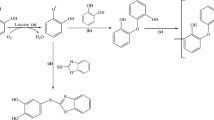
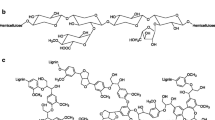
Abstract
Laccase is a ligninolytic enzyme widely distributed in wood-rotting fungi and which is also found in a variety of molds and insects as well as some plants and bacteria. Its biological roles range from depolmerization of lignin, coal and humic acids via the oxidation of various mono- and diaromatic structures, to polymerization reactions and pigment formation in microbial cells or spores. Apart from its action in catabolic, depolymerizing and polymerizing processes, laccases have also been shown to be powerful enzymes for coupling two different molecules to create new low-molecular-weight products in high yield. In addition to their homomolecular coupling capabilities, laccases are also able to couple a hydroxylated aromatic substrate with a nonlaccase substrate of variable structure to create new heteromolecular hybrid molecules. Thus, laccases are increasingly finding applications in biotechnology in the fields of environment-friendly synthesis of fine chemicals and for the gentle derivatization of biologically active compounds e.g., antibiotics, amino acids, antioxidants, and cytostatics. Finally, oligomerization and polymerization reactions can lead to new homo- or heteropolymers and biomaterials. These may be useful in a wide range of applications including the production of polymers with antioxidative properties, the copolymerizing of lignin components with low-molecular mass compounds, the coating of cellulosic cotton fibers or wool, the coloring of hair and leathers, or the cross-linking and oligomerization of peptides.







Similar content being viewed by others
References
Agematu H, Shibamoto N, Nishida H, Okamoto R, Shin T, Murao S (1992) Coriolus versicolor laccase catalyzes the decarboxylation of 2-(4-hydroxyphenyl)glycin and 4-hydroxymandelic acid. Biosci Biotech Biochem 56:1176–1177
Agematu H, Kominato K, Shibamoto N, Yoshioka T, Nishida H, Okamoto R, Shin T, Murao S (1993a) Transformation of 7-(4-hydroxyphenylacetamido)cephalosporanic acid into a new cephalosporin antibiotic, 7-(1-oxaspiro(2.5)octa-6-oxo-4,7-diene-2-carboxamido)cephalosporanic acid, by laccase. Biosci Biotech Biochem 57:1387–1388
Agematu H, Shibamoto N, Nishida H, Okamoto R, Shin T, Murao S (1993b) Oxidative decarboxylations of 4-hydroxymandelic acid and 2-(4-hydroxyphenyl)glycine by laccase from Coriolus versicolor and bilirubin oxidases from Trachyderma tsunodae and Myrothecium verrucaria. Biosci Biotech Biochem 57:1877–1881
Agematu H, Tsuchida T, Kominato K, Shibamoto N, Yoshioka T, Nishida H, Okamoto R, Shin T, Murao S (1993c) Enzymatic dimerization of penicillin X. J Antibiot 46:141–148
Aktas N (2005) Optimization of biopolymerization rate by response surface methodology (RSM). Enzyme Microb Technol 37:441–447
Aktas N, Tanyolac A (2003a) Kinetics of laccase-catalyzed oxidative polymerization of catechol. J Mol Catal B Enzym 22:61–69
Aktas N, Tanyolac A (2003b) Reaction conditions for laccase catalyzed polymerization of catechol. Bioresour Technol 87:209–214
Aktas N, Kibarer G, Tanyolac A (2000) Effects of reaction conditions on laccase-catalyzed α-naphthol polymerization. J Chem Technol Biotechnol 75:840–846
Aktas N, Cicek H, Ünal AT, Kibarer G, Kolankaya N, Tanyolac A (2001) Reaction kinetics for laccase-catalyzed polymerization of 1-naphthol. Bioresour Technol 80:29–36
Aktas N, Sahiner N, Kantoglu Ö, Salih B, Tanyolac A (2003) Biosynthesis and characterization of laccase catalyzed poly(catechol). J Polym Environ 11:123–128
Anyanwutaku I, Petroski R, Rosazza J (1994) Oxidative coupling of mithramycin and hydroquinone catalyzed by copper oxidases and benzoquinone. Implications for the mechanism of action of aureolic acid antibiotics. Bioorg Med Chem 2:543–551
Baldrian P (2006) Fungal laccases—occurrence and properties. FEMS Microbiol Rev 30:215–242
Barreca AM, Fabbrini M, Galli C, Gentili P, Ljunggren S (2003) Laccase/mediated oxidation of a lignin model for improved delignification procedures. J Mol Catal B Enzym 26:105–110
Benfield G, Bocks SM, Bromley K, Brown BR (1964) Studies of fungal and plant laccases. Phytochemistry 3:79–88
