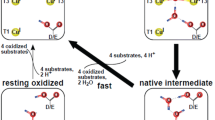
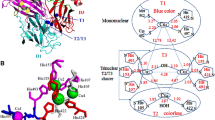
Abstract
Laccases (benzenediol: oxygen oxidoreductase, EC 1.10.3.2) are multi-copper-containing enzymes capable of catalyzing the oxidation of a wide range of phenolic and non phenolic aromatic compounds. The available data indicates that laccases from prokaryotes are promising biological tools for green chemistry based applications, especially in decolorization of industrial textile dye effluents which constitute a major threat to soil and ground water reservoirs worldwide. Another appropriate application of prokaryotic laccases is bio-bleaching of different kind of pulps where there is indiscriminate use of hazardous chlorine based chemicals for brightness of the paper. In recent years, researchers have shown interest in the identification and characterization of laccases from prokaryotic sources. This catalyst is not commonly reported from this kingdom, although prokaryotes have immense environmental adaptability and biochemical versatility. Moreover, true laccases or laccase-like enzymes exist in many gram-negative, gram-positive bacteria and actinomycetes. Corresponding genes have been identified and functionally expressed in genetically developed hosts. This review summarizes the research efforts to characterize laccases and their properties from different prokaryotic sources, including bacteria and actinomycetes.


Similar content being viewed by others
References
Abadulla E, Tzanov T, Costa S, Robra KH, Cavaco-Paulo A, Gubtiz GM (2000) Decolorization and detoxification of textile dyes with laccase from Trametes hirsuta. Appl Environ Microbiol 66:3357–3362
Alexandre G, Zhulin LB (2000) Laccases are widespread in bacteria. Trends Biotechnol 18:41–42
Archibald FS, Bourbonnais R, Jurasek L, Paice MG, Reid ID (1997) Kraft pulp bleaching and delignification by Trametes versicolor. J Biotechnol 53:215–236
Arias ME, Arenas M, Rodríguez J, Soliveri J, Ball SA, Hernández M (2003) Kraft pulp biobleaching and mediated oxidation of a nonphenolic substrate by laccase from Streptomyces cyaneus CECT 3335. Appl Environ Microbiol 69:1953–1958
Arora DS, Sharma RK (2010) Ligninolytic fungal laccases and their biotechnological applications. Appl Biochem Biotechnol 160:1760–1788
Bains J, Capalash N, Sharma P (2003) Laccase from a nonmelanogenic, alkalotolerant γ-proteobacterium JB isolated from industrial waste water drained soil. Biotechnol Lett 25:1155–1159
Bajpai P (2004) Biological bleaching of chemical pulps. Crit Rev Biotechnol 24:1–58
Balakshin M, Chen CL, Gratzl JS, Kirkman AG, Jakob H (2001) Biobleaching of pulp with dioxygen in laccase-mediator system effect of variables on the reaction kinetics. J Mol Catal B Enz 16:205–215
Baldrian P (2006) Fungal laccases occurrence and properties. FEMS Microbiol Rev 30:215–242
Ball AS, Betts WB, McCarthy AJ (1989) Degradation of lignin related compounds by Actinomycetes. Appl Environ Microbiol 55:1642–1644
Bollag JM, Shuttleworth KL, Anderson DH (1988) Laccase-mediated detoxification of phenolic compounds. Appl Environ Microbiol 54(12):3086–3091
Bourbonnais R, Paice MG (1990) Oxidation of non-phenolic substrates. An expanded role for role of laccase in lignin biodegradation. FEBS Lett 267:99–102
Bourbonnais R, Paice MG (1996) Enzymatic delignification of kraft pulp using laccase and a mediator. Tappi J 79:199–204
Bourbonnais R, Paice MG, Freiermuth B, Bodie E, Borneman S (1997) Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Appl Environ Microbiol 63:4627–4632
Bourbonnais R, Rochefort D, Paice MG, Renaud S, Leech D (2000) Transition metal complexes: a new class of laccase mediators for pulp bleaching. Tappi J 83:68–76
Call HP (1994) Process for modifying, breaking down or bleaching lignin, materials containing lignin or like substances. World patent application WO 94/29510
Call HP, Mücke I (1997) History, overview and applications of mediated ligninolytic systems, especially laccase-mediator-systems (Lignozym®-process). J Biotechnol 53:163–202
