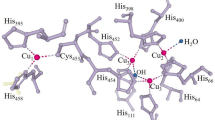
Abstract
Laccases are multicopper-containing enzymes that have the ability to oxidize a wide variety of substrates with a single electron transfer reaction. These are environmentally benign versatile biocatalysts that have gained great interest in the biotechnological community since they utilize molecular oxygen as the last electron acceptor and only produce water as a byproduct. This family of enzymes has been widely used in a broad variety of applications, ranging from food additives and beverage processing to biological diagnostics and even as crosslinking agents in the furniture construction and manufacture of biofuels. Considering the benefits of enzyme immobilization, there has been a dramatic increase in applying immobilized laccases in recent years. Despite the impressive biotechnological promise, the use of laccases in the real world is still constrained by cost–benefit analysis, particularly in terms of practically large-scale production. The enzyme industry is booming research on laccase production, and use neglects to include the economic impact of the operations. Because of their ability to metabolize complex xenobiotics, they are also useful biocatalysts in enzymatic bioremediation processes, such as wastewater treatment. This study discusses the most important and recent breakthroughs in the biocatalytic attributes, sources, and exploitation of laccases in biotechnology for a sustainable industry.
Graphical Abstract


Reprinted from Bilal and Iqbal [32] with permission from Elsevier



Reprinted from Bilal and Iqbal [31] with permission from Springer Nature

Reprinted from Alvarado-Ramírez et al. (2021); an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/)
Similar content being viewed by others
References
Abdel-Raheem AM (1997) Laccase activity of lignicolous aquatic hyphomycetes isolated from the River Nile in Egypt. Mycopathologia 139:145–150
Afreen S, Anwer R, Singh RK, Fatma T (2018) Extracellular laccase production and its optimization from Arthrospira maxima catalyzed decolorization of synthetic dyes. Saudi J Biol Sci 25:1446–1453
Ahn MY, Dec J, Kim JE, Bollag JM (2002) Treatment of 2,4-dichlorophenol polluted soil with free and immobilized laccase. J Environ Qual 31(5):1509–1515
Akram F, Ashraf S, Shah FI, Aqeel A (2022) Eminent industrial and biotechnological applications of laccases from bacterial source: a current overview. Appl Biochem Biotechnol 24:1–21
Alcalde M (2007) Laccases: biological functions, molecular structure and industrial applications In: Industrial enzymes structure, function and applications
Alexandre G, Bally R, Taylor BL, Zhulin IB (1999) Loss of cytochrome c oxidase activity and acquisition of resistance to quinone analogs in a laccase-positive variant of Azospirillum lipoferum. J Bacteriol 181:6730–6738
Bilal M, Iqbal HM, Barceló D (2019) Mitigation of bisphenol A using an array of laccase-based robust bio-catalytic cues–a review. Sci Total Environ 689:160–177
Alexandre GG, Zhulin IB (2000) Laccases are widespread. in bacteria. Trends Biotechnol 18:41–42
Allos MM, Hussein AA (2012) Production, characterization and immobilization of laccase enzyme from Bacillus cereus local isolate. Ministry Higher Educ Sci Res 1:1–106
Alves, J., da Vilar, D.S., Santana, C. E. M., Bharagava, R. N., Bilal, M., Mulla, S. I., ... & Ferreira, L. F. R. (2022). Approaches of overproduction and purification of Pleurotus laccase for the treatment of sugarcane vinasse. In: Innovative bio-based technologies for environmental remediation (pp. 265–280). CRC Press.
Andersen SO, Peter MG, Roepstorff P (1996) Cuticular sclerotization in insects. Comp Biochem Phys B 113:689–705
Ansari MKA, Khatib UM, Owens G, Fatma T (2016) Evaluation of methyl red tolerant cyanobacteria for simultaneous laccase production and dye decolorization. Int J Waste Resour 6:2252–5211
Arakane Y, Muthukrishnan S, Beeman RW, Kanost MR, Kramer KJ (2005) Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proc Natl Acad Sci USA 102:11337–11342
Arimoto M, Yamagishi K, Wang J, Tanaka K, Miyoshi T, Kamei I, Kondo R, Mori T, Kawagishi H, Hirai H (2015) Molecular breeding of lignin-degrading brown-rot fungus Gloeophyllum trabeum by homologous expression of laccase gene. AMB Express 5:81
Arregui L, Ayala M, Gómez-Gil X et al (2019) Laccases: structure, function, and potential application in water bioremediation. Microb Cell Fact 18:1–33
Asgher M, Noreen S, Bilal M (2017) Enhancing catalytic functionality of Trametes versicolor IBL-04 laccase by immobilization on chitosan microspheres. Chem Eng Res Des 119:1–11
Asgher M, Noreen S, Bilal M (2017) Enhancement of catalytic, reusability, and long-term stability features of Trametes versicolor IBL-04 laccase immobilized on different polymers. Int J Biol Macromol 95:54–62
Asgher M, Wahab A, Bilal M, Iqbal HM (2018) Delignification of lignocellulose biomasses by alginate–chitosan immobilized laccase produced from Trametes versicolor IBL-04. Waste Biomass Valorizat 9(11):2071–2079
Aslam S, Ali A, Asgher M, Farah N, Iqbal H, Bilal M (2021) Fabrication and catalytic characterization of laccase-loaded calcium-alginate beads for enhanced degradation of dye-contaminated aqueous solutions. Catal Lett 15:1–13
Aslam S, Asgher M, Khan NA, Bilal M (2021) Immobilization of Pleurotus nebrodensis WC 850 laccase on glutaraldehyde cross-linked chitosan beads for enhanced biocatalytic degradation of textile dyes. J Water Process Eng 40:101971
Bajpai P (2014) Purification of Xylanases. Xylanolytic Enzymes 24:53–61
Bao W, O’Malley DM, Whetten R, Sederoff RRA (1993) laccase associated with lignification in loblolly pine xylem. Science 260:672–674
Barlocher F, Boddy L (2016) Aquatic fungal ecology - How does it differ from terrestrial? Fungal Ecol 19:5–13
Bastos AC, Magan N (2009) Trametes versicolor: potential for atrazine bioremediation in calcareous clay soil, under low water availability conditions. Int Biodeterior Biodegradation 63(4):389–394
Bateman A, Coin L, Durbin R, Finn RD, Hollich V, Griffiths-Jones S, Khanna A, Marshall M, Moxon S, Sonnhammer EL, Studholme DJ, Yeats C, Eddy SR (2004) The Pfam Protein Families Database. Nucleic Acids Res Database 32:D138–D141
Bauer CG, Kuhn A, Gajovic N, Skorobogatko O, Holt PJ, Bruce CN et al (1999) New enzyme sensors for morphine and codeine based on morphine dehydrogenase and laccase. Fresenius J Anal Chem 364:179–183
Bell AA, Wheeler MH (1986) Biosynthesis and functions of fungal melanins. Annu Rev Phytopathol 24:411–451
Berthet S, Thevenin J, Baratiny D, Demont-Caulet N, Debeaujon I, Bidzinski P, Leple JC, Huis R, Hawkins S, Gomez LD et al (2012) Role of plant laccases in lignin polymerization. Adv Bot Res 61:145
Bertrand T, Jolivalt C, Briozzo P, Caminade E, Joly N, Madzak C, Mougin C (2002) Bacterial enzymes and multi-enzymatic systems for cleaning. Biochemistry 41:7325–7333
Bhatt, P., Tiwari, M., Parmarick, P., Bhatt, K., Gangola, S., Adnan, M., ... & Chen, S. (2021). Insights into the catalytic mechanism of ligninolytic peroxidase and laccase in lignin degradation. Bioremed J 24: 1–11.
