Abstract
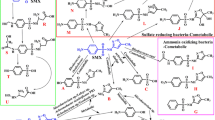
Tetracyclines are antibiotics considered emerging pollutants and currently, wastewater treatment plants are not able to remove them efficiently. Laccases are promising enzymes for bioremediation because they can oxidize a wide variety of substrates. The aim of this study was to evaluate the Botrytis aclada laccase for the oxidation of chlortetracycline and its isomers in the absence of a mediator molecule, at a pH range between 3.0 to 7.0, and to characterize the transformation products by LC–MS. Chlortetracycline and three isomers were detected in both, controls and reaction mixtures at 0 h and in controls after 48 h of incubation but in different proportions depending on pH. An additional isomer was also detected, but only in the presence of BaLac. Based on the transformation products identified in the enzymatic reactions and information from literature, we assembled a network of transformation pathways starting from chlortetracycline and its isomers. The spectrometric analysis of the products indicated the probable occurrence of oxygen insertion, dehydrogenation, demethylation and deamination reactions. Four new products were identified, and we also described a novel transformation product without the chloro group. We observed that increasing pH led to higher diversity of main products. This is the first study using the laccase from fungi Botrytis aclada to oxidate chlortetracycline and its isomers and it can be considered as an ecological alternative to be used in bioremediation processes such as wastewater.




Similar content being viewed by others
References
Adams KJ, Pratt B, Bose N, Dubois LG, John-williams LS, Perrott KM, Ky K, Kapahi P, Sharma V, Maccoss MJ, Moseley MA, Colton CA, Maclean BX, Schilling B, Thompson JW (2020) Skyline for small molecules: a unifying software package for quantitative metabolomics. J Proteome Res 19(4):1447–1458. https://doi.org/10.1021/acs.jproteome.9b00640
Aga DS, Lenczewski M, Snow D, Muurinen J, Sallach JB, Wallace JS (2016) Challenges in the measurement of antibiotics and in evaluating their impacts in agroecosystems: a critical review. J Environ Qual 45(2):407–419. https://doi.org/10.2134/jeq2015.07.0393
Arikan OA (2008) Degradation and metabolization of chlortetracycline during the anaerobic digestion of manure from medicated calves. J Hazard Mater 158(2–3):485–490. https://doi.org/10.1016/j.jhazmat.2008.01.096
Baldrian P (2006) Fungal laccases—occurrence and properties. FEMS Microbiol Rev 30(2):215–242. https://doi.org/10.1111/j.1574-4976.2005.00010.x
Berglund B (2015) Environmental dissemination of antibiotic resistance genes and correlation to anthropogenic contamination with antibiotics. Infect Ecol Epidemiol 5:28564. https://doi.org/10.3402/iee.v5.28564
Bolan N, Szogi A, Chuasavathi T, Seshadri B, Rothrock M, Panneerselvam P (2010) Uses and management of poultry litter. Worlds Poult Sci J 66(4):673–698. https://doi.org/10.1017/S0043933910000656
Borghi AA, Palma MSA (2014) Tetracycline: production, waste treatment and environmental impact assessment. Braz J Pharm Sci 50(1):25–40. https://doi.org/10.1590/S1984-82502011000100003
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1): 248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cabrera-Núñez A, Daniel-Renteria I, Martínez-Sánchez C, Alarcón-Pulido S, Rojas-Ronquillo R, Velázquez-Jiménez S (2018) Aprovechamiento de subproductos avícolas como fuente proteica en la elaboración de dietas para rumiantes. Abanico Veterinario 8(2): 59–67. https://doi.org/10.21929/abavet2018.82.5