Camarero S, Garcia O, Vidal T, Colom J, Rio JC, Gutierrez A (2004) Efficient bleaching of non-wood high-quality paper pulp using laccase-mediator system. Enzyme Microb Technol 35:113–120
Camarero S, Ibarra D, Martínez MJ, Martínez AT (2005) Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Appl Environ Microbiol 71(4):1775–1784
Campos R, Kandelbauer A, Robra K, Cavaco-Paulo A, Gubitz GM (2001) Indigo degradation with purified laccases from Trametes hirsuta and Sclerotium rolfsii. J Biotechnol 89:131–140
Canas AI, Camarero S (2010) Laccases and their natural mediators: biotechnological tools for sustainable eco-friendly processes (available online)
Chakar FS, Ragauskas J (2000) The effects of oxidative alkaline extraction stages after laccase HBT and laccase NHAA treatments: an NMR study of residual lignins. J Wood Chem Technol 20:169–184
Chen SC, Ge W, Buswell JA (2004) Molecular cloning of a new laccase from the edible staw mushroom Volvarielle volvacea: possible involvement in fruit body development. FEMS Microbiol Lett 230:171–176
Claus H (2003) Laccases and their occurrence in prokayotes. Arch Microbiol 179:145–150
Claus H, Faber G, Konig H (2002) Redox-mediated decolorization of synthetic dyes by fungal laccases. Appl Microbiol Biotechnol 59:672–678
Couto SR, Herrera JT (2006) Industrial and biotechnological applications of laccases: a review. Biotechnol Adv 24:500–513
Dalfard AB, Khajeh K, Soudi MR, Naderi-Manesh H, Ranjbar B, Sajedi RH (2006) Isolation and biochemical characterization of laccase and tyrosinase activities in a novel melanogenic soil bacterium. Enzyme Microb Technol 39:1409–1416
Dedeyan B, Klonowska A, Tagger S, Tron T, Iacazio G, Gil G, Petit JL (2000) Biochemical and molecular characterization of a laccase from Marasmius quercophilus. Appl Environ Microbiol 66:925–929
Diamantidis G, Effosse A, Potier P, Bally R (2000) Purification and characterization of the first bacterial laccase in the rhizospheric bacterium Azospirillum lipoferum. Soil Biol Biochem 32:919–927
Dittmer NT, Maureen J, Kanost MR (2010) Characterization of endogenous and recombinant forms of laccase-2: a multicopper oxidase from the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol 39(9):596–606
Driks A (2004) The Bacillus subtilis spore coat. Phytopathology 94:1249–1251
Dubé E, Shareck F, Hurtubise Y, Daneault C, Beauregard M (2008) Homologous cloning, expression, and characterisation of a laccase from Streptomyces coelicolor and enzymatic decolourisation of an indigo dye. Appl Microbiol Biotechnol 79(4):597–603
Duran N, Rosa MA, Annibale A, Gianfreda L (2002) Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: a review. Enzyme Microb Technol 31:907–931
Durao P, Chen Z, Fernandes AT, Hildebrandt P, Murgida DH, Todorovic S, Pereira MM, Melo EP, Martins LO (2008) Copper incorporation into recombinant CotA laccase from Bacillus subtilis: characterization of fully copper loaded enzymes. J Biol Inorg Chem 13:183–193
Endo K, Hosono K, Beppu T, Ueda K (2002) A novel extracytoplasmic phenol oxidase of Streptomyces: its possible involvement in the onset of morphogenesis. Microbiology 148:1767–1776
Fabbrini M, Galli C, Gentili P (2002) Comparing the catalytic efficiency of some mediators of laccase. J Mol Cat B 16:231–240
Fernandez-Larrea J, Stahl U (1996) Isolation and characterization of laccase gene from Podospora anserine. Mol Gen Genet 252:539–551
Ferry Y, Leech D (2005) Amperometric detection of catecholamine neurotransmitters using electrocatalytic substrate recycling at a laccase electrode. Electroanalysis 17:2113–2119
Francis CA, Tebo BM (2002) Enzymatic manganese (II) oxidation by metabolically dormant spores of diverse Bacillus species. Appl Environ Microbiol 68:874–880
Freeman IC, Nayar PG, Begley TP, Villafranca JJ (1993) Stoichiometry and spectroscopic identity of copper centers in phenoxazinone synthase: a new addition to the blue copper oxidase family. Biochemistry 32:4826–4830
Galli C, Gentili P (2004) Chemical messengers: mediated oxidations with the enzyme laccase. J Phys Org Chem 17:973–977