Bilal M, Iqbal HM (2019) Persistence and impact of steroidal estrogens on the environment and their laccase-assisted removal. Sci Total Environ 690:447–459
Bilal M, Iqbal H (2020) Ligninolytic enzymes mediated ligninolysis: an untapped biocatalytic potential to deconstruct lignocellulosic molecules in a sustainable manner. Catal Lett 150(2):524–543
Blánquez P, Caminal G, Sarrà M, Vicent T (2007) The effect of HRT on the decolourisation of the grey lanaset G textile dye by Trametes versicolor. Chem Eng J 126(2–3):163–169
Blánquez P, Casas N, Font X et al (2004) Mechanism of textile metal dye biotransformation by Trametes versicolor. Water Res 38(8):2166–2172
Bulter T, Alcalde M, Sieber V, Meinhold P, Schlachtbauer C, Arnold FH (2003) Screening mutant libraries in Saccharomyces cerevisiae. Appl Environ Microbiol 69:987–995
Chauhan PS, Goradia B, Jha B (2018) Optimization and up scaling of ionic liquid tolerant and thermo-alkali stable laccase from a marine Staphylococcus arlettae S1–20 using tea waste. J Taiwan Inst Chem Eng 86:1–8
Chauhan PS, Goradia B, Saxena A (2017) Bacterial laccase: recent update on production, properties, and industrial applications. Biotech 7(5):1–20
Chauhan PS, Jha B (2018) Pilot scale production of extracellular thermo-alkali stable laccase from Pseudomonas sp. S2 using agro waste and its application in organophosphorous pesticides degradation. J Chem Technol Biotechnol 93(4):1022–1030
Chauhan PS, Kumarasamy M, Sosnik A, Danino D (2019) Enhanced thermostability and anticancer Activity in breast cancer cells of laccase immobilized on pluronic-stabilized nanoparticles. ACS Appl Mater Interfaces 11(43):39436–39448
Chhabra M, Mishra S, Sreekrishnan TR (2015) Immobilized laccase mediated dye decolorization and transformation pathway of azo dye acid red 27. J Environ Health Sci Eng 13:38–45
Claus H (2003) Laccases and their occurrence in prokaryotes. Arch Microbiol 179:145–150
Claus H (2010) Soil heavy metals. Springer; Berlin. Copper-containing oxidases: Occurrence in soil microorganisms, properties, and applications; pp 281–313
Cliffe S, Fawer MS, Maier G, Takata K, Ritter G (1994) Enzyme assays for the phenolic content of natural juices. J Agr Food Chem 42:1824–1828
Cordi L, Minussi RC, Freire RS, Durán N (2007) Fungal laccase: copper induction, semi-purification, immobilization, phenolic effluent treatment and electrochemical measurement. Afr J Biotech 6(10):1255–1259
Couto SR, López E, Sanromán MA (2006) Utilisation of grape seeds for laccase production in solid-state fermentors. J Food Eng 74(2):263–267
Couto SR, Moldes D, Liébanas A, Sanromán A (2003) Investigation of several bioreactor configurations for laccase production by Trametes versicolor operating in solid-state conditions. Biochem Eng J 15(1):21–26
Couto SR, Sanromán MA (2005) Application of solid-state fermentation to ligninolytic enzyme production. Biochem Eng J 22(3):211–219
Couto SR, Sanromán MA, Hofer D, Gübitz GM (2004) Production of laccase by Trametes hirsuta grown in an immersion bioreactor and its application in the decolorization of dyes from a leather factory. Eng Life Sci 4(3):233–238
Couto SR, Sanromán MA, Hofer D, Gübitz GM (2004) Stainless steel sponge: a novel carrier for the immobilisation of the white-rot fungus Trametes hirsuta for decolourization of textile dyes. Biores Technol 95(1):67–72
Couto SR, Toca Herrera JL (2006) Industrial and biotechnological applications of laccases: a review. Biotechnol Adv 24(5):500–513
Couto SR, Toca-Herrera JL (2007) Laccase production at reactor scale by filamentous fungi. Biotechnol Adv 25(6):558–569
Dacunzo F, Galli C, Gentili P, Sergi F (2006) Mechanistic and steric issues in the oxidation of phenolic and non-phenolic compounds by laccase or laccase-mediator systems: the case of bifunctional substrates. New J Chem 30:583–591
Das M, Royer TV, Leff LG (2007) Diversity of fungi, bacteria, and actinomycetes on leaves decomposing in a stream. Appl Environ Microbiol 73:756–767
Diamantidis G, Effosse A, Potier P, Bally R (2000) Purification and characterization of the first bacterial laccase in the rhizospheric bacterium Azospirillum lipoferum. Soil Biol Biochem 32:919–927
Dittmer NT, Gorman MJ, Kanost MR (2009) Characterization of endogenous and recombinant forms of laccase-2, a multicopper oxidase from the tobacco hornworm Manduca sexta. Insect Biochem Mol Biol 39:596–606
Dittmer NT, Kanost MR (2010) Insect multicopper oxidases: diversity, properties, and physiological roles. Insect Biochem Mol Biol 40:179–188
Dittmer NT, Suderman RJ, Jiang H, Zhu YC, Gorman MJ, Kramer KJ, Kanost MR (2004) Characterization of cDNAs encoding putative laccase-like multicopper oxidases and developmental expression in the tobacco hornworm, Manduca sexta, and the malaria mosquito Anopheles gambiae. Insect Biochem Mol 34:29–41
Domínguez A, Couto SR, Sanromán MA (2005) Dye decolorization by Trametes hirsuta immobilized into alginate beads. World J Microbiol Biotechnol 21(4):405–409
Driouich A, Laine AC, Vian B, Faye L (1992) Characterization and localization of laccase forms in stem and cell-cultures of sycamore. Plant J 2:13–24
Dwivedi UN, Singh P, Pandey VP, Kumar A (2011) Structure-function relationship among bacterial, fungal and plant laccases. J Mol Catal B 68:117–128
D’Souza-Ticlo D, Sharma D, Raghukumar C (2009) A thermostable metal-tolerant laccase with bioremediation potential from a marine-derived fungus. Mar Biotechnol 11(6):725–737
Eggert C, Temp U, Eriksson KEL (1996) The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol 62(4):1151–1158
