.
The geometry of the RAT meter (Fig. 1) is based on the setup used by Schultz (1996). It uses a light source that unambiguously measures transmittance through the specimen using a photodiode directly under the floor of the measuring chamber. Typically, transmittance near the blue and red in vivo peaks for Chlorophylls is very low (Fig. 2). Reflectance is a more complex issue. The photometer setup used by Schultz (1996) to estimate reflectance has the detector diode set at an angle of 45o to the normal light beam (Fig. 1 in Schultz 1996), essentially the same as the setup used in the RAT meter (Fig. 1) and Figure 1 in Ritchie and Runcie (2014). This leads to theoretical and experimental problems outlined by Ritchie and Runcie (2014) but an algal or photosynthetic bacterial film on a glass fibre disk is probably closer to a Lambertian surface than a leaf of a vascular plant, in part due to the lack of a reflective waxy cuticle.
The results of experimental measurements of Abt445 nm in this study show that the experimentally measured absorptances of glass fibre disks fully loaded with Synechococcus, Acaryochloris, Chlorella or the Chl c-containing Chaetoceros and Isochrysis have absorptances similar to the default absorptance value (Abt%F = 84) currently in common use (Synechococcus: Abt%445 nm, ∞ = 77.3±0.94, n = 26; Acaryochloris: Abt%445 nm, ∞ = 79.5±2.18 (n = 44); Chlorella: Abt%445 nm, ∞a = 77.0±2.06 (n = 28); Chaetoceros Abt%445 nm, ∞ = 80.6±1.88 (n = 28); Isochrysis Abt%445 nm, ∞ = 83.2±8.06 (n = 33). Thus, the ETR based estimates of photosynthesis of Chlorella and Chaetoceros made assuming a standard absorptance value of 84% in studies before RAT measurements were available (Ritchie 2008; Ritchie and Runcie 2013; Seatae et al. 2014) were not greatly in error because high enough Chl a m-2 loading values were used. This, however, is only fortuitous. In contrast, the standard absorptance value (Abt%F) of 84 used routinely for vascular plants is a considerable underestimate of absorptance in the case of the leaves of most vascular plants in blue light, where blue-light absorptances typically range from 90 to 95% or higher (Ritchie and Runcie 2014).
The absorptance of a film of unicellular algae or photosynthetic bacteria depends on how many cells are loaded onto the glass fibre disk: this is limited by blockage of the filter. In the case of the experimental organisms used in the present study, the Abt%445 nm vs. concentration of chlorophyll (as mg Chl a m-2) follows an exponential saturation curve of the form (Abt%445 nm = Abt%445 nm, ∞× (1- e (-k× [Chl])). The asymptotic absorptance value varies from one organism to another and under different wavelengths of light. In some cases, the asymptotic value is very different to the default AbtF of 0.84 used as a standard setting in many PAM machines. The kinetic constant (k), and hence the ½ point for saturating loading (Ln(2)/k), varies much more widely than the asymptotic value from one alga to another and in different light regimes.
Figures 3, 4, 5 and 6 show that Abt%445 nm, max is very close to the asymptotic value (Abt%445 nm, ∞) if chlorophyll a loadings are above 100 mg Chl a m-2 for Synechococcus, Acaryochloris and Chlorella and > 60 mg Chl a m-2 for Chaetoceros. These values for Abt445 nm could be used as standard in many circumstances because a Chl a or Chl d determination is part of most routine photosynthetic studies and so if the chlorophyll a or chlorophyll d loadings onto glass fibre filters exceeded the saturating values for Chl a m-2 the saturating values for absorptance could be used instead of actual absorptance readings. There would be little error in estimates of ETR. In the case of Isochrysis it appears to be difficult to reach an optically saturating loading even in blue light (Fig. 7). Absorptances of macroalgae show more variability than one might expect: Figueroa et al. (2009) noted a very troublesome seasonal variability in Ulva which as a flat sheet only two cells thick which one would think presented a very uniform material for photosynthetic studies. Enríquez et al. (2005) showed that there was an exponential saturation curve relationship between chlorophyll a content (mg m-2) of seagrass leaves (Thalassia testudinum) and absorptance (Abt%400-700 nm). Similar shaped curves were found in Red Maple (Acer rubrum) (Bauerle et al. 2004). Figures 3, 4, 5, 6 and 7 on unicellular algae loaded onto glass fibre disks show more clearly the relationship between absorptances of photosynthetic surfaces and chlorophyll content than selecting leaves at various stages of development and age and measuring their absorptance and chlorophyll content. These figures help to show that the RGB-RAT gives valid estimates of absorptances.