Georis J, Lomascolo A, Camarero S, Dorgeo V, Herpoel I, Asther M (2003) Pycnoporus cinnabarinus laccases: an interesting tool for food or non-food applications. Fac Landbouwkd Toegep Biol Wet 68:263–266
Givaudan A, Effose A, Faure D, Potier P, Bouillant ML, Bally R (1993) Polyphenol oxidase in Azospirillum lipoferum isolated from rice rhizosphere: evidence for laccase activity in non-motile strains of Azospirillum lipoferum. FEMS Microbiol Lett 108:205–210
Gudelj M, Fruhwirth G, Paar A, Lottspeich F, Robra KH, Cavaco-Paulo A, Gübitz GM (2001) A catalase-peroxidase from a newly isolated thermoalkalophilic Bacillus sp. with potential for the treatment of textile bleaching effluents. Extremophiles 5:423–429
Guijarro JM, Pérez J, Dorado JM, Guillén F, Moya R, Hernández M, Arias ME (2009) Detoxification of azo dyes by a novel pH-versatile, salt-resistant laccase from Streptomyces ipomoea. Int Microbiol 12:13–21
Gupta N, Farinas ET (2010) Directed evolution of CotA laccase for increased substrate specificity using Bacillus subtilis spores. Prot Eng Des Sel 23:679–682
Harkin JM, Larsen MJ, Obst JR (1974) Use of syringaldazine for detection of laccase in sporophores of wood rotting fungi. Mycologia 66:469–476
Heinzkill M, Messner K (1997) The ligninolytic system of fungi. In: Anke T (ed) Fungal biotechnology. Weinheim: Chapman and Hall, pp 213−227
Held C, Kandelbauer A, Schroeder M, Cavaco-Paulo A, Guebitz GM (2005) Biotransformation of phenolics with laccase containing bacterial spores. Environ Chem Lett 3:74–77
Hernández-Coronado MJ, Hernández M, Rodríguez J, Arias ME (1998) Gas chromatography/mass spectrometry as a suitable alternative technique to evaluate the ability of Streptomyces to degrade lignin from lignocellulosic residues. Rapid Commun Mass Spectrom 12:1744–1748
Huber M, Lerch K (1987) The influence of copper on the induction of tyrosinase and laccase in Neurospora crassa. FEBS Lett 219:335–338
Hullo MF, Moszer I, Danchin A, Martin-Verstraete I (2001) Cot A of Bacillus substilis is a copper-dependent laccase. J Bacteriol 183:5426–5430
Jing D (2010) Improving the simultaneous production of laccase and lignin peroxidase from Streptomyces lavendulae by medium optimization. Bioresour Technol 101:7592–7597
Johannes C, Majcherczyk A (2000) Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl Environ Microbiol 66:524–528
Jordaan J (2005) Isolation and characterization of a novel thermostable and catalytically efficient laccase from Peniophora sp. strain UD4. Doctor thesis of philosophy of Rhodes University
Kandioller G, Christov L (2001) Evaluation of the delignification and bleaching abilities of selected laccases with HBT on different pulps. In: Argyropoulos DS (ed) Oxidative delignification chemistry fundamentals and catalysis. ACS symposium series, 750. Oxford University Press, USA, pp 427–443
Kiiskinen LL, Ratto M, Kruus K (2004) Screening for novel laccase producing microbes. J Appl Microbiol 97:640–645
Kim C, Lorentz W, Hoopes JT, Dean FF (2001) Oxidation of phenolate siderophores by the multicopper oxidase encoded by the Escherichia coli yacK gene. J Bacteriol 183:4866–4875
Koschorreck K, Richter SM, Ene AB, Roduner E, Schmid RD, Urlacher VB (2008) Cloning and characterization of a new laccase from Bacillus licheniformis catalyzing dimerization of phenolic acids. Appl Microbiol Biotechnol 79(2):217–224
Koschorreck K, Schmid RD, Urlacher VB (2009) Improving the functional expression of a Bacillus licheniformis laccase by random and site-directed mutagenesis. BMC Biotechnol 9:12
Lorenzo M, Moldes D, Couto R, Sanromán MA (2002) Improving laccase production by employing different lignocellulosic wastes in submerged cultures of Trametes versicolor. Bioresour Technol 82:109–113
Malhotra K, Sharma P, Capalash N (2004) Copper and dyes enhance laccase production in γ-proteobacterium JB. Biotechnol Lett 26:1047–1050
Martins LO, Soares CM, Pereira MM, Teixeira M, Costa T, Jones GH, Henriques AO (2002) Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J Biol Chem 277:18849–18859