Endo K, Hayashi Y, Hibi T, Hosono K, Beppu T, Ueda K (2003) Enzymological characterization of EpoA, a laccase-like phenol oxidase produced by Streptomyces griseus. J Biochem 133:671–677
Endo K, Hosono K, Beppu T, Ueda K (2002) A novel extracytoplasmic phenol oxidase of Streptomyces: Its possible involvement in the onset of morphogenesis. Microbiology 148:1767–1776
Enguita FJ, Marcal D, Martins LO, Grenha R, Henriques AO, Lindley PF, Carrondo MA (2004) Substrate and dioxygen binding to the endospore coat laccase from Bacillus subtilis. J Biol Chem 279:23472–23476
Erickson T, Liu L, Gueyikian A, Zhu X, Gibbons J, Williamson PR (2001) Multiple virulence factors of Cryptococcus neoformans are dependent on VPH1. Mol Microbiol 42:1121–1131
Faraco V, Giardina P, Palmieri G, Sannia G (2002) Metal-activated laccase promoters. Prog Biotechnol 21:105–111
Faure D, Bouillant ML, Jacoud C, Bally R (1996) Phenolic derivatives related to lignin metabolism as substrates for Azospirillum laccase activity. Phytochemistry 42:357–359
Feng SZ, Su YR, Dong MZ, He XY, Kumaresan D, O’Donnell A, Wu JS, Chen XB (2015) Laccase activity is proportional to the abundance of bacterial laccase-like genes in soil from subtropical arable land. World J Microbiol Biotechnol 31:2039–2045
Fernandes AT, Damas JM, Todorovic S, Huber R, Baratto MC, Pogni R, Soares CM, Martins LO (2010) The multicopper oxidase from the archaeon Pyrobaculum aerophilum shows nitrous oxide reductase activity. Febs J 277:3176–3189
Fernandes AT, Soares CM, Pereira MM, Huber R, Grass G, Martins LO (2007) A robust metallo-oxidase from the hyperthermophilic bacterium Aquifex aeolicus. FEBS J 274:2683–2694
Flores C, Vidal C, Trejo-Hernandez MR, Galindo E, Serrano-Carre L (2009) Selection of Trichoderma strains capable of increasing laccase production by Pleurotus ostreatus and Agaricus bisporus in dual cultures. J Appl Microbiol 106:249–257
Frasconi M, Favero G, Boer H et al (2010) Kinetic and biochemical properties of high and low redox potential laccases from fungal and plant origin. Biochim Biophys Acta Proteins Proteomics 1804:899–908
Frases S, Salazar A, Dadachova E, Casadevall A (2007) Cryptococcus neoformans can utilize the bacterial melanin precursor homogentisic acid for fungal melanogenesis. Appl Environ Microbiol 73:615–621
Di Fusco M, Tortolini C, Deriu D, Mazzei F (2010) Laccase-based biosensor for the determination of polyphenol index in wine. Talanta 81:235–240
Garavaglia S, Cambria MT, Miglio M, Ragusa S, Iacobazzi V, Palmieri F, D’Ambrosio C, Scaloni A, Rizzi M (2004) The structure of Rigidoporus lignosus laccase containing a full complement of copper ions, reveals an asymmetrical arrangement for the T3 copper pair. J Mol Biol 342:1519–1531
Ghindilis AL, Gavrilova VP, Yaropolov AI (1992) Laccase-based biosensor for determination of polyphenols-determination of catechols in tea. Biosens Bioelectron 7:127–131
Gianfreda L, Xu F, Bollag J-M (1999) Laccases: A useful group of oxidoreductive enzymes. Bioremediat J 3:1–26
Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G (2010) Laccases: a never-ending story. Cell Mol Life Sci 67:369–385
Givaudan A, Effosse A, Faure D, Potier P, Bouillant ML, Bally R (1993) Polyphenol oxidase in Azospirillum lipoferum isolated from rice rhizosphere-evidence for laccase activity in nonmotile strains of Azospirillum lipoferum. FEMS Microbiol Lett 108:205–210
Goldman DL, Khine H, Abadi J, Lindenberg DJ, Pirofski L, Niang R, Casadevall A (2001) Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 107:E66
De Gonzalo G, Colpa DI, Habib MHM, Fraaije MW (2016) Bacterial enzymes involved in lignin degradation. J Biotechnol 236:110–119
De Gregorio E, Spellman PT, Rubin GM, Lemaitre B (2001) Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc Natl Acad Sci USA 98:12590–12595
Grinhut T, Hadar Y, Chen Y (2007) Degradation and transformation of humic substances by saprotrophic fungi: Processes and mechanisms. Fungal Biol Rev 21:179–189
Grotewold E, Taccioli GE, Aisemberg GO, Judewicz ND (1998) Purification of an extracellular fungal laccase. Mircen J Appl Microbiol Biotechnol 4:357–363
Gunne M, Hoppner A, Hagedoorn PL, Urlacher VB (2014) Structural and redox properties of the small laccase Ssl1 from Streptomyces sviceus. FEBS J 281:4307–4318. https://doi.org/10.1111/febs.12755
Gunne M, Urlacher VB (2012) Characterization of the alkaline laccase Ssl1 from Streptomyces sviceus with unusual properties discovered by genome mining. PLoS ONE 7:e52360
Gupta G, Rajendran V, Atanassov P (2003) Laccase biosensor on monolayer-modified gold electrode. Electroanal 15:1577–1583
Hakulinen N, Kiiskinen LL, Kruus K, Saloheimo M, Paananen A, Koivula A, Rouvinen J (2002) Crystal structure of a laccase from Melanocarpus albomyces with an intact trinuclear copper site. Nat Struct Biol 9:601–605
Hakulinen N, Rouvinen J (2015) Three-dimensional structures of laccases. Cell Mol Life Sci 72:857–868
Han MJ, Choi HT, Song HG (2005) Purification and characterization of laccase from the white rot fungus Trametes versicolor. J Microbiol 43(6):555–560
Harakava R (2005) Genes encoding enzymes of the lignin biosynthesis pathway in Eucalyptus. Genet Mol Biol 28:601–607
Harvey BM (1997) Master’s Thesis. University of Canterbury; Christchurch, New Zealand. Laccases in Higher Plants
Hattori M, Konishi H, Tamura Y, Konno K, Sogawa K (2005) Laccase-type phenoloxidase in salivary glands and watery saliva of the green rice leafhopper, Nephotettix cincticeps. J Insect Physiol 51:1359–1365
Hattori M, Tsuchihara K, Noda H, Konishi H, Tamura Y, Shinoda T, Nakamura M, Hasegawa T (2010) Molecular characterization and expression of laccase genes in the salivary glands of the green rice leafhopper, Nephotettix cincticeps (Hemiptera: Cicadellidae) Insect Biochem. Mol Biol 40:331–338