Cañas AI, Camarero S (2010) Laccases and their natural mediators: biotechnological tools for sustainable eco-friendly processes. Biotechnol Adv 28(6):694–705. https://doi.org/10.1016/j.biotechadv.2010.05.002
Cessna AJ, Kuchta SL, Bailey J, Waiser M, Tumber V (2020) Isomerization of chlortetracycline in prairie wetland water. J Environ Qual 49: 1435–1444. https://doi.org/10.1002/jeq2.20079
Chan R, Chiemchaisri C, Chiemchaisri W, Boonsoongnern A, Tulayakul P (2022) Occurrence of antibiotics in typical pig farming and its wastewater treatment in Thailand. Emerg Contam 8:21–29. https://doi.org/10.1016/j.emcon.2021.12.003
Chang BV, Hsu FY, Liao HY (2014) Biodegradation of three tetracyclines in swine wastewater. J Environ Sci Health B 49(6): 449–455. https://doi.org/10.1080/03601234.2014.894784
Chen WR, Huang CH (2011) Transformation kinetics and pathways of tetracycline antibiotics with manganese oxide. Environ Pollut 159(5):1092–1100. https://doi.org/10.1016/j.envpol.2011.02.027
Cornejo J, Pokrant E, Araya D, Briceño C, Hidalgo H, Maddaleno A, Araya-Jordán C, San Martin B (2017) Residue depletion of oxytetracycline (OTC) and 4-epi-oxytetracycline (4-epi-OTC) in broiler chicken’s claws by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Food Add Contam A 34(4):494–500. https://doi.org/10.1080/19440049.2016.1263876
Cornejo J, Yevenes K, Avello C, Pokrant E, Maddaleno A, Martin BS, Lapierre L (2018) Determination of chlortetracycline residues, antimicrobial activity and presence of resistance genes in droppings of experimentally treated broiler chickens. Molecules (basel, Switzerland) 23(6):1264. https://doi.org/10.3390/molecules23061264
De Cazes M, Belleville MP, Petit E, Llorca M, Rodríguez-Mozaz S, De Gunzburg J, Barceló D, Sanchez-Marcano J (2014) Design and optimization of an enzymatic membrane reactor for tetracycline degradation. Catalysis Today 236(PART A): 146–152. https://doi.org/10.1016/j.cattod.2014.02.051
Ding H, Wu Y, Zou B, Lou Q, Zhang W, Zhong J, Lu L, Dai G (2016) Simultaneous removal and degradation characteristics of sulfonamide, tetracycline, and quinolone antibiotics by laccase-mediated oxidation coupled with soil adsorption. J Hazard Mater 307:350–358. https://doi.org/10.1016/j.jhazmat.2015.12.062
Eichhorn P, Aga DS (2004) Identification of a photooxygenation product of chlortetracycline in hog lagoons using LC/ESI-ion trap-MS and LC/ESI-time-of-flight-MS. Anal Chem 76(20):6002–6011. https://doi.org/10.1021/ac0494127
Fernández RA, Dassie SA (2005) Transfer of tetracyclines across the H2O|1,2-dichloroethane interface: analysis of degraded products in strong acid and alkaline solutions. J Electroanal Chem 585(2):240–249. https://doi.org/10.1016/j.jelechem.2005.08.015
Forsberg KJ, Patel S, Wencewicz TA, Dantas G (2015) The tetracycline destructases: a novel family of tetracycline-inactivating enzymes. Chem Biol 22(7):888–897. https://doi.org/10.1016/j.chembiol.2015.05.017
García-Delgado C, Eymar E, Camacho-Arévalo R, Petruccioli M, Crognale S, D’Annibale A (2018) Degradation of tetracyclines and sulfonamides by stevensite- and biochar-immobilized laccase systems and impact on residual antibiotic activity. J Chem Technol Biotechnol 93(12):3394–3409. https://doi.org/10.1002/jctb.5697
Gaugain M, Gautier S, Bourcier S, Jacques AM, Laurentie M, Abjean JP, Hurtaud-Pessel D, Verdon E (2015) 6-Iso-chlortetracycline or keto form of chlortetracycline? Need for clarification for relevant monitoring of chlortetracycline residues in food. Food Add Contam A 32(7):1105–1115. https://doi.org/10.1080/19440049.2015.1036381