Maté D, García-Ruiz E, Camarero S, Alcalde M (2011) Directed evolution of fungal laccases. Curr Genomics 12(2):113–122
Miyazaki K (2005) A hyperthermophilic laccase from Thermus thermophilus HB27. Extremophiles 9:415–425
Mohammadian M, Fathi-Roudsari M, Mollania N, Badoei-Dalfard A, Khajeh K (2010) Enhanced expression of a recombinant bacterial laccase at low temperature and micro aerobic conditions: purification and biochemical characterization. J Ind Microbiol Biotechnol 37(8):863–869
Morozova OV, Shumakovich GP, Shleev SV, Yaropolov YI (2007) Laccase-mediator systems and their applications: a review. Appl Biochem Microbiol 43:523–535
Munoz C, Guillen AT, Martinez MJ (1997) Induction and characterization of laccase in the ligninolytic fungus Pleurotus eryngi. Curr Microbiol 34:15
Naclerio G, Falasca A, Petrella E, Nerone V, Federica C, Fulvio C (2010) Potential role of Bacillus endospores in soil amended by olive mill wastewater. Water Sci Technol (online available)
Nicolas JJ, Richard-Forget CF, Goupy MP, Amiot MJ, Aubert YS (1994) Enzymatic browning reactions in apple and apple products. Crit Rev Food Nutr 34:109–157
Niladevi KN, Sukumaran RK, Prema P (2007) Utilization of rice straw for laccase production by Streptomyces psammoticus in solid-state fermentation. J Ind Microbiol Biotechnol 34:665–674
Ninawe S, Kuhad RC (2006) Bleaching of wheat straw-rich soda pulp with xylanase from a thermoalkalophilic Streptomyces cyaneus SN32. Bioresour Technol 97:2291–2295
Palmer AE, Quintanar L, Severance S, Wang TP, Kosman DJ, Solomon EI (2002) Spectroscopic characterization and O2 reactivity of the trinuclear copper cluster of mutants of the multicopper oxidase Fet3p. Biochemistry 41:6438–6448
Palmieri G, Giardina P, Bianco C, Fontanella B, Sannia G (2000) Copper induction of laccase isoenzymes in ligninolytic fungus Pleurotus ostreatus. Appl Environ Microbiol 66:920–924
Palmieri G, Cennamo G, Faraco V, Amoresano A, Sannia G, Giardina P (2003) Atypical laccase isoenzymes from copper supplemented Pleurotus ostreatus cultures. Enzym Microb Technol 33:220–230
Pan JB, Zhao M, Lu L, Du MH, Li GF, Li J, Wang TN, Tang XL (2011) Isolation and characterization of laccase activity in a novel Bacillus amyloliquefaciens LC02. Adv Material Res 183–185:773–777
Renato P, Catarina K, Marcos S (2005) Screening of inducers for laccase production by Lentinula edodes in liquid medium. Braz J Microbiol 36(4):1–8
Roberts SA, Weichsel A, Grass G, Thakali K, Hazzard JT, Tollin G, Rensing C, Montfort WR (2003) Crystal structure and electron transfer kinetics of CueO: a multicopper oxidase required for copper homeostasis in Escherichia coli. Proc Natl Acad Sci USA 99:2766–2771
Ruijssenaars HJ, Hartmans S (2004) A cloned Bacillus halodurans multicopper oxidase exhibiting alkaline laccase activity. Appl Microbiol Biotechnol 65:177–182
Sanchez-Amat A, Solano F (1997) A pluripotent polyphenol oxidase from the melanogenic marine Altermonas sp. shares catalytic capabilities of tyrosinases and laccases. Biochem Biophysic Res Commun 240:787–792
Scharf ME, Tartar A (2008) Termite digestomes as sources for novel lignocellulases. Biofuels Bioprod Bioref 2:540–552
Selvam K, Swaminathan K, Chae K-S (2003) Decolourization of azo dyes and a dye industry effluent by a white rot fungus Thelephora sp. Biores Technol 88:115–119
Setti L, Giuliani S, Spinozzi G, Pifferi PG (1999) Laccase catalyzedoxidativem coupling of 3-methyl 2-benzothiazolinone hydrazone and methoxyphenols. Enzym Microb Technol 25:285–289
Sharma P, Goel R, Capalash N (2007) Bacterial laccases. World J Microbiol Biotechnol 23:823–832
Sheel T, Hofer M, Ludwig S, Holker U (2000) Differential expression of manganese peroxidase and laccase in white-rot fungi in the presence of manganese or aromatic compounds. Appl Microbiol Biotechnol 54:686–691
Shleev S, Pita M, Yaropolov AI, Ruzgas T, Gorton L (2006) Direct heterogeneous electron transfer reactions of Trametes hirsuta laccase at bare and thiol-modified gold electrodes. Electroanal 18:1901–1908