Berka RM, Schneider P, Golightly EJ, Brown SH, Madden M, Brown KM, Halkier T, Mondorf K, Xu F (1997) Characterization of the gene encoding an extracellular laccase of Myceliophthora thermophila and analysis of the recombinant enzyme expressed in Aspergillus oryzae. Appl Environ Microbiol 63:3151–3157
Berrio J, Plou FJ, Ballesteros A, Martinez AT, Martinez MJ (2007) Immobilization of Pycnoporus coccineus laccase on Eupergit C: stabilization and treatment of olive oil mill wastewaters. Biocatal Biotransformation 25:130–134
Bhalerao UT, Muralikrishna C, Rani BR (1994) Laccase enzyme catalysed efficient synthesis of 3-substituted-1,2,4-triazolo(4,3-b)(4,1,2)benzothiadiazine-8-ones. Tetrahedron 50:4019–4024
Bocks SM, Brown BR, Todd AH (1962) The biosynthesis of extended quinones. Proc Chem Soc, issue March:117
Bollag JM, Liu SY (1985) Copolymerization of halogenated phenols and syringic acid. Pestic Biochem Physiol 23:261–272
Bollag JM, Liu SY, Minard RD (1982) Enzymatic oligomerization of vanillic acid. Soil Biol Biochem 14:157–163
Bourbonnais R, Paice MG, Reid ID, Lanthier P, Yaguchi M (1995) Lignin oxidation by laccase isoenzymes from Trametes versicolor and role of the mediator 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) in kraft lignin depolymerization. Appl Environ Microbiol 61:1876–1880
Brown BR, Todd AH (1963) A new perylene synthesis. J Chem Soc 5564–5567
Bruno FF, Akkara JA, Kaplan DL, Sekher P, Marx KA, Tripathy SK (1995) Enzyme-mediated two-dimensional polymerization of aromatic derivatives on a Langmuir Trough. Ind Eng Chem Res 34:4009–4015
Bruyneel F, Enaud E, Billottet L, Vanhulle S, Marchand-Brynaert J (2008) Regioselective synthesis of 3-hydroxyorthanilic acid and its biotransformation into a novel phenoxazinone dye by use of laccase. Eur J Org Chem 1:72–79
Burke RM, Cairney JWG (2002) Laccases and other polyphenol oxidases in ecto- and ericoid mycorrhizal fungi. Mycorrhiza 12:105–116
Burton SG (2003) Laccases and phenol oxidases in organic synthesis—a review. Curr Org Chem 7:1317–1331
Calafell M, Diaz C, Hadzhiyska H, Gibert JM, Daga JM, Tzanov T (2007) Bio-catalyzed coloration of cellulose fibers. Biocatal Biotransformation 25:336–340
Cherkashin EA, Stepanova EV, Landesman EO, Koroleva OV, Tishkov VI (2007) Comparative analysis of gene sequences of three high-redox-potential laccases from basidiomycetes. Dokl Biochem Biophys 417:348–351
Cho NS, Cho HY, Shin SJ, Choi YJ, Leonowicz A, Ohga S (2008) Production of fungal laccase and its immobilization and stability. J Fac Agr Kyushu U 53:13–18
Chung JE, Kurisawa M, Tachibana Y, Uyama H, Kobayashi S (2003a) Enzymatic synthesis and antioxidant property of poly(allylamine)-catechin conjugate. Chem Lett 32:620–621
Chung J, Kurisawa M, Uyama H, Kobayashi S (2003b) Enzymatic synthesis and antioxidant property of gelatin-catechin conjugates. Biotechnol Lett 25:1993–1997
Ciecholewski S, Hammer E, Manda K, Bose G, Nguyen VTH, Langer P, Schauer F (2005) Laccase-catalyzed carbon-carbon bond formation: oxidative dimerization of salicylic esters by air in aqueous solution. Tetrahedron 61:4615–4619
Claus H (2003) Laccases and their occurrence in prokaryotes. Arch Microbiol 179:145–150
Claus H (2004) Laccases: structure, reactions, distribution. Micron 35:93–96
Cohen MS, Gabriele PD (1982) Degradation of coal by the fungi Polyporus versicolor and Poria monticola. Appl Environ Microbiol 44:23–27
Couto SR, Toca-Herrera JL (2006) Industrial and biotechnological applications of laccases: a review. Biotechnol Adv 24:500–513
Couto SR, Toca-Herrera JL (2007) Laccase production at reactor scale by filamentous fungi. Biotechnol Adv 25:558–569
Covington AD, Evans CS, Lilley TH, Suparno O (2005) Collagen and polyphenols: new relationships and new outcomes. Part 2. Phenolic reactions for simultaneous tanning and coloring. J Am Leather Chem As 100:336–343
Crecchio C, Ruggiero P, Pizzigallo MDR (1995) Polyphenoloxidases immobilized in organic gels: properties and applications in the detoxification of aromatic compounds. Biotechnol Bioeng 48:585–591
Desentis-Mendoza RM, Hernandez-Sanchez H, Moreno A, Rojas del CE, Chel-Guerrero L, Tamariz J, Jaramillo-Flores ME (2006) Enzymatic polymerization of phenolic compounds using laccase and tyrosinase from Ustilago maydis. Biomacromolecules 7:1845–1854