Gianfreda L, Xu F, Bollag JM (1999) Laccases: a useful group of oxidoreductive enzymes. Bioremediat J 3(1):1–26. https://doi.org/10.1080/10889869991219163
Halling-Sørensen B, Sengeløv G, Tjørnelund J (2002) Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline-resistant bacteria. Arch Environ Contam Toxicol 42(3):263–271. https://doi.org/10.1007/s00244-001-0017-2
Hautphenne C, Penninckx M, Debaste F (2016) Product formation from phenolic compounds removal by laccases: a review. In: Environmental technology and innovation, vol 5, pp 250–266. Elsevier. https://doi.org/10.1016/j.eti.2016.04.001
Huang ST, Wu CY, Lee N, Cheng CW, Yang MJ, Hung YA, Wong TW, Liang JY (2018) Effects of 462 nm light-emitting diode on the inactivation of Escherichia coli and a multidrug-resistant by tetracycline photoreaction. J Clin Med 7(9):278. https://doi.org/10.3390/jcm7090278
Jeon J, Chang Y (2012) Laccase-mediated oxidation of small organics: bifunctional roles for versatile applications. Trends Biotechnol 31: 335–341. https://doi.org/10.1016/j.tibtech.2013.04.002
Jeong J, Song W, Cooper WJ, Jung J, Greaves J (2010) Degradation of tetracycline antibiotics: mechanisms and kinetic studies for advanced oxidation/reduction processes. Chemosphere 78(5):533–540. https://doi.org/10.1016/j.chemosphere.2009.11.024
Kang DH, Gupta S, Rosen C, Fritz V, Singh A, Chander Y, Murray H, Rohwer C (2013) Antibiotic uptake by vegetable crops from manure-applied soils. J Agric Food Chem 61(42):9992–10001. https://doi.org/10.1021/jf404045m
Keßler DN, Fokuhl VK, Petri MS, Spielmeyer A (2019) Abiotic transformation products of tetracycline and chlortetracycline in salt solutions and manure. Chemosphere 224:487–493. https://doi.org/10.1016/j.chemosphere.2019.02.169
Khan MH, Bae H, Jung JY (2010) Tetracycline degradation by ozonation in the aqueous phase: proposed degradation intermediates and pathway. J Hazard Mater 181(1–3):659–665. https://doi.org/10.1016/j.jhazmat.2010.05.063
Kittl R, Gonaus C, Pillei C, Haltrich D, Ludwig R (2012a) Constitutive expression of Botrytis aclada laccase in Pichia pastoris. Bioengineered 3(4):232–235. https://doi.org/10.4161/bioe.20037
Kittl R, Mueangtoom K, Gonaus C, Khazaneh ST, Sygmund C, Haltrich D, Ludwig R (2012b) A chloride tolerant laccase from the plant pathogen ascomycete Botrytis aclada expressed at high levels in Pichia pastoris. J Biotechnol 157(2):304–314. https://doi.org/10.1016/j.jbiotec.2011.11.021
Kudanga T, Le Roes-Hill M (2014) Laccase applications in biofuels production: current status and future prospects. Appl Microbiol Biotechnol 98(15):6525–6542. https://doi.org/10.1007/s00253-014-5810-8
Kudanga T, Nemadziva B, Le Roes-Hill M (2017) Laccase catalysis for the synthesis of bioactive compounds. Appl Microbiol Biotechnol 101(1):13–33. https://doi.org/10.1007/s00253-016-7987-5
Kwak J, Yoon S, Mahanty B, Kim CG (2017) Redox-mediator-free degradation of sulfathiazole and tetracycline using Phanerochaete chrysosporium. J Environ Sci Health A 52(13):1211–1217. https://doi.org/10.1080/10934529.2017.1356191
Li YX, Zhang XL, Li W, Lu XF, Liu B, Wang J (2013) The residues and environmental risks of multiple veterinary antibiotics in animal faeces. Environ Monit Assess 185(3):2211–2220. https://doi.org/10.1007/s10661-012-2702-1