Heinzkill M, Bech L, Halkier T, Schneider P, Anke T (1998) Characterization of laccases and peroxidases from wood-rotting fungi (family Coprinaceae). Appl Environ Microbiol 64(5):1601–1606
Hess J, Leitner C, Galhaup C et al (2002) Enhanced formation of extracellular laccase activity by the white-rot fungus Trametes multicolor. Appl Biochem Biotechnol Part A 98:229–241
Hoegger PJ, Kilaru S, James TY, Thacker JR, Kues U (2006) Phylogenetic comparison and classification of laccase and related multicopper oxidase protein sequences. FEBS J 273:2308–2326
Hoegger PJ, Kilaru S, James TY, Thacker JR, Kües U (2006) Phylogenetic comparison and classification of laccase and related multicopper oxidase protein sequences. FEBS J 273:2308–2326
Hongoh Y (2011) Toward the functional analysis of uncultivable, symbiotic microorganisms in the termite gut. Cell Mol Life Sci 68:1311–1325
Hoopes JT, Dean JF (2004) Ferroxidase activity in a laccase-like multicopper oxidase from Liriodendron tulipifera. Plant Physiol Biochem 42:27–33
Hou H, Zhou J, Wang J, Du C, Yan B (2004) Enhancement of laccase production by Pleurotus ostreatus and its use for the decolorization of anthraquinone dye. Process Biochem 39(11):1415–1419
Hussain A, Bilal M, Rafeeq H, Jabeen Z, Afsheen N, Sher F, ... & Iqbal HM (2022) Role of laccase in the pulp and paper industry. In Nanotechnology in Paper and Wood Engineering (pp 35–60). Elsevier
Junghanns C, Parra R, Keshavarz T, Schlosser D (2008) Towards higher laccase activities produced by aquatic ascomycetous fungi through combination of elicitors and an alternative substrate. Eng Life Sci 8:277–285
Kahraman SS, Gurdal IH (2002) Effect of synthetic and natural culture media on laccase production by white rot fungi. Biores Technol 82(3):215–217
Kawai S, Umezawa T, Higuchi T (1988) Degradation mechanisms of phenolic b-1 lignin substructure model compounds by laccase of Coriolus versicolor. Arch Biochem Biophys 262:99–110
Keum YS, Li QX (2004) Fungal laccase-catalyzed degradation of hydroxy polychlorinated biphenyls. Chemosphere 56(1):23–30
Kiiskinen LL, Kruus K, Bailey M, Ylösmäki E, Siika-aho M, Saloheimo M (2004) Expression of Melanocarpus albomyces laccase in Trichoderma reesei and characterization of the purified enzyme. Microbiology 150(9):3065–3074
Kiiskinen LL, Rättö M, Kruus K (2004) Screening for novel laccase-producing microbes. J Appl Microbiol 97(3):640–646
Kim JE, Han KH, Jin J, Kim H, Kim JC, Yun SH, Lee YW (2005) Putative polyketide synthase and laccase genes for biosynthesis of aurofusarin in Gibberella zeae. Appl Environ Microbiol 71:1701–1708
Kim C, Lorenz WW, Hoopes JT, Dean JFD (2001) Oxidation of phenolate siderophores by the multicopper oxidase encoded by the Escherichia coli yacK Gene. J Bacteriol 183:4866–4875
Koschorreck K, Richter SM, Ene AB, Roduner E, Schmid RD, Urlacher VB (2008) Cloning and characterization of a new laccase from Bacillus licheniformis catalyzing dimerization of phenolic acids. Appl Microbiol Biotechnol 79:217–224
Kramer KJ, Kanost MR, Hopkins TL, Jiang HB, Zhu YC, Xu RD, Kerwin JL, Turecek F (2001) Oxidative conjugation of catechols with proteins in insect skeletal systems. Tetrahedron 57:385–392
Kumar VS, Phale PS, Durani S, Wangikar PP (2003) Combined sequence and structure analysis of the fungal laccase family. Biotechnol Bioeng 83:386–394
Kumar A, Singh AK, Bilal M, Chandra R (2021) Sustainable production of thermostable laccase from agro-residues waste by Bacillus aquimaris AKRC02. Catal Lett 24:1–17
Kuo HC, Detry N, Choi J, Lee YH (2015) Potential roles of laccases on virulence of Heterobasidion annosum s.s. Microb Pathog 81:16–21
Laccase XF (1999) In: Flickinger MC, Drew SW (eds) Encyclopedia of Bioprocess Technology. Wiley, Hoboken
Lakshmanan D, Sadasivan C (2016) Trichoderma viride laccase plays a crucial role in defense mechanism against antagonistic organisms. Front Microbiol 7:1–8
Lee IY, Jung KH, Lee CH, Park YH (1999) Enhanced production of laccase in Trametes vesicolor by the addition of ethanol. Biotech Lett 21(11):965–968
Lema JM, Roca E, Sanroman A, Nunez MJ, Moreira MT, Feijoo G (2001) Pulsating bioreactors. In: Cabral JMS, Mota M, Tramper J (eds) Multiphase bioreactor design. Taylor and Francis, London, pp 309–329
Li Q, Wang X, Korzhev M, Schroder HC, Link T, Tahir MN, Diehl-Seifert B, Muller WE (2015) Potential biological role of laccase from the sponge Suberites domuncula as an antibacterial defense component. Biochim Biophys Acta 1850:118–128
Liu L, Tewari RP, Williamson PR (1999) Laccase protects Cryptococcus neoformans from antifungal activity of alveolar macrophages. Infect Immun 67:6034–6039. https://doi.org/10.1128/IAI.67.11.6034-6039.1999
Lorenzo M, Moldes D, Couto SR, Sanromán A (2002) Improving laccase production by employing different lignocellulosic wastes in submerged cultures of Trametes versicolor. Biores Technol 82(2):109–113
Lu SXF, Jones CL, Lonergan GT (1996) Correlation between fungal morphology and laccase expression under the influence of cellobiose induction. In: Proceedings of the 10th international biotechnology symposium and 9th international symposium on yeasts; Sydney
Luke AK, Burton SG (2001) A novel application for Neurospora crassa: progress from batch culture to a membrane bioreactor for the bioremediation of phenols. Enzyme Microb Technol 29(6–7):348–356
Ma S, Liu N, Jia H, Dai D, Zang J, Cao Z (2018) Expression, purification, and characterization of a novel laccase from Setosphaeria turcica in Eschericha coli. J Basic Microbiol 58:68–75
MacCabe A.P. (eds). Springer, Dordrecht, pp 461–476.