Duran N, Esposito E (2000) Potential applications of oxidative enzyms and phenoloxidase-like compounds in wastewater and soil treatment: a review. Appl Catal B Environ 28:83–99
Duran N, Rosa MA, D’Annibale A, Gianfreda L (2002) Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: a review. Enzyme Microb Technol 31:907–931
Eckenrode F, Peczynska-Czoch W, Rosazza J (1982) Microbial transformations of natural anitumor agents XVIII: conversions of vindoline with copper oxidases. J Pharm Sci 71:1246–1250
Eggert C, Temp U, Dean JFD, Eriksson KEL (1995) Laccase-mediated formation of the phenoxazione derivative, cinnabarinic acid. FEBS Lett 376:202–206
Elegir G, Daina S, Zoia L, Bestetti G, Orlandi M (2005) Laccase mediator system: oxidation of recalcitrant lignin model structures present in residual kraft lignin. Enzyme Microb Technol 37:340–346
Es-Safi NE, Ghidouche S, Ducrot PH (2007) Flavonoids: hemisynthesis, reactivity, characterization and free radical scavenging activity. Molecules 12:2228–2258
Faergemand M, Otte J, Qvist KB (1998) Cross-linking of whey proteins by enzymatic oxidation. J Agric Food Chem 4:1326–1333
Fakoussa RM, Hofrichter M (1999) Biotechnology and microbiology of coal degradation. Appl Microbiol Biotechnol 52:25–40
Fukuda T, Uchida H, Takashima Y, Uwajima T, Kawabata T, Suzuki M (2001) Degradation of bisphenol A by purified laccase from Trametes villosa. Biochem Biophys Res Commun 284:704–706
Fukuda T, Uchida H, Suzuki M, Miyamoto H, Morinaga H, Nawata H, Uwajima T (2004) Transformation products of bisphenol A by a recombinant Trametes villosa laccase and their estrogenic activity. J Chem Technol Biotechnol 79:1212–1218
Ganachaud C, Garfagnoli V, Tron T, Iacazio G (2008) Trimerisation of indole through laccase catalysis. Tetrahedron Lett 49:2476–2478
Gianfreda L, Iamarino G, Scelza R, Rao MA (2006) Oxidative catalysts for the transformation of phenolic pollutants: a brief review. Biocatal Biotransformation 24:177–187
Güresir M, Aktas N, Tanyolac A (2005) Influence of reaction conditions on the rate of enzymatic polymerization of pyrogallol using laccase. Process Biochem 40:1175–1182
Gutierrez A, del Rio JC, Rencoret J, Ibarra D, Martinez AT (2006) Main lipophilic extractives in different paper pulp types can be removed using the laccase-mediator system. Appl Microbiol Biotechnol 72:845–851
Hahn V, Mikolasch A, Manda K, Gördes D, Thurow K, Schauer F (2008) Laccase-catalyzed carbon–nitrogen bond formation: coupling and derivatization of unprotected l-phenylalanine with different para-hydroquinones. Amino Acids doi:https://doi.org/10.1007/s00726-008-0154-2
Hatakka A (1994) Lignin-modifying enzymes from selected white-rot fungi: production and role in lignin degradation. FEMS Microbiol Rev 13:125–135
Hernandez-Luna CE, Guiterrez-Soto G, Salcedo-Martinez SM (2008) Screening for decolorizing basidiomycetes in Mexico. Screening and selection of ligninolytic basidiomycetes with decolorizing ability in Northeast Mexico. World J Microbiol Biotechnol 24:465–473
Hoegger PJ, Kilaru S, James TY, Thacker JR, Kües U (2006) Phylogenetic comparison and classification of laccase and related multicopper oxidase protein sequences. FEBS J 273:2308–2326
Hölker U, Schmiers H, Große S, Winkelhöfer M, Polsakiewicz M, Ludwig S, Dohse J, Höfer M (2002) Solubilization of low-rank coal Trichoderma atroviride: evidence for the involvement of hydrolytic and oxidative enzymes by using 14C-labelled lignite. J Ind Microbiol Biotechnol 28:207–212
Hosny M, Rosazza JPN (2002) Novel oxidations of (+)-catechin by horseradish peroxidase and laccase. J Agric Food Chem 50:5539–5545
Husain Q (2006) Potential applications of the oxidoreductive enzymes in the decolorization and detoxification of textile and other synthetic dyes from polluted water: a review. Crit Rev Biotechnol 26:201–221
Hwang HM, Hu X, Zhao X (2007) Enhanced bioremediation of polycyclic aromatic hydrocarbons by environmentally friendly techniques. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 25:313–352
Hu X, Zhao X, Hwang H (2007) Comparative study of immobilized Trametes versicolor laccase on nanoparticles and kaolinite. Chemosphere 66:1618–1626
Ikeda R, Sugihara J, Uyama H, Kobayashi S (1996a) Enzymatic oxidative polymerization of 2,6-dimethylphenol. Macromolecules 29:8702–8705