Liu Y, He X, Fu Y, Dionysiou DD (2016) Degradation kinetics and mechanism of oxytetracycline by hydroxyl radical-based advanced oxidation processes. Chem Eng J 284:1317–1327. https://doi.org/10.1016/j.cej.2015.09.034
Llorca M, Rodríguez-Mozaz S, Couillerot O, Panigoni K, de Gunzburg J, Bayer S, Czaja R, Barceló D (2015) Identification of new transformation products during enzymatic treatment of tetracycline and erythromycin antibiotics at laboratory scale by an on-line turbulent flow liquid-chromatography coupled to a high resolution mass spectrometer LTQ-Orbitrap. Chemosphere 119:90–98. https://doi.org/10.1016/j.chemosphere.2014.05.072
Lueangjaroenkit P, Teerapatsakul C, Sakka K, Sakka M, Kimura T, Kunitake E, Chitradon L (2019) Two manganese peroxidases and a laccase of Trametes polyzona KU-RNW027 with novel properties for dye and pharmaceutical product degradation in redox mediator-free system. Mycobiology 47(2):217–229. https://doi.org/10.1080/12298093.2019.1589900
MacLean BX, Pratt BS, Egertson JD, MacCoss MJ, Smith RD, Baker ES (2018) Using Skyline to analyze data-containing liquid chromatography, ion mobility spectrometry, and mass spectrometry dimensions. J Am Soc Mass Spectrom 29(11):2182–2188. https://doi.org/10.1007/s13361-018-2028-5
Marco-Urrea E, Pérez-Trujillo M, Vicent T, Caminal G (2009) Ability of white-rot fungi to remove selected pharmaceuticals and identification of degradation products of ibuprofen by Trametes versicolor. Chemosphere 74(6):765–772. https://doi.org/10.1016/j.chemosphere.2008.10.040
Marco-Urrea E, Pérez-Trujillo M, Cruz-Morató C, Caminal G, Vicent T (2010) Degradation of the drug sodium diclofenac by Trametes versicolor pellets and identification of some intermediates by NMR. J Hazard Mater 176(1–3):836–842. https://doi.org/10.1016/j.jhazmat.2009.11.112
Markley JL, Wencewicz TA (2018) Tetracycline-inactivating enzymes. Front Microbiol 9(MAY):1–22. https://doi.org/10.3389/fmicb.2018.01058
Maroga Mboula V, Héquet V, Gru Y, Colin R, Andrès Y (2012) Assessment of the efficiency of photocatalysis on tetracycline biodegradation. J Hazard Mater 209–210:355–364. https://doi.org/10.1016/j.jhazmat.2012.01.032
McCormick JRD, Fox SM, Smith LL, Bitler BA, Reichenthal J, Origoni VE, Muller WH, Winterbottom R, Doerschuk AP (1957) Studies of the reversible epimerization occurring in the tetracycline family. The preparation, properties and proof of structure of some 4-epi-tetracyclines. J Am Chem Soc 79(11): 2849–2858. https://doi.org/10.1021/ja01568a050
McIlvaine TC (1921) A buffer solution for colorimetric comparison. J Biol Chem 49(1):183–186. https://doi.org/10.1016/S0021-9258(18)86000-8
Mikolasch A, Schauer F (2009) Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Appl Microbiol Biotechnol 82(4):605–624. https://doi.org/10.1007/s00253-009-1869-z
Morozova OV, Shumakovich GP, Gorbacheva MA, Shleev SV, Yaropolov AI (2007) “Blue” laccases. Biochem Biokhimiia 72(10):1136–1150. https://doi.org/10.1134/s0006297907100112
Niu J, Ding S, Zhang L, Zhao J, Feng C (2013) Visible-light-mediated Sr-Bi2O3 photocatalysis of tetracycline: kinetics, mechanisms and toxicity assessment. Chemosphere 93(1):1–8. https://doi.org/10.1016/j.chemosphere.2013.04.043
Oberoi AS, Jia Y, Zhang H, Khanal SK, Lu H (2019) Insights into the fate and removal of antibiotics in engineered biological treatment systems: a critical review. Environ Sci Technol 53(13):7234–7264. https://doi.org/10.1021/acs.est.9b01131