Asymptotic values for absorptance were found experimentally at the maximum loading of cells in the cases of Synechococcus in blue, red and “white” light but not in green light. Acaryochloris absorbs strongly in blue and green light and so absorptances as asymptotic or nearly so in blue, green and “white” light (Figs. 2 and 3) but the red light of the RGB diode used in the RAT machine is not suitable for estimating absorptance of a Chl d-containing organism at the red absorptance peak for in vivo Chl d (Figs. 2 and 4). Absorptances of Chlorella and the Chl c-containing Chaetoceros and Isochrysis are also strongly asymptotic in blue light. The overall mean asymptote for all the species tested for blue light was Abt%445 nm, ∞ ≈ 80 mg Chl a m-2 and in all species was almost asymptotic in green and “white” light. Chlorella and especially Chaetoceros and Isochrysis did not reach an asymptote in red light: in such cases an asymptotic absorptance cannot be assumed for a high loading of algae onto a glass fibre disk and the absorptance has to be measured experimentally.
Some algae contain photoprotectant compounds similar to anthocyanin found in many vascular plants. Merzlyak et al. (2008) have shown in various tree species that anthocyanin primarily affects absorptance in green and orange light rather than absorptance in blue and red light. Similar conclusions were drawn for Coleus varieties heavily pigmented with anthocyanin (Burger and Edwards 1996). Thus anthocyanin does not greatly affect absorptances in blue and red light and so does not cause difficulties in the use of blue or red-diode based PAM machines to estimate ETR in such plants. Anthocyanin does affect the overall absorptance in the PPFD range (Abt%400-700 nm) and so the correct absorptance value needs to be estimated when using a PAM with an incandescent light source. Not only algae but some animals with photosynthetic endosymbionts also have non-photosynthetic photoprotectant fluorescent and non-fluorescent compounds which strongly absorb blue light but not red light and so would affect the amount of photosynthetically useable blue light (Leutenegger et al. 2007). The perietin synthesized by the fungal partner in many lichen associations also strongly absorbs UV-blue light (Solhaug et al. 2010). A red-diode based PAM is perhaps the more appropriate PAM to use in such circumstances.
Use of a RGB (Red-Green-Blue) LED diode allows separate measurements of absorptance in “white”, red, green and blue light and is particularly valuable for work with cyanobacteria and rhodophytes which have phycobilin pigments absorbing in the green and orange parts of the spectrum. The disadvantage of using an RGB diode of similar specifications as that used in the present study (SML-LX1610RGBW/A) is that the red wavelength peak is 625 – 630 nm (a suitable wavelength for human perception of red colour) which is not very close to the in vivo red peak of Chl a (670-680 nm, Fig. 2) (but is close to the peaks for Chl c1 and c2) and is definitely not suitable for measuring at the near-infrared absorptance peak of Chl d in vivo in Acaryochloris (Fig. 4). Absorptance values for red, green and blue light also allow an estimation of absorptance in sunlight (5778 K) using methods as described in our previous paper (Ritchie and Runcie 2014) either by the calculation of a mean absorptance from the blue, green and red light absorptances or by adjusting the intensity of the three colour channels to achieve an approximation to “white” light. Some types of PAM machine and other photosynthetic apparatus use red light sources: in such cases the RGB diode source in the RAT device could be replaced by a diode with a more suitable wavelength preferably of the same spectral specification as the light source of the photosynthetic measurement apparatus in question. Unfortunately, the commercially available RGB-diodes are designed for human-eye sensitivity: for photosynthetic purposes a red-diode with a peak at about 670 to 680 nm would be more appropriate.