Ikeda R, Uyama H, Kobayashi S (1996b) Novel synthetic pathway to a poly(phenylene oxide). Laccase-catalyzed oxidative polymerization of syringic acid. Macromolecules 29:3053–3054
Ikeda R, Tanaka H, Uyama H, Kobayashi S (1998) Laccase-catalyzed polymerization of acrylamide. Macromol Rapid Commun 19:423–425
Ikeda R, Tanaka H, Oyabu H, Uyama H, Kobayashi M (2001) Preparation of artificial urushi via an environmentally benign process. Bull Chem Soc Jpn 74:1067–1073
Ikehata K, Buchanan ID, Smith DW (2004) Recent developments in the production of extracellular fungal peroxidases and laccases for waste treatment. J Environ Eng Sci 3:1–19
Intra A, Nicotra S, Riva S, Danieli B (2005) Significant and unexpected solvent influence on the selectivity of laccase-catalyzed coupling of tetrahydro-2-naphthol derivatives. Adv Synth Catal 347:973–977
Jiang DS, Long SY, Huang J, Xiao HY, Zhou JY (2005) Immobilization of Pycnoporus sanguineus laccase on magnetic chitosan microspheres. Biochem Eng J 25:15–23
Jolivalt C, Raynal A, Caminade E, Kokel B, Le Goffic F, Mougin C (1999) Transformation of N′,N′-dimethyl-N-(hydroxyphenyl)ureas by laccase from the white rot fungus Trametes versicolor. Appl Microbiol Biotechnol 51:676–681
Jonas U, Hammer E, Schauer F, Bollag JM (1998) Transformation of 2-hydroxydibenzofuran by laccases of the white rot fungi Trametes versicolor and Pycnoporus cinnabarinus and characterization of oligomerization products. Biodegradation 8:321–328
Jonas U, Hammer E, Haupt ETK, Schauer F (2000) Characterisation of coupling products formed by biotransformation of biphenyl and diphenyl ether by the white rot fungus Pycnoporus cinnabarinus. Arch Microbiol 174:393–398
Jung H, Xu F, Li K (2002) Purification and characterization of laccase from wood-degrading fungus Trichophyton rubrum LKY-7. Enzyme Microb Technol 30:161–168
Kanitskaya LV, Seleznev SN, Medvedeva SA, Kupriyanovich YN, Turchaninov VK (2003) Dehydrogenation polymerization of 2,6-dimethoxyphenol. Polym Sci Ser A 45:92–98
Karamyshev AV, Shleev SV, Koroleva OV, Yaropolov AI, Sakharov IY (2003) Laccase-catalyzed synthesis of conducting polyaniline. Enzyme Microb Technol 33:556–564
Kim SY, Zille A, Murkovic M, Güebitz G, Cavaco-Paulo A (2007) Enzymatic polymerization on the surface of functionalized cellulose fibers. Enzyme Microb Technol 40:1782–1787
Kim S, Lopez C, Güebitz G, Cavaco-Paulo A (2008) Biological coloration of flax fabrics with flavonoids using laccase from Trametes hirsuta. Eng Life Sci 8:324–330
Klonowska A, Gaudin C, Fournel A, Asso M, Le Petit J, Giorgi M, Tron T (2002) Characterization of a low redox potential laccase from the basidiomycete C30. Eur J Biochem 269:6119–6125
Klonowska A, Gaudin C, Asso A, Fournel A, Reglier M, Tron T (2005) LAC3, a new low redox potential laccase from Trametes sp. strain C30 obtained as a recombinant protein in yeast. Enzyme Microb Technol 36:34–41
Kobayashi S, Higashimura H (2003) Oxidative polymerization of phenols revisited. Prog Polym Sci 28:1015–1048
Kobayashi S, Uyama H (1998) Enzymatic polymerization for synthesis of polyester and polyaromatics. Enzymes in Polymer Synthesis ACS Symp Series 684:58–73
Kobayashi S, Uyama H, Ikeda R (2001) Artificial urushi. Chem Eur J 7:4755–4760
Koschorreck K, Richter SM, Swierczek A, Beifuss U, Schmid RD, Urlacher VB (2008) Comparative characterization of four laccases from Trametes versicolor concerning phenolic C-C coupling and oxidation of PAHs. Arch Biochem Biophys 474:213–219
Kulys J, Vidziunaite R, Schneider P (2003) Laccase-catalyzed oxidation of naphthol in the presence of soluble polymers. Enzyme Microb Technol 32:455–463
Kunamneni A, Ghazi I, Camarero S, Ballesteros A, Plou FJ, Alcalde M (2008) Decolorization of synthetic dyes by laccase immobilized on epoxy-activated carriers. Process Biochem 43:169–178
Kupriyanovich YN, Medvedeva SA, Rokhin AV, Kanitskaya LV (2007) Regioselectivity of ferulic acid polymerization catalyzed by oxidases. Russ J Bioorg Chem 33:516–522
Kurisawa M, Chung JE, Uyama H, Kobayashi S (2003a) Enzymatic synthesis and antioxidant properties of poly(rutin). Biomacromolecules 4:1394–1399
Kurisawa M, Chung JE, Uyama H, Kobayashi S (2003b) Laccase-catalyzed synthesis and antioxidant property of poly(catechin). Macromol Biosci 3:758–764