Osipov E, Polyakov K, Kittl R, Shleev S, Dorovatovsky P, Tikhonova T, Hann S, Ludwig R, Popov V (2014) Effect of the L499M mutation of the ascomycetous Botrytis aclada laccase on redox potential and catalytic properties. Acta Crystallogr D Biol Crystallogr 70(11):1–11. https://doi.org/10.1107/S1399004714020380
Osipov EM, Polyakov KM, Tikhonova TV, Kittl R, Dorovatovskii PV, Shleev SV, Popov VO, Ludwig R (2015) Incorporation of copper ions into crystals of T2 copper-depleted laccase from Botrytis aclada. Acta Crystallogr Sect F Struct Biol Commun 71:1465–1469. https://doi.org/10.1107/S2053230X1502052X
Park J, Gasparrini AJ, Reck MR, Symister CT, Elliott JL, Vogel JP, Wencewicz TA, Dantas G, Tolia NH (2017) Plasticity, dynamics, and inhibition of emerging tetracycline resistance enzymes. Nat Chem Biol 13(7):730–736. https://doi.org/10.1038/nchembio.2376
Pokrant E, Trincado L, Yévenes K, Terraza G, Maddaleno A, Martín BS, Zavala S, Hidalgo H, Lapierre L, Cornejo J (2021) Determination of five antimicrobial families in droppings of therapeutically treated broiler chicken by high-performance liquid chromatography-tandem mass spectrometry. Poultry Sci 100(9): 101313. https://doi.org/10.1016/j.psj.2021.101313
Prabakaran R, Valavan SE (2021) Wealth from poultry waste: an overview. World’s Poult Sci J 77(2):389–401. https://doi.org/10.1080/00439339.2021.1901557
Pulicharla R, Das RK, Kaur Brar S, Drogui P, Surampalli RY (2018) Degradation kinetics of chlortetracycline in wastewater using ultrasonication assisted laccase. Chem Eng J 347(March):828–835. https://doi.org/10.1016/j.cej.2018.04.162
Qi W, Long J, Feng C, Feng Y, Cheng D, Liu Y, Xue J, Li Z (2019) Fe3+ enhanced degradation of oxytetracycline in water by pseudomonas. Water Res 160:361–370. https://doi.org/10.1016/j.watres.2019.05.058
Qian S, Liu S, Jiang Z, Deng D, Tang B, Zhang J (2019) Electrochemical degradation of tetracycline antibiotics using a Ti/SnO2-Sb2O3 /PbO2 anode: kinetics, pathways, and biotoxicity change. J Electrochem Soc 166(6):E192–E199. https://doi.org/10.1149/2.1411906jes
Qiu W, Chen B, Tang L, Zheng C, Xu B, Liu Z, Magnuson JT, Zhang S, Schlenk D, Xu EG, Xing B (2022) Antibiotic chlortetracycline causes transgenerational immunosuppression via NF-κB. Environ Sci Technol 56(7):4251–4261. https://doi.org/10.1021/acs.est.1c07343
Riva S (2006) Laccases: blue enzymes for green chemistry. Trends Biotechnol 24(5):219–226. https://doi.org/10.1016/j.tibtech.2006.03.006
SAG. SERVICIO AGRÍCOLA Y GANADERO (2015) Sistema en línea de búsqueda de medicamentos veterinarios autorizados por el SAG. http://medicamentos.sag.gob.cl/ConsultaUsrPublico/BusquedaMedicamentos_1.asp. Accessed 26 July 2022
Salazar-Rábago JJ, Sánchez-Polo M, Rivera-Utrilla J, Leyva-Ramos R, Ocampo-Pérez R (2016) Role of 1[O2]* in chlortetracycline degradation by solar radiation assisted by ruthenium metal complexes. Chem Eng J 284:896–904. https://doi.org/10.1016/j.cej.2015.09.010
Shang Z, Salim AA, Khalil Z, Bernhardt PV, Capon RJ (2016) Fungal biotransformation of tetracycline antibiotics. J Org Chem 81(15):6186–6194. https://doi.org/10.1021/acs.joc.6b01272
Søeborg T, Ingerslev F, Halling-Sørensen B (2004) Chemical stability of chlortetracycline and chlortetracycline degradation products and epimers in soil interstitial water. Chemosphere 57(10):1515–1524. https://doi.org/10.1016/j.chemosphere.2004.09.020
Solliec M, Roy-Lachapelle A, Gasser MO, Coté C, Généreux M, Sauvé S (2016) Fractionation and analysis of veterinary antibiotics and their related degradation products in agricultural soils and drainage waters following swine manure amendment. Sci Total Environ 543(Pt A):524–535. https://doi.org/10.1016/j.scitotenv.2015.11.061