Singh D, Chen S (2008) The white-rot fungus Phanerochaete chrysosporium: conditions for the production of lignin-degrading enzymes. Appl Microbiol Biotechnol 81:399–417
Singh G, Capalash N, Goel R, Sharma P (2007) A pH-stable laccase from alkali-tolerant γ-proteobacterium JB: purification, characterization and indigo carmine degradation. Enzym Microb Technol 41:794–799
Singh G, Ahuja N, Batish M, Capalash N, Sharma P (2008) Biobleaching of wheat straw rich soda pulp with alkalophilic laccase from γ-proteobacterium JB: optimization of process parameters using response surface methodology. Bioresour Technol 99:7472–7479
Singh G, Batish M, Sharma P, Capalash N (2009a) Xenobiotics enhance laccase activity in alkali-tolerant γ-proteobacterium JB. Brazilian J Microbiol 40:26–30
Singh G, Capalash N, Sharma P (2009b) Performance of an alkalophilic and halo tolerant laccase from γ-proteobacterium JB in the presence of industrial pollutants. J Gen Appl Microbiol 55:283–289
Singh G, Bhalla A, Capalash N, Sharma P (2010) Characterization of immobilized laccase from γ-proteobacterium JB: approach towards the development of biosensor for the detection of phenolic compounds. Ind J Sci Technol 3:48–53
Suzuki TK, Endo M, Ito H, Tsujibo K, Inamori Y (2003) A thermo stable laccase from Streptomyces lavendulae REN-7: purification, characterization, nucleotide sequence and expression. Biosci Biotechnol Biochem 67:2167–2175
Takami H, Takaki Y, Uchiyama I (2002) Genome sequence of Oceanobacillus iheyensis isolated from the Iheya Ridge and its unexpected adaptive capabilities to extreme environments. Nucleic Acids Res 30:3927–3935
Tartar A, Wheeler MM, Zhou X, Coy MR, Boucias DG, Scharf ME (2009) Parallel metatranscriptome analyses of host and symbiont geneexpression in the gut of the termite Reticulitermes flavipes. Biotechnol Biofuel 2(25):1–19
Thurston CF (1994) The structure and function of fungal laccases. Microbiol (UK) 140:19–26
Viikari L, Ranua M, Kantelinen A, Linko M, Sundqvist J (1986) Bleaching with enzymes. In: Proceedings of the third conference on biotechnology in the pulp and paper industry, 16–19 June. Swedish Wood Research Institute, Stockholm, pp 67–69
Wang C, Zhao M, Li D, Cui D, Lu L, Wei X (2010) Isolation and characterization of a novel Bacillus subtilis WD23 exhibiting laccase activity from forest soil. Afr J biotechnol 9(34):5496–5502
Witayakran S, Ragauskas A (2009) Synthetic applications of laccase in green chemistry. Adv Synth Catal 351(9):1169–1450
Wong YX, Yu J (1999) Laccase-catalyzed decolorization of synthetic dyes. Water Res 33:3512–3520
Wu J, Kim KS, Lee JH, Lee YC (2010) Cloning, expression in Escherichia coli, and enzymatic properties of laccase from Aeromonas hydrophila WL-11. J Environ Sci (China) 22(4):635–640
Xiao YZ, Chen Q, Hang J, Shi YY (2004) Selective induction, purification and characterization of a laccase isozyme from the basidiomycete Trametes sp. AH28–2. Mycologia 96(1):26–35
Xu P, Yu B, Li FL, Cai XF, Ma CQ (2006) Microbial degradation of sulfur, nitrogen and oxygen heterocycles. Trends Microbiol 14:398–405
Yatsu J, Asano T (2009). Cuticle laccase of the silkworm, Bombyx mori: purification, gene identification and presence of its inactive precursor in the cuticle. Insect Biochem Molec Biol (online available)
Ye M, Li G, Liang WQ, Liu YH (2010) Molecular cloning and characterization of a novel metagenome-derived multicopper oxidase with alkaline laccase activity and highly soluble expression. Appl Microbiol Biotechnol 87(3):1023–1031
Zilly A, Souza CGM, Barbosa-Tessmann IP, Peralta RM (2002) Decolorization of industrial dyes by a Brazilian strain of Pleurotus pulmonariusproducing laccase as the sole phenol-oxidizing enzyme. Folia Microbiol 47(3):273–277
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, G., Bhalla, A., Kaur, P. et al. Laccase from prokaryotes: a new source for an old enzyme. Rev Environ Sci Biotechnol 10, 309–326 (2011). https://doi.org/10.1007/s11157-011-9257-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-011-9257-4