Leonowicz A, Edgehill RU, Bollag JM (1984) The effect of pH on the transformation of syringic and vanillic acids by the laccases of Rhizoctonia praticola and Trametes versicolor. Arch Microbiol 137:89–96
Leonowicz A, Matuszewska A, Luterek J, Ziegenhagen D, Wojtas-Wasilewska M, Cho NS, Hofrichter M, Rogalski J (1999) Biodegradation of lignin by white rot fungi. Fungal Genet Biol 27:175–185
Leonowicz A, Cho NS, Luterek J, Wilkolazka A, Wojtas-Wasilewska M, Matuszewska A, Hofrichter M, Wesenberg D, Rogalski J (2001) Fungal laccase: properties and activity on lignin. J Basic Microbiol 41:185–227
Liu SY, Bollag JM (1985) Enzymatic binding of the pollutant 2,6-xylenol to a humus constituent. Water Air Soil Pollut 25:97–106
Liu SY, Minard RD, Bollag JM (1981) Oligomerization of syringic acid, a lignin derivative, by a phenoloxidase. Soil Sci Soc Am J 45:1100–1105
Lu L, Zhao M, Wang Y (2007) Immobilization of laccase by alginate-chitosan microcapsules and its use in dye decolorization. World J Microbiol Biotechnol 23:159–166
Lugaro G, Carrera G, Cremonesi P, Casellato MM, Antonini E (1973) The oxidation of steroid hormones by fungal laccase in emulsion of water and organic solvent. Arch Biochem Biophys 159:1–6
Mai C, Milstein O, Hüttermann A (2000) Chemoenzymatical grafting of acrylamide onto lignin. J Biotechnol 79:173–183
Mai C, Schormann W, Hüttermann A (2001) Chemo-enzymatically induced copolymerization of phenolics with acrylate compounds. Appl Microbiol Biotechnol 55:177–186
Manda, K (2006) Biotransformation von Aminosäuren, Peptiden und dihydroxylierten Aromaten durch Laccasen aus Pilzen zur Herstellung von Hybridmolekülen. Ph.D. thesis, Greifswald, Germany
Manda K, Hammer E, Mikolasch A, Niedermeyer T, Dec J, Jones AD, Benesi AJ, Schauer F, Bollag JM (2005) Laccase-induced cross-coupling of 4-aminobenzoic acid with para-dihydroxylated compounds 2,5-dihydroxy-N-(2-hydroxyethyl)-benzamide and 2,5-dihydroxybenzoic acid methyl ester. J Mol Catal B Enzym 35:86–92
Manda K, Hammer E, Mikolasch A, Gördes D, Thurow K, Schauer F (2006) Laccase-induced derivatization of unprotected amino acid l-tryptophan by coupling with p-hydroquinone 2,5-dihydroxy-N-(2-hydroxyethyl)-benzamide. Amino Acids 31:409–419
Manda K, Gördes D, Mikolasch A, Hammer E, Schmidt E, Thurow K, Schauer F (2007) Carbon-oxygen bond formation by fungal laccases: cross-coupling of 2,5-dihydroxy-N-(2-hydroxyethyl)-benzamide with the solvents water, methanol, and other alcohols. Appl Microbiol Biotechnol 76:407–416
Marco MP, Barcelo D (1996) Environmental applications of analytical biosensors. Meas Sci Technol 7:1547–1562
Martinez AT, Speranza M, Ruiz-Duenas FJ, Ferreira P, Camarero S, Guillen F, Martinez MJ, Gutierrez A, del Rio JC (2005) Biodegradation of lignocellulosics: microbial chemical, and enzymatic aspects of the fungal attack of lignin. Int Microbiol 8:195–204
Mattinen ML, Kruus K, Buchert J, Nielsen JH, Andersen HJ, Steffensen CL (2005) Laccase-catalyzed polymerization of tyrosine-containing peptides. FEBS J 272:3640–3650
Mattinen ML, Hellman M, Permi P, Autio K, Kalkkinen N, Buchert J (2006) Effect of protein structure on laccase-catalyzed protein oligomerization. J Agric Food Chem 54:8883–8890
Mayer AM, Staples RC (2002) Laccase: new functions for an old enzyme. Phytochemistry 60:551–565
Michalek H, Szarkowska L (1959) The quinone–amino acid complexes and polyphenolase. Acta Biochim Pol 6:399–409
Mikolasch A, Schauer F (2003) Biotransformation of N-(2-alkylamino-4-phenylimidazol-1-yl)-acetamides and kinetic studies by using cells and laccase from Trametes versicolor. J Basic Microbiol 43:508–521
Mikolasch A, Hammer E, Jonas U, Popowski K, Stielow A, Schauer F (2002) Synthesis of 3-(3,4-dihydroxyphenyl)-propionic acid derivatives by N-coupling of amines using laccase. Tetrahedron 58:7589–7593
Mikolasch A, Niedermeyer THJ, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Gesell M, Hessel S, Jülich WD, Lindequist U (2006) Novel penicillins synthesized by biotransformation using laccase from Trametes spec. Chem Pharm Bull 54:632–638
Mikolasch A, Niedermeyer THJ, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Gesell Salazar M, Hessel S, Jülich WD, Lindequist U (2007) Novel cephalosporins synthesized by amination of 2,5-dihydroxybenzoic acid derivatives using fungal laccases. Chem Pharm Bull 55:412–416