Solomon EI, Sundaram UM, Machonkin TE (1996) Multicopper oxidases and oxygenases. Chem Rev 96(7):2563–2605. https://doi.org/10.1021/cr950046o
Spielmeyer A, Petri MS, Höper H, Hamscher G (2020) Long-term monitoring of sulfonamides and tetracyclines in manure amended soils and leachate samples—a follow-up study. Heliyon 6(8): e04656. https://doi.org/10.1016/j.heliyon.2020.e04656
Suda T, Hata T, Kawai S, Okamura H, Nishida T (2012) Treatment of tetracycline antibiotics by laccase in the presence of 1-hydroxybenzotriazole. Biores Technol 103(1):498–501. https://doi.org/10.1016/j.biortech.2011.10.041
Sun K, Huang Q, Li S (2017) Transformation and toxicity evaluation of tetracycline in humic acid solution by laccase coupled with 1-hydroxybenzotriazole. J Hazard Mater 331:182–188. https://doi.org/10.1016/j.jhazmat.2017.02.058
Sversut RA, Vieira JC, Kassab NM, Silva DB, Salgado HRN (2019) Forced degradation behavior of two-drug combinations: isolation and characterization of major degradation products by LC–MS. Microchem J 150: 104074. https://doi.org/10.1016/j.microc.2019.104074
Taheran M, Naghdi M, Brar SK, Knystautas EJ, Verma M, Surampalli RY (2017) Covalent immobilization of laccase onto nanofibrous membrane for degradation of pharmaceutical residues in water. ACS Sustain Chem Eng 5(11):10430–10438. https://doi.org/10.1021/acssuschemeng.7b02465
Taheran M, Naghdi M, Brar SK, Knystautas EJ, Verma M, Tyagi RD, Surampalli RY (2018) Biodegradation of chlortetracycline by Trametes versicolor—produced laccase: by-product identification. J Environ Eng (United States) 144(4). https://doi.org/10.1061/(ASCE)EE.1943-7870.0001362
Teixidó M, Granados M, Prat MD, Beltrán JL (2012) Sorption of tetracyclines onto natural soils: data analysis and prediction. Environ Sci Pollut Res Int 19(8):3087–3095. https://doi.org/10.1007/s11356-012-0954-5
Tian Q, Dou X, Huang L, Wang L, Meng D, Zhai L, Shen Y, You C, Guan Z, Liao X (2020) Characterization of a robust cold-adapted and thermostable laccase from Pycnoporus sp. SYBC-L10 with a strong ability for the degradation of tetracycline and oxytetracycline by laccase-mediated oxidation. J Hazard Mat 382: 121084. https://doi.org/10.1016/j.jhazmat.2019.121084
Varga B, Somogyi V, Meiczinger M, Kováts N, Domokos E (2019) Enzymatic treatment and subsequent toxicity of organic micropollutants using oxidoreductases—a review. J Clean Prod 221:306–322. https://doi.org/10.1016/j.jclepro.2019.02.135
Wang W, Han Q, Zhu Z, Zhang L, Zhong S, Liu B (2019) Enhanced photocatalytic degradation performance of organic contaminants by heterojunction photocatalyst BiVO4/TiO2/RGO and its compatibility on four different tetracycline antibiotics. Adv Powder Technol 30(9):1882–1896. https://doi.org/10.1016/j.apt.2019.06.006
Wang L, Chai B (2022) Fate of antibiotic resistance genes and changes in bacterial community with increasing breeding scale of layer manure. Front Microbiol 13: 857046. https://doi.org/10.3389/fmicb.2022.857046
Xu L, Zhang H, Xiong P, Zhu Q, Liao C, Jiang G (2021) Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: a review. Sci Total Environ 753: 141975. https://doi.org/10.1016/j.scitotenv.2020.141975
Yang W, Moore IF, Koteva KP, Bareich DC, Hughes DW, Wright GD (2004) TetX is a flavin-dependent monooxygenase conferring resistance to tetracycline antibiotics. J Biol Chem 279(50):52346–52352. https://doi.org/10.1074/jbc.M409573200