The RGB RAT Machine described in the present study was designed as a simple portable devise suitable for fieldwork (Ritchie and Runcie 2014). Integrating sphere spectrophotometers are now more readily available than previously (Ritchie and Sma-Air 2020b) but require special attachments to measure the transmission (T%λ) and reflectance (R%λ) and hence absorptance (Abt%λ) characteristics of translucent disk material. Using such an integrating sphere setup of a scanning spectrophotometer would allow measurements of absorptance at the red peak of Chl d-type oxygenic organisms and for photosynthetic bacteria at near infrared wavelengths as well as over their entire photosynthetically active spectrum. Since the original development of the RAT machine our laboratory has used it for estimating absorptances for PAM-based estimates of electron transport rates in a wide variety of systems (for example: Chandaravithoon et al. 2018, 2020, Ritchie 2013, 2014, Ritchie and Heemboo 2021, Ritchie and Runcie 2013, 2014, Ritchie and Sma-Air 2022).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request as EXCEL FILES.
Abbreviations
- Abt%λ nm :
-
percentage absorptance at wavelength λ
- Abt%F :
-
default Leaf Absorptance Factor as a percentage
- CL:
-
confidence limit
- E:
-
irradiance (mol photons m-2 s-1) PPFD
- ETR:
-
Absolute Electron Transport Rate
- rETR:
-
Relative Electron Transport Rate
- PAM:
-
fluorometry
- Pulse:
-
Amplitude Modulation fluorometry
- PPFD:
-
Photosynthetic Photon Fluence Density (400 – 700 nm)
- Pg :
-
Gross Photosynthesis
References
Allen MM 1973 Methods for cyanophyceae. In: Stein JR (ed.): Handbook of Phycological Methods: culture methods and growth measurements. Cambridge University Press, Cambridge pp 127–138.
Bauerle WL, Weston DJ, Bowdena JD, Dudley JB, Toler JE (2004) Leaf absorptance of photosynthetically active radiation in relation to chlorophyll meter estimates among woody plant species. Scientia Horticulturae-Amsterdam 101:169–178
Beach KS, Borgeas HB, Smith CM (2006) Ecophysiological implications of the measurement of transmittance and reflectance of tropical macroalgae. Phycologia 45:450–457
Beer S, Ilan M, Eschel A, Weil A, Brickner I (1998) Use of pulse amplitude modulated (PAM) fluorometry for in situ measurements of photosynthesis in two Red Sea faviid corals. Mar Biol 131:697–612
Beer S, Larsson C, Poryan O, Axelsson L (2000) Photosynthetic rates of Ulva (Chlorophyta) measured by pulse amplitude modulated (PAM) fluorometry. Eur J Phycol 35:69–74
Björkman O, Demmig B (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77K among vascular plants of diverse origins. Planta 170:489–504
Burger J, Edwards GE (1996) Photosynthetic efficiency, and photodamage by UV and visible radiation, in red versus green leaf coleus varieties. Plant Cell Physiol 37:395–399
Carr H, Björk M (2003) A methodological comparison of photosynthetic oxygen evolution and estimated electron transport rate in tropical Ulva (Chlorophyceae) species under different light and inorganic carbon conditions. J Phycol 39:1125–1131
Carreres-Prieto D, García JT, Cerdán-Cartagena F, Suardiaz-Muro J (2020) Performing calibration of transmittance by single RGB-LED within the visible spectrum. Sensors 20:3492
Cebrian J, Enriquez S, Fortes M, Agawin N, Vermaat JE, Duarte CM (1999) Epiphyte accrual on Posidonia oceanica (L.) Delile leaves: implications for leaf absorption. Bot Mar 42:123–128
Chandaravithoon P, Nakphet S, Ritchie RJ (2018) Oxygenic and anoxygenic photosynthesis in a sewage pond. J Appl Phycol 30:3089–3102
Chandaravithoon P, Ritchie RJ, Runcie JW (2020) Measuring photosynthesis of both oxygenic and anoxygenic photosynthetic organisms using pulse amplitude modulation (PAM) fluorometry in wastewater ponds. J Appl Phycol 32:2615–2629
Enríquez S, Méndez ER, Iglesias-Prieto R (2005) Multiple scattering on coral skeletons enhances light absorption by symbiotic algae. Limnol Oceanogr 50:1025–1032