MacVittie K, Conlon T, Katz E (2015) A wireless transmission system powered by an enzyme biofuel cell implanted in an orange. Bioelectrochemistry 106:28–33
Machczynski MC, Vijgenboom E, Samyn B, Canters GW (2004) Characterization of SLAC: a small laccase from Streptomyces coelicolor with unprecedented activity. Protein Sci 13:2388–2397
Madhavi V, Lele SS (2009) Laccase: properties and applications. BioResources 4(4):1694–1717
Madhavi V, Lele SS (2009) Laccase: properties and applications. BioResources 4:1694–1717
Maestre-Reyna M, Liu W-C, Jeng W-Y, Lee C-C, Hsu C-A, Wen T-N et al (2015) Structural and functional roles of glycosylation in fungal laccase from Lentinus sp. PLoS ONE 10:e012060
Martin C, Corvini PF, Vinken R, Junghanns C, Krauss G, Schlosser D (2009) Quantification of the influence of extracellular laccase and intracellular reactions on the isomer-specific biotransformation of the xenoestrogen technical nonylphenol by the aquatic hyphomycete Clavariopsis aquatica. Appl Environ Microbiol 75:4398–4409
Martinez AT, Speranza M, Ruiz-Duenas FJ, Ferreira P, Camarero S, Guillen F, Martinez MJ, Gutierrez A, del Rio JC (2005) Biodegradation of lignocellulosics: Microbial chemical, and enzymatic aspects of the fungal attack of lignin. Int Microbiol Off J Span Soc Microbiol 8:195–204
Martins LO, Durao P, Brissos V, Lindley PF (2015) Laccases of prokaryotic origin: enzymes at the interface of protein science and protein technology. Cell Mol Life Sci 72:911–922
Martins LO, Soares CM, Pereira MM, Teixeira M, Costa T, Jones GH, Henriques AO (2002) Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J Biol Chem 277:18849–18859
Maryan AS, Montazer M (2009) The effect of cellulase and laccase enzymes on denim color. J Color Sci Technol 3:53–64
Masai E, Katayama Y, Fukuda M (2007) Genetic and biochemical investigations on bacterial catabolic pathways for lignin-derived aromatic compounds. Biosci Biotechnol Biochem 71:1–15
Mathiasen TE (1995) Laccase and beer storage. PCT international application, WO 9521240 A2, [Ref list]
Mayer AM, Staples RC (2002) Laccase: New functions for an old enzyme. Phytochemistry 60:551–565
McCaig BC, Meagher RB, Dean JF (2005) Gene structure and molecular analysis of the laccase-like multicopper oxidase (LMCO) gene family in Arabidopsis thaliana. Planta 221:619–636
Mellano MA, Cooksey DA (1988) Nucleotide sequence and organization of copper resistance genes from Pseudomonas syringae pv. tomato. J Bacteriol 170:2879–2883
Melo EP, Fernandes AT, Durao P, Martins LO (2007) Insight into stability of CotA laccase from the spore coat of Bacillus subtilis. Biochem Soc Trans 35:1579–1582
Mikolasch A, Schauer F (2009) Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Appl Microbiol Biotechnol 82:605–624
Minussi RC, Pastore GM, Durán N (2002) Potential applications of laccase in the food industry. Trends Food Sci Technol 13(6–7):205–216
Minussi RC, Rossi M, Bologna L, Rotilio D, Pastore GM, Durán N (2007) Phenols removal in musts: strategy for wine stabilization by laccase. J Mol Catal B 45(3–4):102–107
Miyazaki K (2005) A hyperthermophilic laccase from Thermus thermophilus HB27. Extremophiles 9:415–425
Mohorčič M, Friedrich J, Pavko A (2004) Decolourization of the diazo dye reactive black 5 by immobilised Bjerkundera adusta in a stirred tank bioreactor. Acta Chim Slov 51(4):619–628
Montazer M, Maryan AS (2008) Application of laccases with cellulases on denim for clean effluent and repeatable biowashing. J Appl Polym Sci 110:3121–3129
Moo-Young M, Moreira AR, Tengerdy RP (1983) Principles of solid state fermentation: the filamentous fungi. In: Smith JE, Berry DR, Kristiansen B (eds) London. Edward Arnold, UK, pp 117–144
Moreno LF, Feng P, Weiss VA, Vicente VA, Stielow JB, de Hoog S (2017) Phylogenomic analyses reveal the diversity of laccase-coding genes in Fonsecaea genomes. PLoS ONE 12:e0171291
Morozova OV, Shumakovich GP, Shleev SV, Yaropolov YI (2007) Laccase-mediator systems and their applications: a review. Appl Biochem Microb 43:523–535
Morsi R, Bilal M, Iqbal HM, Ashraf SS (2020) Laccases and peroxidases: the smart, greener and futuristic biocatalytic tools to mitigate recalcitrant emerging pollutants. Sci Total Environ 714:136572
Mot AC, Coman C, Hadade N et al (2020) “Yellow” laccase from Sclerotinia sclerotiorum is a blue laccase that enhances its substrate affinity by forming a reversible tyrosyl-product adduct. PLoS ONE 15:1–19
Munk L, Sitarz AK, Kalyani DC, Mikkelsen JD, Meyer AS (2015) Can laccases catalyze bond cleavage in lignin? Biotechnol Adv 33:13–24
Muñoz C, Guillén F, Martínez AT, Martínez MJ (1997) Induction and characterization of laccase in the ligninolytic fungus Pleurotus eryngii. Curr Microbiol 34(1):1–5
Nakamura T (1958) Purification and physico-chemical properties of laccase. Biochim Biophys Acta 30:44–52
Nakamura K, Go N (2005) Function and molecular evolution of multicopper blue proteins. Cell Mol Life Sci 62:2050–2066
Niu BL, Shen WF, Liu Y, Weng HB, He LH, Mu JJ, Wu ZL, Jiang P, Tao YZ, Meng ZQ (2008) Cloning and RNAi-mediated functional characterization of MaLac2 of the pine sawyer Monochamus alternatus. Insect Mol Biol 17:303–312
Noreen S, Asgher M, Qamar SA, Bilal M, Iqbal H (2021) Poly (vinyl Alcohol)-alginate immobilized trametes versicolor IBL-04 laccase as eco-friendly biocatalyst for dyes degradation. Catal Lett 10:1–11
Noreen S, Perveen S, Shafiq N, Aslam S, Iqbal HM, Ashraf SS, Bilal M (2021) Laccase-loaded functionalized graphene oxide assemblies with improved biocatalytic properties and decolorization performance. Environ Technol Innov 24:101884