Yang J, Lin Y, Yang X, Ng TB, Ye X, Lin J (2017) Degradation of tetracycline by immobilized laccase and the proposed transformation pathway. J Hazard Mater 322:525–531. https://doi.org/10.1016/j.jhazmat.2016.10.019
Yang Y, Zeng Z, Zhang C, Huang D, Zeng G, Xiao R, Lai C, Zhou C, Guo H, Xue W, Cheng M, Wang W, Wang J (2018) Construction of iodine vacancy-rich BiOI/Ag@AgI Z-scheme heterojunction photocatalysts for visible-light-driven tetracycline degradation: transformation pathways and mechanism insight. Chem Eng J 349:808–821. https://doi.org/10.1016/j.cej.2018.05.093
Yang S, Liu Y, Shen C, Li F, Yang B, Huang M, Yang M, Wang Z, Sand W (2020) Rapid decontamination of tetracycline hydrolysis product using electrochemical CNT filter: mechanism, impacting factors and pathways. Chemosphere 244: 125525. https://doi.org/10.1016/j.chemosphere.2019.125525
Yaropolov AI, Skorobogat’ko OV, Vartanov SS, Varfolomeyev SD (1994) Laccase. Appl Biochem Biotechnol 49: 257–280. https://doi.org/10.1007/BF02783061
Yévenes K, Pokrant E, Pérez F, Riquelme R, Avello C, Maddaleno A, San Martín B, Cornejo J (2018) Assessment of three antimicrobial residue concentrations in broiler chicken droppings as a potential risk factor for public health and environment. Int J Environ Res Public Health 16(1):24. https://doi.org/10.3390/ijerph16010024
Yuan F, Hu C, Hu X, Wei D, Chen Y, Qu J (2011) Photodegradation and toxicity changes of antibiotics in UV and UV/H(2)O(2) process. J Hazard Mater 185(2–3):1256–1263. https://doi.org/10.1016/j.jhazmat.2010.10.040
Zdarta J, Machałowski T, Degórska O, Bachosz K, Fursov A, Ehrlich H, Ivanenko VN, Jesionowski T (2020) 3D Chitin scaffolds from the marine demosponge Aplysina archeri as a support for laccase immobilization and its use in the removal of pharmaceuticals. Biomolecules 10(4). https://doi.org/10.3390/biom10040646
Zhao Y, Geng J, Wang X, Gu X, Gao S (2011) Tetracycline adsorption on kaolinite: pH, metal cations and humic acid effects. Ecotoxicology (london, England) 20(5):1141–1147. https://doi.org/10.1007/s10646-011-0665-6
Zhao F, Zhang D, Xu C, Liu J, Shen C (2020) The enhanced degradation and detoxification of chlortetracycline by Chlamydomonas reinhardtii. Ecotoxicol Environ Safety 196(April): 110552. https://doi.org/10.1016/j.ecoenv.2020.110552
Zhong SF, Yang B, Lei HJ, Xiong Q, Zhang QQ, Liu F, Ying GG (2022) Transformation products of tetracyclines in three typical municipal wastewater treatment plants. Sci Total Environ 830: 154647. https://doi.org/10.1016/j.scitotenv.2022.154647
Acknowledgements
This research was supported by the National Agency for Research and Development (ANID)/Scholarship Program/DOCTORADO BECAS CHILE/2016—21160533 for the Ph.D. financial support (to NGF), and Valorización de la Investigación Universitaria FONDEF-VIU16P0084 (to JCC and RC).
Author information
Authors and Affiliations
Contributions
Conceptualization: NGF, AB, RC; methodology: NGF, JCC; formal analysis: NGF, AB; investigation: NGF, JCC; writing—original draft and visualization: NGF, AB; writing—review and editing: AB, EP, RC; resources: AB, EP, RC; supervision: RC.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Fátima, N.G., Barriga, A., Cáceres, J.C. et al. Oxidation of chlortetracycline and its isomers by Botrytis aclada laccase in the absence of mediators: pH dependence and identification of transformation products by LC–MS. Biodegradation 35, 155–171 (2024). https://doi.org/10.1007/s10532-023-10046-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-023-10046-1