Figueroa FL, Conde-Álvarez R, Gómez I (2003) Relations between electron transport rates determined by pulse amplitude modulated chlorophyll fluorescence and oxygen evolution in macroalgae under different light conditions. Photosynth Res 75:259–275
Figueroa FL, Martínez B, Israel A, Neori A, Malta E-J, Ang P Jr, Inken S, Marquardt R, Rachamim T, Arazi U, Frenk S, Korbee N (2009) Acclimation of Red Sea macroalgae to solar radiation: photosynthesis and thallus absorptance. Aquat Biol 7:159–172
Frost-Christensen H, Sand-Jensen K (1992) The quantum efficiency of photosynthesis in macroalgae and submerged angiosperms. Oecologia 91:377–384
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Hennige SJ, Suggett DJ, Warner ME, McDougall KE, Smith DJ (2009) Photobiology of Symbiodinium revisited: bio-physical and bio-optical signatures. Coral Reefs 28:179–195
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and phytoplankton. Biochem Physiol Pflanz (BPP) 167:191–194
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol 42:313–349
Larkum AWD, Chen M, Li Y, Schliep M, Trampe E, West J, Salih A, Kühl M (2012) A novel epiphytic chlorophyll d-containing cyanobacterium isolated from a mangrove-associated red alga. J Phycol 48:1320–1327
Larkum AWD, Ritchie RJ, Raven JA (2018) REVIEW: living off the sun: chlorophylls, bacteriochlorophylls and rhodopsins. Photosynthetica 56:11–43
Leutenegger A, Kredel S, Gundel S, D'Angelo C, Salih A, Wiedenmann J (2007) Analysis of fluorescent and non-fluorescent sea anemones from the Mediterranean Sea during a bleaching event. J Exp Mar Biol Ecol 353:221–234
Longstaff BJ, Kildea T, Runcie JW, Dennison WC, Hurd C, Kanna T, Raven JA, Larkum AWD (2002) An in situ study of photosynthetic oxygen exchange and electron transport rate in the marine macroalga Ulva lactuca (Chlorophyta). Photosynth Res 74:281–293
McLachlan J (1973) Growth media – marine. In: Stein JR (ed) Handbook of Phycological methods: culture methods and growth measurements. Cambridge University Press, Cambridge, pp 25–51
Melis A (1989) Spectroscopic methods in photosynthesis: photosystem stoichiometry and chlorophyll antenna size. Phil Trans R Soc B 323:397–409
Mercado JM, Jiménez C, Niell FX, Figueroa FL (1996) Comparison of methods for measuring light absorption by algae and their application to the estimation of the package effect. Scient Mar 60:39–45
Merzlyak MN, Chivkunova OB, Solovchenko AE, Naqvi KR (2008) Light absorption by anthocyanins in juvenile, stressed, and senescing leaves. J Exp Bot 59:3903–3911
Oxborough K, Moore CM, Suggett DJ, Lawson T, Chan HG, Geider RJ (2012) Direct estimation of functional PSII reaction center concentration and PSII electron flux on a volume basis: a new approach to the analysis of fast repetition rate fluorometry (FRRf) data. Limnol Oceanogr Methods 10:142–154
Ritchie RJ (2006) Consistent sets of spectrophotometric equations for acetone, methanol and ethanol solvents. Photosynth Res 89:27–41
Ritchie RJ (2008) Fitting light saturation curves measured using PAM fluorometry. Photosynth Res 96:201–215
Ritchie RJ (2010) Modelling photosynthetically active radiation and maximum potential gross photosynthesis. Photosynthetica 48:596–609
Ritchie RJ (2013) The use of solar radiation by a photosynthetic bacterium living as a mat or in a shallow pond or flatbed reactor. Photochem Photobiol 89:1143–1162
Ritchie RJ (2014) Photosynthesis in an encrusting lichen (Dirinaria picta (Sw.) Schaer. Ex Clem., Physiaceae) and its symbiont, Trebouxia sp, using PAM fluorometry. Int J Plant Sci 175:450–466
Ritchie RJ, Heemboo M (2021) Trentepohlia sp., a terrestrial chlorophyte growing on galvanised iron lamp posts. Phycologia 60:48–61
Ritchie RJ, Larkum AWD, Ribas A (2017) Could photosynthesis function on Proxima Centauri b? Int J Astrobiol 17:147–176