Mikolasch A, Hessel S, Gesell Salazar M, Neumann H, Manda K, Gördes D, Schmidt E, Thurow K, Hammer E, Lindequist U, Beller M, Schauer F (2008a) Synthesis of new N-analogous corollosporine derivatives with antibacterial activity by laccase-catalyzed amination. Chem Pharm Bull 56:781–786
Mikolasch A, Matthies A, Lalk M, Schauer F (2008b) Laccase-induced C–N coupling of substituted p-hydroquinones with p-aminobenzoic acid in comparison with known chemical routes. Appl Microbiol Biotechnol 80:389–397
Mikolasch A, Wurster M, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Jülich WD, Lindequist U (2008c) Novel β-lactam antibiotics synthesized by amination of catechols using fungal laccase. Chem Pharm Bull 56:902–907
Milstein O, Hüttermann A, Fründ R, Lüdemann HD (1994) Enzymatic co-polymerization of lignin with low-molecular mass compounds. Appl Microbiol Biotechnol 40:760–767
Minard RD, Liu SY, Bollag JM (1981) Oligomers and quinones from 2,4-dichlorophenol. J Agr Food Chem 29:250–253
Minussi RC, Pastore GM, Duran N (2002) Potential applications of laccase in the food industry. Trends Food Sci Tech 13:205–216
Mita N, Tawaki S, Uyama H, Kobayashi S (2003) Laccase-catalyzed oxidative polymerization of phenols. Macromol Biosci 3:253–257
Morozova OV, Shumakovich GP, Gorbacheva MA, Shleev SV, Yaropolov AI (2007a) “Blue” laccases. Biochemistry (Mosc) 72:1136–1150
Morozova OV, Shumakovich GP, Shleev SV, Yaropolov YI (2007b) Laccase-mediator systems and their applications: a review. Appl Biochem Microbiol 43:523–535
Nakamura K, Go N (2005) Function and molecular evolution of multicopper blue proteins. Cell Mol Life Sci 62:2050–2066
Nicotra S, Cramarossa MR, Mucci A, Pagnoni UM, Riva S, Forti L (2004a) Biotransformation of resveratrol: synthesis of trans-dehydrodimers catalyzed by laccases from Myceliophtora thermophyla and from Trametes pubescens. Tetrahedron 60:595–600
Nicotra S, Intra A, Ottolina G, Riva S, Danieli B (2004b) Laccase-mediated oxidation of the steroid hormone 17ß-estradiol in organic solvents. Tetrahedron Asymmetry 15:2927–2931
Niedermeyer THJ, Mikolasch A, Lalk M (2005) Nuclear amination catalyzed by fungal laccases: reaction products of p-hydroquinones and primary aromatic amines. J Org Chem 70:2002–2008
Niladevi KN, Prema P (2008) Immobilization of laccase from Streptomyces psammoticus and its application in phenol removal using packed bed reactor. World J Microbiol Biotechnol 24:1215–1222
O’Malley DM, Whetten R, Bao W, Chen CL, Sederoff RR (1993) The role of laccase in lignification. Plant J 4:751–757
Ong E, Pollock WBR, Smith M (1997) Cloning and sequence analysis of two laccase complementary DNAs from the ligninolytic basidiomycete Trametes versicolor. Gene 196:113–119
Osiadacz J, Al-Adhami AJH, Bajraszewska D, Fischer P, Peczynska-Czoch W (1999) On the use of Trametes versicolor laccase for the conversion of 4-methyl-3-hydroxyanthranilic acid to actinocin chromophore. J Biotechnol 72:141–149
Pal S, Bollag JM, Huang PM (1994) Role of abiotic and biotic catalysts in the transformation of phenolic compounds through oxidative coupling reaction. Soil Biol Biochem 26:813–820
Palmieri G, Giardina P, Desiderio B, Marzullo L, Giamberini M, Sannia G (1994) A new enzyme immobilization procedure using copper alginate gel: application to a fungal phenol oxidase. Enzyme Microb Technol 16:151–158
Peshkova S, Li K (2003) Investigation of chitosan-phenolics systems as wood adhesives. J Biotechnol 102:199–207
Pilz R, Hammer E, Schauer F, Kragl U (2003) Laccase-catalysed synthesis of coupling products of phenolic substrates in different reactors. Appl Microbiol Biotechnol 60:708–712
Poeckel D, Niedermeyer THJ, Pham HTL, Mikolasch A, Mundt S, Lindequist U, Lalk M, Werz O (2006) Inhibition of human 5-lipoxygenase and anti-neoplastic effects by 2-amino-1,4-benzoquinones. Med Chem 2:591–595
Ponzoni C, Beneventi E, Cramarossa MR, Raimondi S, Trevisi G, Pagnoni UM, Riva S, Forti L (2007) Laccase-catalyzed dimerization of hydroxystilbenes. Adv Synth Catal 349:1497–1506
Quaratino D, Federici F, Petruccioli M, Fenice M, D’Annibale A (2007) Production, purification and partial characterization of a novel laccase from the white-rot fungus Panus tigrinus CBS 577.79. Antonie van Leeuwenhoek 91:57–69