Noreen S, Perveen S, Bilal M, & Iqbal HM (2022) Laccases: catalytic and functional attributes for robust biocatalysis. In Nanomaterials for Biocatalysis (pp. 567–594). Elsevier
Nosanchuk JD, Casadevall A (2003) The contribution of melanin to microbial pathogenesis. Cell Microbiol 5:203–223
Nothaft H, Szymanski CM (2010) Protein glycosylation in bacteria: Sweeter than ever. Nat Rev Microbiol 8:765–778
Omalley DM, Whetten R, Bao WL, Chen CL, Sederoff RR (1993) The role of laccase in lignification. Plant J 4:751–757
Otto B, Schlosser D (2014) First laccase in green algae: Purification and characterization of an extracellular phenol oxidase from Tetracystis aeria. Planta 240:1225–1236
Palanisami S, Saha SK, Lakshmanan U (2010) Laccase and polyphenol oxidase activities of marine cyanobacteria: a study with Poly R-478 decolourization. World J Microbiol Biotechnol 26:63–69
Palmer AE, Szylagyi RK, Cherry JR, Jones A, Xu F, Solomon EI (2003) Spectroscopic characterization of the Leu513His variant of fungal laccase: effect of increased axial ligand interaction on the geometric and electronic structure of the type 1 Cu sit. Inorg Chem 42:4006–4017
Palmieri G, Giardina P, Bianco C et al (1997) A novel white laccase from Pleurotus ostreatus. J Biol Chem 272:31301–31307
Palmieri G, Giardina P, Bianco C, Fontallella B, Sannina G (2000) Copper induction of laccase isoenzyme in the lignolytic fungus Pleurotus ostreatus. Appl Microbiol Biotechnol 66:920–924
Palmieri G, Giardina P, Bianco C, Scaloni A, Capasso A, Sannia G (1997) J Biol Chem 272:31301–31307
Pandey A, Selvakumar P, Soccol CR, Nigam P (1999) Solid state fermentation for the production of industrial enzymes. Curr Sci 77(1):149–162
Pannu JS, Kapoor RK (2014) Microbial laccases: A mini-review on their production, purification and applications. Int J Pharm Arch 3:528–536
Paraschiv G, Ferdes M, Ionescu M, Moiceanu G, Zabava BS, Dinca MN (2022) Laccases—versatile enzymes used to reduce environmental pollution. Energies 15(5):1835
Passarini MR, Ottoni CA, Santos C, Lima N, Sette LD (2015) Induction, expression and characterisation of laccase genes from the marine-derived fungal strains Nigrospora sp. CBMAI 1328 and Arthopyrenia sp. CBMAI 1330. AMB Express 5:19–25
Pawlik A, Wojcik M, Rulka K, Motyl-Gorzel K, Osinska-Jaroszuk M, Wielbo J, Marek-Kozaczuk M, Skorupska A, Rogalski J, Janusz G (2016) Purification and characterization of laccase from Sinorhizobium meliloti and analysis of the lacc gene. Int J Biol Macromol 92:138–147
Perveen S, Noreen S, Shahid S, Mehboob H, Aslam S, Iqbal H, Bilal M (2022) Carrier-free cross-linked laccase crystals for biocatalytic degradation of textile industrial effluents. Appl Biochem Biotechnol 24:1–15
Piontek K, Antorini M, Choinowski T (2002) Crystal structure of a laccase from the fungusTrametes versicolor at 1.90-Å resolution containing a full complement of coppers. J Biol Chem 277:37663–37669
Polaina J., Alvarado-Ramírez, L., Rostro-Alanis, M., Rodríguez-Rodríguez, J., Sosa-Hernández, J. E., Melchor-Martínez, E. M., Iqbal, H., & Parra-Saldívar, R. (2021) Enzyme (single and multiple) and nanozyme biosensors: recent developments and their novel applications in the water-food-health nexus. Biosensors 11(11):410
Polak J (2012) Structure/Redox potential relationship of simple organic compounds as potential precursors of dyes for laccase-mediated transformation. Biotechnol Progr 28:93–102
Polak J, Jarosz-Wilkołazka A (2007) Reakcje katalizowane przez lakazę–mechanizm i zastosowanie w biotechnologii. Biotechnologia 4:82–94
Pourcel L, Routaboul JM, Kerhoas L, Caboche M, Lepiniec L, Debeaujon I (2005) TRANSPARENT TESTA10 encodes a laccase-like enzyme involved in oxidative polymerization of flavonoids in Arabidopsis seed coat. Plant Cell 17:2966–2980
Pozdnyakova NN, Rodakiewicz-Nowak J, Turkovskaya OV (2004) Catalytic properties of yellow laccase from Pleurotus ostreatus D1. J Mol Catal B 30:19–24
Ramsay JA, Nguyen T (2002) Decolourization of textile dyes by Trametes versicolour and its effect on dye toxicity. Biotech Lett 24:1757–1761
Ranocha P, Chabannes M, Chamayou S, Danoun S, Jauneau A, Boudet AM, Goffner D (2002) Laccase down-regulation causes alterations in phenolic metabolism and cell wall structure in poplar. Plant Physiol 129:145–155
Ranocha P, McDougall G, Hawkins S, Sterjiades R, Borderies G, Stewart D, Cabanes-Macheteau M, Boudet AM, Goffner D (1999) Biochemical characterization, molecular cloning and expression of laccases: a divergent gene family - in poplar. Eur J Biochem 259:485–495
Reiss R, Ihssen J, Thöny-Meyer L (2011) Bacillus pumilus laccase: A heat stable enzyme with a wide substrate spectrum. BMC Biotechnol 11:9
Rezaei S, Tahmasbi H, Mogharabi M, Ameri A, Forootanfar H, Khoshayand MR (2015) Laccase-catalyzed decolorization and detoxification of Acid Blue 92: statistical optimization, microtoxicity, kinetics, and energetics. J Environ Health Sci Eng 13:31–36
Ribeiro DS, Henrique SMB, Oliveira LS, Macedo GA, Fleuri LF (2010) Enzymes in juice processing: a review. Int J Food Sci Technol 45(4):635–641
Richardson A, McDougall GJ (1997) A laccase-type polyphenol oxidase from lignifying xylem of tobacco. Phytochemistry 44:229–235
Roberts SA, Weichsel A, Grass G, Thakali K, Hazzard JT, Tollin G, Rensing C, Montfort WR (2002) Crystal structure and electron transfer kinetics of CueO, a multicopper oxidase required for copper homeostasis in Escherichia coli. Proc Natl Acad Sci USA 99:2766–2771
Rochefort D, Leech D, Bourbonnais R (2004) Electron transfer mediator systems for bleaching of paper pulp. Green Chem 6:14–24
