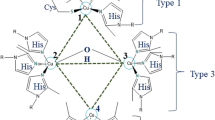
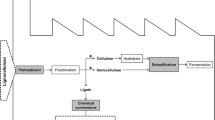
Abstract
The desire to reduce dependence on the ever diminishing fossil fuel reserves coupled with the impetus towards green energy has seen increased research in biofuels as alternative sources of energy. Lignocellulose materials are one of the most promising feedstocks for advanced biofuels production. However, their utilisation is dependent on the efficient hydrolysis of polysaccharides, which in part is dependent on cost-effective and benign pretreatment of biomass to remove or modify lignin and release or expose sugars to hydrolytic enzymes. Laccase is one of the enzymes that are being investigated not only for potential use as pretreatment agents in biofuel production, mainly as a delignifying enzyme, but also as a biotechnological tool for removal of inhibitors (mainly phenolic) of subsequent enzymatic processes. The current review discusses the major advances in the application of laccase as a potential pretreatment strategy, the underlying principles as well as directions for future research in the search for better enzyme-based technologies for biofuel production. Future perspectives could include synergy between enzymes that may be required for optimal results and the adoption of the biorefinery concept in line with the move towards the global implementation of the bioeconomy strategy.




Similar content being viewed by others
References
Adelakun OE, Kudanga T, Parker A, Green IR, Le Roes-Hill M, Burton SG (2012) Laccase-catalyzed dimerization of ferulic acid amplifies antioxidant activity. J Mol Catal B: Enzym 74:29–35
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861
Amirta R, Tanabe T, Watanabe T, Honda Y, Kuwahara M, Watanabe T (2006) Methane fermentation of Japanese cedar wood pretreated with a white rot fungus, Ceriporiopsis subvermispora. J Biotechnol 123:71–77
Andreu G, Vidal T (2011) Effects of laccase-natural mediator systems on kenaf pulp. Bioresour Technol 102:5932–5937
Aracri E, Colom JF, Vidal T (2009) Application of laccase-natural mediator systems to sisal pulp: an effective approach to biobleaching or functionalizing pulp fibres? Bioresour Technol 100:5911–5916
Arora DS, Sharma RK (2010) Ligninolytic fungal laccases and their biotechnological applications. Appl Biochem Biotechnol 160:1760–1788
Asgher M, Shahid M, Kamal S, Iqbal HMN (2014) Recent trends and valorization of immobilization strategies and ligninolytic enzymes by industrial biotechnology. J Mol Catal B: Enzym 101:56–66
Balakshin M, Capanema E, Chang H, Jameel H (2011) Quantification of lignin–carbohydrate linkages with high-resolution NMR spectroscopy. Planta 233:1097–1110
Baldrian P (2006) Fungal laccases—occurrence and properties. FEMS Microbiol Rev 30:215–242
Bensah EC, Mensah M (2013) Chemical pretreatment methods for the production of cellulosic ethanol: technologies and innovations. Int J Chem Eng 2013:1–21
Bourbonnais R, Rochefort D, Paice MG, Leech D (2001) Development of stable redox complexes to mediate delignification of Kraft pulp by laccase. In: Argyropoulos DS (ed) Oxidative delignification chemistry. ACS Symposium Series 785. American Chemical Society, Washington, DC, pp 391–399
Breen A, Singleton FL (1999) Fungi in lignocellulose breakdown and biopulping. Curr Opin Biotechnol 10:252–258
Brijwani K, Rigdon A, Vadlani PV (2010) Fungal laccases: production, function, and applications in food processing. Enzym Res 2010:1–10
Bruni E (2010) Improved anaerobic digestion of energy crops and agricultural residues. PhD thesis, Technical University of Denmark
Bruni E, Jensen AP, Angelidaki I (2010) Comparative study of mechanical, hydrothermal, chemical and enzymatic treatments of digested biofibers to improve biogas production. Bioresour Technol 101:8713–8717
Burton SG, Le Roes-Hill M (2008) Oxidizing enzymes in multi-step biotransformation processes. In: Garcia-Junceda E (ed) Multi-step enzyme catalysis: Biotransformations and chemoenzymatic synthesis. Wiley-VCH, Weinheim, pp 41–60
Burton SG, Cowan DA, Woodley JM (2002) The search for the ideal biocatalyst. Nat Biotechnol 20:37–45
Camarero S, Galletti GC, Martínez AT (1997) Demonstration of in situ oxidative degradation of lignin side-chains by two white-rot fungi using analytical pyrolysis of methylated wheat straw. Rapid Commun Mass Spectrom 11:331–334