Ritchie RJ, Runcie JW (2013) Measurement of the photosynthetic electron transport rate in an anoxygenic photosynthetic bacterium Afifella (Rhodopseudomonas) salina using PAM fluorometry. Photochem Photobiol 89:370–383
Ritchie RJ, Runcie JW (2014) A portable reflectance-absorptance-transmittance (RAT) meter for vascular plant leaves. Photosynthetica 52:614–626
Ritchie RJ, Sma-Air S (2020a) Solvent-free chlorophyll spectrometry in unicellular algal research. J Appl Phycol 32:2711–2723
Ritchie RJ, Sma-Air S (2020b) Using integrating sphere spectrophotometry in unicellular algal research. J Appl Phycol 32:2947–2958
Ritchie RJ, Sma-Air S (2022) Photosynthesis of an endolithic Chlorococcum alga (Chlorophyta, Chlorococcaceae) from travertine calcium carbonate rocks of a tropical limestone spring. Appl Phycol 3:1–15
Ritchie RJ, Sma-Air S, Dummee V (2022) DMSO formula for chlorophyll determination in dinoflagellates (Chl a + c2). J Appl Phycol 34:335–341
Ritchie RJ, Sma-Air S, Phongphattarawat S (2021) Using DSMO for chlorophyll spectroscopy. J Appl Phycol 33:2047–2055
Rodríguez-Román A, Hernández-Pech X, Thomé PE, Enríquez S, Iglesias-Prieto R (2006) Photosynthesis and light utilization in the Caribbean coral Montastraea faveolata recovering from a bleaching event. Limnol Oceanogr 51:2702–2710
Runcie JW, Durako MJ (2004) Among-shoot variability and leaf-specific absorptance characteristics affect diel estimates of in situ electron transport of Posidonia australis. Aquat Bot 80:209–220
Schreiber U, Bilger W, Neubaue C (1995) Chlorophyll fluorescence as a non-intrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze, ED, Caldwell, MM (eds.): Ecophysiology of Photosynthesis. Ecological Studies, 100. Springer, Berlin. pp 49–70.
Schultz HR (1996) Leaf absorptance of visible radiation in Vitis vinifera L. estimates of age and shade effects with a simple field method. Scientia Horticulturae-Amsterdam 66:93–102
Seatae P, Bunthawin S, Ritchie RJ (2014) Environmental persistence of chlorine from prawn farm discharge monitored by measuring the light reactions of photosynthesis of phytoplankton. Aquacult Int 22:321–338
Solhaug KA, Larsson P, Gauslaa Y (2010) Light screening in lichen cortices can be quantified by chlorophyll fluorescence techniques for both reflecting and absorbing pigments. Planta 231:1003–1011
Stambler N, Dubinsky Z (2005) Corals as light collectors: an integrating sphere approach. Coral Reefs 24:1–9
Zar JH (2014) Biostatistical analysis, 5th edn. Pearson New International Edition Pearson, Harlow, England, UK, p 761
Acknowledgements
We wish to thank Prof A.W.D. Larkum (University of Technology, Sydney, Australia) for his keen interest in the study and helpful input and discussion of the absorptance problem. The Faculty of Environment and Technology of Prince of Songkla University-Phuket provided access to the facilities for the project as part of its policy of promoting the aquaculture industry in Southern Thailand. Andaman Environment and Natural Disaster Research Centre (ANED) also provided facilities and support. The authors declare they have no competing interests in this project.
Contribution statement
The two authors Raymond J. Ritchie (RJR) & John W. Runcie (JWR) conceived the project, RJR and Suhailar Sma-Air did most of the experimental work based in Thailand, JWR built the machine and all authors jointly wrote the paper. The authors declare they have no competing interests in this project. All authors have seen and approved of the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PPTX 149 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ritchie, R.J., Sma-Air, S. & Runcie, J.W. Light absorptance of algal films for photosynthetic rate determinations. J Appl Phycol 34, 2463–2475 (2022). https://doi.org/10.1007/s10811-022-02782-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02782-3