Rodríguez-Delgado MM, Alemán-Nava GS, Rodríguez-Delgado JM, Dieck-Assad G, Martínez-Chapa SO, Barceló D, Parra R (2015) Laccase-based biosensors for detection of phenolic compounds. Trend Anal Chem 74:21–45
Romero S, Blánquez P, Caminal G et al (2006) Different approaches to improving the textile dye degradation capacity of Trametes versicolor. Biochem Eng J 31(1):42–47
Roriz MS, Osma JF, Teixeira JA, Couto SR (2009) Application of response surface methodological approach to optimise Reactive Black 5 decolouration by crude laccase from Trametes pubescens. J Hazard Mater 169(1–3):691–696
Rosales E, Couto SR, Sanromán MA (2007) Increased laccase production by Trametes hirsuta grown on ground orange peelings. Enzyme Microb Technol 40(5):1286–1290
Ruijssenaars HJ, Hartmans S (2004) A cloned Bacillus halodurans multicopper oxidase exhibiting alkaline laccase activity. Appl Microbiol Biotechnol 65:177–182
Ruiz-Duenas FJ, Martinez AT (2009) Microbial degradation of lignin: How a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this. Microb Biotechnol 2:164–177
Sanchez-Amat A, Solano F (1997) A pluripotent polyphenol oxidase from the melanogenic marine Alteromonas sp shares catalytic capabilities of tyrosinases and laccases. Biochem Biophys Res Commun 240:787–792
Sapmak A, Boyce KJ, Andrianopoulos A, Vanittanakom N (2015) The pbrB gene encodes a laccase required for DHN-melanin synthesis in conidia of Talaromyces (Penicillium) marneffei. PLoS ONE 10:1–89
Sapmak A, Kaewmalakul J, Nosanchuk JD, Vanittanakom N, Andrianopoulos A, Pruksaphon K, Youngchim S (2016) Talaromyces marneffei laccase modifies THP-1 macrophage responses. Virulence 7:702–717
Sarker A, Islam T, Bilal M, Kim JE (2022) A pilot study for enhanced transformation of a metabolite 3, 5-dichloroaniline derived from dicarboximide fungicides through immobilized laccase mediator system. Environ Sci Pollut Res 15:1–16
Sato Y, Bao WL, Sederoff R, Whetten R (2001) Molecular cloning and expression of eight laccase cDNAs in loblolly pine (Pinus taeda). J Plant Res 114:147–155
Schneider KP, Gewessler U, Flock T, Heinzle A, Schenk V, Kaufmann F et al (2012) Signal enhancement in polysaccharide-based sensors for infections by incorporation of chemically modified laccase. New Biotechnol 29:502–509
Schouten A, Maksimova O, Cuesta-Arenas Y, van den Berg G, Raaijmakers JM (2008) Involvement of the ABC transporter BcAtrB and the laccase BcLCC2 in defence of Botrytis cinerea against the broad-spectrum antibiotic 2,4-diacetylphloroglucinol. Environ Microbiol 10:1145–1157
Sedarati MR, Keshavarz T, Leontievsky AA, Evans CS (2003) Transformation of high concentrations of chlorophenols by the white-rot basidiomycete Trametes versicolor immobilized on nylon mesh. Electron J Biotechnol 6(2):27–37
Sharma KK, Kuhad RC (2008) Laccase: Enzyme revisited and function redefined. Indian J Microbiol 48:309–316
Shleev S, Christenson A, Serezhenkov V, Burbaev D, Yaropolov A, Gorton L, Ruzgas T (2005) Electrochemical redox transformations of T1 and T2 copper sites in native Trametes hirsuta laccase at gold electrode. Biochem J 385:745–754
Si W, Wu Z, Wang L, Yang M, Zhao X (2015) Enzymological characterization of Atm, the first laccase from Agrobacterium sp S5–1, with the ability to enhance in vitro digestibility of maize straw. PLoS ONE 10:0128204
Singh G, Bhalla A, Kaur P, Capalash N, Sharma P (2011) Laccase from prokaryotes: A new source for an old enzyme. Rev Environ Sci Biotechnol 10:309–326
Sitarz AK, Mikkelsen JD, Meyer AS (2016) Structure, functionality and tuning up of laccases for lignocellulose and other industrial applications. Crit Rev Biotechnol 36:70–86
Sjaarda CP, Abubaker KS, Castle AJ (2015) Induction of lcc2 expression and activity by Agaricus bisporus provides defence against Trichoderma aggressivum toxic extracts. Microb Biotechnol 8:918–929
Solano F, Lucas-Elío P, López-Serrano D, Fernández E, Sanchez-Amat A (2001) Dimethoxyphenol oxidase activity of different microbial blue multicopper proteins. FEMS Microbiol Lett 204:175–181
Sole M, Kellner H, Brock S, Buscot F, Schlosser D (2008) Extracellular laccase activity and transcript levels of putative laccase genes during removal of the xenoestrogen technical nonylphenol by the aquatic hyphomycete Clavariopsis aquatica. FEMS Microbiol Lett 288:47–54
Sondhi S, Sharma P, George N, Chauhan PS, Puri N, Gupta N (2015) An extracellular thermo-alkali-stable laccase from Bacillus tequilensis SN4, with a potential to biobleach softwood pulp. Biotech 5(2):175–185
Sondhi S, Sharma P, Saini S, Puri N, Gupta N (2014) Purification and characterization of an extracellular, thermo-alkali-stable, metal tolerant laccase from Bacillus tequilensis SN4. PLoS ONE 9:e96951
Souza C, Zilly A, Peralta RM (2002) Production of laccase as the sole phenoloxidase by a Brazilian strain of Pleurotus pulmonarius in solid state fermentation. J Basic Microbiol 42(2):83–90
Sterjiades R, Dean JF, Eriksson KE (1992) Laccase from sycamore maple (Acer pseudoplatanus) polymerizes monolignols. Plant Physiol 99:1162–1168
Sugumaran M, Giglio L, Kundzicz H, Saul S, Semensi V (1992) Studies on the enzymes involved in puparial cuticle sclerotization in Drosophila melanogaster. Arch Insect Biochem Physiol 19:271–283
Suzuki T, Endo K, Ito M, Tsujibo H, Miyamoto K, Inamori Y (2003) A thermostable laccase from Streptomyces lavendulae REN-7: purification, characterization, nucleotide sequence, and expression. Biosci Biotechnol Biochem 67:2167–2175
Tavares APM, Coelho MAZ, Agapito MSM, Coutinho JAP, Xavier AMRB (2006) Optimization and modeling of laccase production by Trametes versicolor in a bioreactor using statistical experimental design. Appl Biochem Biotechnol 134(3):233–248