Cañas AI, Camarero S (2010) Laccases and their natural mediators: biotechnological tools for sustainable eco-friendly processes. Biotechnol Adv 28:694–705
Chandel AK, Kapoor RK, Singh A, Kuhad RC (2007) Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Bioresour Technol 98:1947–1950
Chang VS, Holtzapple MT (2000) Fundamental factors affecting enzymatic reactivity. Appl Biochem Biotechnol 84–86:5–37
Chang K-L, Thitikorn-amorn J, Chen S-H, Hsieh J-F, Ratanakhanokchai K, Huang P-J, Lin T-C, Chen S-T (2011) Improving the remaining activity of lignocellulolytic enzymes by membrane entrapment. Bioresour Technol 102:519–523
Chen Y, Sarkanen S (2003) Macromolecular lignin replication—a mechanistic working hypothesis. Phytochem Rev 2:235–255
Chen Y, Sarkanen S (2010) Macromolecular replication during lignin biosynthesis. Phytochemistry 71:453–462
Chen Q, Marshall MN, Geib SM, Tien M, Richard TL (2012) Effects of laccase on lignin depolymerization and enzymatic hydrolysis of ensiled corn stover. Bioresour Technol 117:186–192
Claus H (2003) Laccases and their occurrence in prokaryotes. Arch Microbiol 179:145–150
Claus H, Faber G, Konig H (2002) Redox-mediated decolorization of synthetic dyes by fungal laccases. Appl Microbiol Biotechnol 59:672–678
Crestini C, Melone F, Saladino R (2011) Novel multienzyme oxidative biocatalyst for lignin bioprocessing. Bioorg Med Chem 19:5071–5078
DeMartini JD, Pattathil S, Avci U, Szekalski K, Mazumder K, Hahn MG, Wyman CE (2011) Application of monoclonal antibodies to investigate plant cell wall deconstruction for biofuels production. Energy Environ Sci 4:4332–4339
Desai SS, Nityanand C (2011) Microbial laccases and their applications: a review. Asian J Biotechnol 3:98–124
Dias AA, Freitas GS, Marques GSM, Sampaio A, Fraga IS, Rodrigues MAM, Evtuguin DV, Bezerra RMF (2010) Enzymatic saccharification of biologically pre-treated wheat straw with white-rot fungi. Bioresour Technol 101:6045–6050
Ding SY, Liu YS, Zeng Y, Himmel ME, Baker JO, Bayer EA (2012) How does plant cell wall nanoscale architecture correlate with enzymatic digestibility? Science 338:1055–1060
Dinis MJ, Bezerra RMF, Nunes F, Dias AA, Guedes CV, Ferreira LMM, Cone JW, Marques GSM, Barros ARN, Rodrigues MAM (2009) Modification of wheat straw lignin by solid state fermentation with white-rot fungi. Bioresour Technol 100:4829–4835
Du X (2013) Deepening the insights of lignin structure: Lignin–carbohydrate complex (LCC) fractionation and characterization and Kraft lignin amination. Dissertation, KTH Royal Institute of Technology
Du X, Maria E, Martín E, Li J (2013a) Improvement of kraft pulp bleaching by treatments with laccase, urea, and refining. Holzforschung 67:651–658
Du X, Li J, Gellerstedt G, Rencoret J, Del Río JC, Martínez AT, Gutiérrez A (2013b) Understanding pulp delignification by laccase-mediator systems through isolation and characterization of lignin–carbohydrate complexes. Biomacromolecules 14:3073–3080
Du X, Pérez-Boada M, Fernández C, Rencoret J, del Río JC, Jiménez-Barbero J, Li J, Gutiérrez A, Martínez AT (2014) Analysis of lignin–carbohydrate and lignin–lignin linkages after hydrolase treatment of xylan–lignin, glucomannan–lignin and glucan–lignin complexes from spruce wood. Planta 239:1079–1090
Durán N, Esposito E (2000) Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: a review. Appl Catal B 28:83–99
Durán N, Rosa MA, D’Annibale A, Gianfreda L (2002) Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: a review. Enzyme Microb Technol 31:907–931
Dwivedi UN, Singh P, Pandey VP, Kumar A (2011) Structure–function relationship among bacterial, fungal and plant laccases. J Mol Catal B: Enzym 68:117–128
Ede RM, Kilpeläinen I (1995) Homo- and hetero-nuclear 2D NMR techniques—unambiguous structural probes for non-cyclic benzyl aryl ethers in soluble lignin samples. Res Chem Intermed 21:313–328
Esteghlalian AR, Svivastava V, Gilkes N, Gregg DJ, Saddler JN (2001) An overview of factors influencing the enzymatic hydrolysis of lignocellulosic feedstocks. In: Himmel ME, Baker W, Saddler JN (eds) Glycosyl hydrolases for biomass conversion. ACS, Washington, pp 100–111
Eugenio ME, Miranda J, Martin-Sampedro R, Villar JC (2010b) Influence of laccase bleaching process variables on bleached pulps properties. In: Proceeding of VI Iberoamerican Congress on Pulp and Paper Research (CIADICYP), Lisboa, Portugal, pp 160–161