Rättö M, Ritschkoff AC, Viikari L (2004) Enzymatically polymerized phenolic compounds as wood preservatives. Holzforschung 58:440–445
Rebrikov DN, Stepanova EV, Koroleva OV, Budarina ZI, Zakharova MV, Yurkova TV, Solonin AS, Belova OV, Pozhidaeva ZA, Leont’evsky AA (2006) Laccase of the lignolytic fungus Trametes hirsuta: purification and characterization of the enzyme, and cloning and primary structure of the gene. Appl Biochem Microbiol 42:564–572
Rittstieg K, Suurnakki A, Suortti T, Kruus K, Guebitz G, Buchert J (2002) Investigations on the laccase-catalyzed polymerization of lignin model compounds using size-exclusion HPLC. Enzyme Microb Technol 31:403–410
Rittstieg K, Suurnäkki A, Suortti T, Kruus K, Guebitz GM, Buchert J (2003) Polymerization of guaiacol and a phenolic β-O-4-substructure by Trametes hirsuta laccase in the presence of ABTS. Biotechnol Prog 19:1505–1509
Riva S (2006) Laccases: blue enzymes for green chemistry. Trends Biotechnol 24:219–226
Rüttimann-Johnson C, Lamar RT (1996) Polymerization of pentachlorphenol and ferulic acid by fungal extracellular lignin-degrading enzymes. Appl Environ Microbiol 62:3890–3893
Schäfer A, Specht M, Hetzheim A, Francke W, Schauer F (2001) Synthesis of substituted imidazoles and dimerization products using cells and laccase from Trametes versicolor. Tetrahedron 57:7693–7699
Schroeder M, Aichernig N, Guebitz GM, Kokol V (2007) Enzymatic coating of lignocellulosic surfaces with polyphenols. Biotechnol J 2:334–341
Schultz A, Jonas U, Hammer E, Schauer F (2001) Dehalogenation of chlorinated hydroxybiphenyls by fungal laccase. Appl Environ Microbiol 67:4377–4381
Selinheimo E, Lampila P, Mattinen ML, Buchert J (2008) Formation of protein–oligosaccharide conjugates by laccase and tyrosinase. J Agric Food Chem 56:3118–3128
Shah V, Nerud F (2002) Lignin degrading system of white-rot fungi and its exploitation for dye decolorization. Can J Microbiol 48:857–870
Shiba T, Xiao L, Miyakoshi T, Chen CL (2000) Oxidation of isoeugenol and coniferyl alcohol catalyzed by laccases isolated from Rhus vernicifera Stokes and Pycnoporus coccineus. J Mol Catal B Enzym 10:605–615
Shin H, Guebitz G, Cavaco-Paulo A (2001) “In situ” enzymatically prepared polymers for wool coloration. Macromol Mater Eng 286:691–694
Silva C, Silva CJ, Zille A, Guebitz GM, Cavaco-Paulo A (2007) Laccase immobilization on enzymatically functionalized polyamide 6,6 fibres. Enzyme Microb Technol 41:867–875
Simmons KE, Minard RD, Bollag JM (1989) Oxidative co-oligomerization of guaiacol and 4-chloroaniline. Environ Sci Technol 23:115–121
Sjoblad RD, Minard RD, Bollag JM (1976) Polymerization of 1-naphthol and related phenolic compounds by an extracellular fungal enzyme. Pestic Biochem Physiol 6:457–463
Solomon EI, Sundaram UM, Machonkin TE (1996) Multicopper oxidases and oxygenases. Chem Rev 96:2563–2606
Solomon EI, Chen P, Metz M, Lee SK, Palmer AE (2001) Oxygen binding, activation, and reduction to water by copper proteins. Angew Chem 40:4570–4590
Song HK, Palmore GTR (2005) Conductive polypyrrole via enzyme catalysis. J Phys Chem B 109:19278–19287
Suparno O, Covington AD, Evans CS (2007) Application of diphenols for dyeing. J Soc Leath Tech Ch 91:139–141
Tanaka T, Yamamoto M, Takahashi M, Fujii T, Taniguchi M (2003) Enzymatic oxidative polymerization of 4-chloroguaiacol by laccase. J Chem Eng Jpn 36:1101–1106
Tatsumi K, Freyer A, Minard RD, Bollag JM (1994) Enzymatic coupling of chloroanilines with syringic acid, vanillic acid and protocatechuic acid. Soil Biol Biochem 26:735–742
Teerapatsakul C, Bucke C, Parra R, Keshavarz T, Chitradon L (2008) Dye decolorisation by laccase entrapped in copper alginate. World J Microbiol Biotechnol 24:1367–1374
Temp U, Meyrahn H, Eggert C (1999) Extracellular phenol oxidase patterns during depolymerisation of low-rank coal by three basidiomycetes. Biotechnol Lett 21:281–287
Tsai HF, Wheeler MH, Chang YC, Kwon-Chung KJ (1999) A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J Bacteriol 181:6469–6477
Tsujimoto T, Uyama H, Kobayashi S (2001) Polymerization of vinyl monomers using oxidase catalysts. Macromol Biosci 1:228–232
Tsujimoto T, Ando N, Oyabu H, Uyama H, Kobayashi S (2007) Laccase-catalyzed curing of natural phenolic lipids and product properties. J Macromol Sci A 44:1055–1060