Thomas BR, Yonekura M, Morgan TD, Czapla TH, Hopkins TL, Kramer KJ (1989) A trypsin-solubilized laccase from pharate pupal integument of the tobacco hornworm Manduca sexta. Insect Biochem 19:611
Tong P, Hong Y, Xiao Y, Zhang M, Tu X, Cui T (2007) High production of laccase by a new basidiomycete. Biotech Lett 29(2):295–301
Tsai HF, Wheeler MH, Chang YC, Kwon-Chung KJ (1999) A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J Bacteriol 181:6469–6477
Ünal A, Kolankaya N (2001) Dechlorination of bleached kraft pulp by laccase enzyme produced from some white-rot fungi. Turk Electron J Biotechnol 25:67–72
Udayasoorian C, Prabu PC (2005) Biodegradation of phenols by lignolytic fungus Trametes versicolour. J Biol Sci 5:824–827
Valeriano VS, Silva AMF, Santiago MF, Bara MTF, Garcia TA (2009) Production of laccase by Pycnoporus sanguineus using 2,5-xylidine and ethanol. Braz J Microbiol 40(4):790–794
Velazquez-Cedeno M, Farnet AM, Billette C, Mata G, Savoie JM (2007) Interspecific interactions with Trichoderma longibrachiatum induce Pleurotus ostreatus defence reactions based on the production of laccase isozymes. Biotechnol Lett 29:1583–1590
Villalba-Rodríguez AM, Parra-Arroyo L, González-González RB, Parra-Saldívar R, Bilal M, Iqbal HM (2022) Laccase-assisted biosensing constructs–robust modalities to detect and remove environmental contaminants. Case Stud Chem Environ Eng 14:100180
Virk AP, Sharma P, Capalash N (2012) Use of laccase in pulp and paper industry. Biotechnol prog 28:21–32
Viswanath B, Subhosh Chandra M, Pallavi H, Rajasekhar RB (2008) Screening and assessment of laccase producing fungi isolated from different environmental samples. Afr J Biotech 7(8):1129–1133
Voriskova J, Baldrian P (2013) Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J 7:477–486
Wang J, Feng J, Jia W, Chang S, Li S, Li Y (2015) Lignin engineering through laccase modification: A promising field for energy plant improvement. Biotechnol Biofuels 8:145
Wei YX, Pu JJ, Zhang H, Liu YN, Zhou FX, Zhang KL, Liu XM (2017) The laccase gene (LAC1) is essential for Colletotrichum gloeosporioides development and virulence on mango leaves and fruits. Physiol Mol Plant Pathol 99:55–64
Westereng B, Cannella D, Agger JW, Jorgensen H, Andersen ML, Eijsink VGH, Felby C (2015) Enzymatic cellulose oxidation is linked to lignin by long-range electron transfer. Sci Rep 5:15
Williamson PR (1994) Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: Identification as a laccase. J Bacteriol 176:656–664
Williamson PR (2016) Role of laccase in the virulence of Talaromyces marneffei: A common link between AIDS-related fungal pathogens? Virulence 7:627–629
Williamson PR, Wakamatsu K, Ito S (1998) Melanin biosynthesis in Cryptococcus neoformans. J Bacteriol 180:1570–1572
Wong DW (2009) Structure and action mechanism of ligninolytic enzymes. Appl Biochem Biotechnol 157(2):174–209
Wosilait WD, Nason A, Terrell AJ (1954) Pyridine nucleotide-quinone reductase 2: role in electron transport. J Biol Chem 206:271–282
Wu Y-R, Luo ZH, Chow RKK, Vrijmoed L (2010) Purification and characterization of an extracellular laccase from the anthracene-degrading fungus Fusarium solani MAS2. Bioresour Technol 101:9772–9777
Xavier AMRB, Evtuguin DV, Ferreira RMP, Amado F (2001) Laccase production for lignin oxidase activity. In: Proceedings of the 8th International Conference on Biotechnology; Helsinki
Xu F (1999). In: Flickinger MC, Drew SW (eds) The encyclopedia of bioprocessing technology: fermentation, biocatalysis, and bioseparation. Wiley, New York, pp 1545–1554
Xu F, Palmer A, Yaver DS, Berka RM, Gambetta GA, Brown SH, Solomon EI (1999) Targeted mutations in a Trametes villosa laccase: axial perturbations of the T1 copper. J Biol Chem 274:12372–12375
Xu L, Zhu M, Chen X, Wang H, Zhang G (2015) A novel laccase from fresh fruiting bodies of the wild medicinal mushroom Tricholoma matsutake. Acta Biochim Pol 62:35–40
Yang J, Li W, Bun Ng T et al (2017a) Laccases: production, expression regulation, and applications in pharmaceutical biodegradation. Front Microbiol.
Yatsu J, Asano T (2009) Cuticle laccase of the silkworm, Bombyx mori: purification, gene identification and presence of its inactive precursor in the cuticle. Insect Biochem Mol Biol 39:254–262
Zhao D, Cui DZ, Mu JS et al (2014) Induction of a white laccase from the deuteromycete Myrothecium verrucaria NF-05 and its potential in decolorization of dyes. Biocatal Biotransformation 32:214–221
Zhu XD, Gibbons J, Garcia-Rivera J, Casadevall A, Williamson PR (2001) Laccase of Cryptococcus neoformans is a cell wall-associated virulence factor. Infect Immun 69:5589–5596
Zofair SFF, Ahmad S, Hashmi MA, Khan SH, Khan MA, Younus H (2022) Catalytic roles, immobilization and management of recalcitrant environmental pollutants by laccases: Significance in sustainable green chemistry. J Environ Manage 309:114676
Zouari N, Romette JL, Thomas D (1988) A continuous-flow method for the rapid-determination of sanitary quality of grape must at industrial-scales. J Chem Technol Biot 41:243–248
Acknowledgements
Khalifa University is graciously acknowledged for partially supporting this work under the international collaboration project awarded to Syed Salman Ashraf and Muhammad Bilal (CIRA-2020-046).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ayodeji, F.D., Shava, B., Iqbal, H.M.N. et al. Biocatalytic Versatilities and Biotechnological Prospects of Laccase for a Sustainable Industry. Catal Lett 153, 1932–1956 (2023). https://doi.org/10.1007/s10562-022-04134-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04134-9