Uchida H, Fukuda T, Miyamoto H, Kawabata T, Suzuki M, Uwajima T (2001) Polymerization of bisphenol A by purified laccase from Trametes villosa. Biochem Biophys Res Commun 287:355–358
Uyama H (2001) Enzymatic synthesis and applications of new polymeric materials. Kobunshi Ronbunshu 58:382–396
Uyama H, Kobayashi S (2002) Enzyme-catalyzed polymerization to functional polymers. J Mol Catal B Enzym 19:117–127
Uyama H, Kobayashi S (2003) Enzymatic synthesis of polyphenols. Curr Org Chem 7:1387–1397
Uyama H, Kobayashi S (2006) Enzymatic synthesis and properties of polymers from polyphenols. Adv Polym Sci 194:51–67
Waite JH (1983) Evidence for a repeating 3,4-dihydroxyphenylalanine-and hydroxyproline-containing decapeptide in the adhesive protein of the mussel, Mytilus edulis L. J Biol Chem 258:2911–2915
Waite JH (1985) Catechol oxidase in the byssus of the common mussel, Mytilus edulis L. J Mar Biol Assoc UK 65:359–371
Waite JH (1987) Nature’s underwater adhesive specialist. Int J Adhesion Adhes 7:9–14
Waite JH (1990a) Marine adhesive proteins: natural composite thermosets. Int J Biol Macromol 12:139–144
Waite JH (1990b) The phylogeny and chemical diversity of quinone-tanned glues and varnishes. Comp Biochem Physiol B 97:19–29
Waite JH (1992) The formation of mussel byssus: anatomy of a natural manufacturing process. Results Probl Cell Differ 19:27–54
Waite JH, Tanzer ML (1981) Specific colorimetric detection of o-diphenols and 3,4-dihydroxyphenylalanine-containing peptides. Anal Biochem 111:131–136
Waite JH, Housley TJ, Tanzer ML (1985) Peptide repeats in a mussel glue protein: theme and variations. Biochemistry 24:5010–5014
Waite JH, Hansen DC, Little KT (1989) The glue protein of ribbed mussels (Geukensia demissa): a natural adhesive with some features of collagen. J Comp Physiol B 159:517–525
Wells A, Teria M, Eve T (2006) Green oxidations with laccase-mediator systems. Biochem Soc Trans 34:304–308
Wesenberg D, Kyriakides I, Agathos SN (2003) White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol Adv 22:161–187
Widsten P, Kandelbauer A (2008) Laccase applications in the forest products industry: a review. Enzyme Microb Technol 42:293–307
Willmann G, Fakoussa RM (1997) Extracellular oxidative enzymes of coal-attacking fungi. Fuel Process Technol 52:27–41
Williamson PR, Wakamatsu K, Ito S (1998) Melanin biosynthesis in Cryptococcus neoformans. J Bacteriol 180:1570–1572
Xu F (1999) Laccase. In: Flickinger MC, Drew SW (eds) Encyclopedia of bioprocess technology: fermentation, biocatalysis and bioseparation, first edn. Wiley, New York, pp 1545–1554
Xu P, Uyama H, Whitten JE, Kobayashi S, Kaplan DL (2005) Peroxidase-catalyzed in situ polymerization of surface orientated caffeic acid. J Am Chem Soc 127:11745–11753
Yamaguchi H, Maeda Y, Sakata I (1992) Applications of phenol dehydrogenative polymerization by laccase to bonding among woody-fibers. Mokuzai Gakkaishi 38:931–937
Yamaguchi H, Maeda Y, Sakata I (1994) Bonding among woody fibers by use of enzymatic phenol dehydrogenative polymerization — mechanism of generation of bonding strength. Mokuzai Gakkaishi 40:185–190
Yaropolov AI, Skorobogatko OV, Vartanov SS, Varfolomeyev SD (1994) Laccase — properties, catalytic mechanism, and applicability. Appl Biochem Biotechnol 49:257–280
Zhou P, Fu S (2004) The polymerization of 4-phenylphenol catalyzed by laccase. Acta Polym Sin 4:614–616
Zhu Y, Kaskel S, Shi J, Wage T, van Pee KH (2007) Immobilization of Trametes versicolor laccase on magnetically separable mesoporous silica spheres. Chem Mater 19:6408–6413
Zhuklistova NE, Zhukova YN, Lyashenko AV, Zaitsev VN, Mikhailov AM (2008) Three-dimensional organization of three-domain copper oxidases: a review. Crystallogr Rep 53:92–109
Zille A, Munteanu F, Guebitz GM, Artur CP (2005) Laccase polymerization of amino-phenol compounds. Abstracts of Papers of the American Chemical Society 229:U955–U955
Acknowledgment
We thank R. Jack (Institute of Immunology, University of Greifswald) for help in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mikolasch, A., Schauer, F. Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Appl Microbiol Biotechnol 82, 605–624 (2009). https://doi.org/10.1007/s00253-009-1869-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-1869-z