Eugenio ME, Santos SM, Carbajo JM, Martín JA, Martín-Sampedro R, González AE, Villar JC (2010b) Kraft pulp biobleaching using an extracellular enzymatic fluid produced by Pycnoporus sanguineus. Bioresour Technol 101:1866–1870
Fabbrini M, Galli C, Gentili P (2002) Comparing the catalytic efficiency of some mediators of laccase. J Mol Catal B: Enzym 16:231–240
Fillat A, Colom JF, Vidal T (2010) A new approach to the biobleaching of flax pulp with laccase using natural mediators. Bioresour Technol 101:4104–4110
Gamble GR, Snook ME, Henrikson G, Akin DE (2000) Phenolic constituents in flax bast tissue and inhibition of cellulase and pectinase. Biotechnol Lett 22:741–746
Ge H, Gao Y, Hong Y, Zhang M, Xiao Y, Teng M, Niu L (2010) Structure of native laccase B from Trametes sp. AH28–2. Acta Crystallogr F66:254–258
Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G (2010) Laccases: a never-ending story. Cell Mol Life Sci 67:369–385
Gregg D, Saddler JN (1996) A techno-economic assessment of the pretreatment and fractionation steps of a biomass-to-ethanol process. Appl Biochem Biotechnol 57–58:711–727
Guillén F, Muñoz C, Gomez-Toribio V, Martínez AT, Martínez MJ (2000) Oxygen activation during oxidation of methoxyhydroquinones by laccase from Pleurotus eryngii. Appl Environ Microbiol 66:170–175
Gutiérrez A, Rencoret J, Cadena EM, Rico A, Barth D, del Río JC, Martínez ÁT (2012) Demonstration of laccase-based removal of lignin from wood and non-wood plant feedstocks. Bioresour Technol 119:114–122
Hammel KE, Kapich AN, Jensen KA Jr, Ryan ZC (2002) Reactive oxygen species as agents of wood decay by fungi. Enzyme Microb Technol 30:445–453
Haykir I (2009) A comparative study on lignocellulose pretreatments for bioethanol production from cotton stalk. New Biotechnol 25(Suppl 1):S253–S254
Hendriks ATWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100:10–18
Johannes C, Majcherczyk A (2000) Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl Environ Microbiol 66:2524–2528
Jönsson LJ, Palmqvist E, Nilvebrant NO, Hahn-Hägerdal B (1998) Detoxification of wood hydrolysates with laccase and peroxidase from the white-rot fungus Trametes versicolor. Appl Microbiol Biotechnol 49:691–697
Jung HJG, Jorgensen MA, Linn JG, Engels FM (2000) Impact of accessibility and chemical composition on cell wall polysaccharide degradability of maize and lucerne stems. J Sci Food Agric 80:419–427
Jurado M, Prieto A, Martínez-Alcalá A, Martínez ÁT, Martinez MJ (2009) Laccase detoxification of steam-exploded wheat straw for second generation bioethanol. Bioresour Technol 100:6378–6384
Kalyani D, Dhiman SS, Kim H, Jeya M, Kim I-W, Lee J-K (2012) Characterization of a novel laccase from the isolated Coltricia perennis and its application to detoxification of biomass. Process Biochem 47:671–678
Kaparaju P, Felby C (2010) Characterization of lignin during oxidative and hydrothermal pre-treatment processes of wheat straw and corn stover. Bioresour Technol 101:3175–3181
Karhunen P, Rummakko P, Sipilä J, Brunow G, Kilpeläinen I (1995a) Dibenzodioxocins; a novel type of linkage in softwood lignins. Tetrahedron Lett 36:169–170
Karhunen P, Rummakko P, Sipilä J, Brunow G, Kilpeläinen I (1995b) The formation of dibenzodioxocin structures by oxidative coupling. A model reaction for lignin biosynthesis. Tetrahedron Lett 36:4501–4504
Kawai S, Umezawa T, Higuchi T (1988a) Degradation mechanisms of phenolic beta-1 lignin substructure model compounds by laccase of Coriolus versicolor. Arch Biochem Biophys 262:99–110
Kawai S, Umezawa T, Shimada M, Higuchi T (1988b) Aromatic ring cleavage of 4,6-di(tert-butyl)guaiacol, a phenolic lignin model compound, by laccase of Coriolus versicolor. FEBS Lett 236:309–311
Kawai S, Nakagawa M, Ohashi H (2002) Degradation mechanism of a nonphenolic b-O-4 lignin model dimer by Trametes versicolor laccase in the presence of 1-hydroxybenzotriozole. Enzym Microb Technol 30:482–489
Kilpeläinen I, Sipilä J, Brunow G, Lundquist K, Ede RM (1994) Application of two-dimensional NMR spectroscopy to wood lignin structure determination and identification of some minor structural units of hard- and softwood lignins. J Agric Food Chem 42:2790–2794
Kolb M, Sieber V, Amann M, Faulstich M, Schieder D (2012) Removal of monomer delignification products by laccase from Trametes versicolor. Bioresour Technol 104:298–304
Kramer KJ, Kanost MR, Hopkins TL, Jiang H, Zhu YC, Xu R, Kerwin JL, Turecek F (2001) Oxidative conjugation of catechols with proteins in insect skeletal systems. Tetrahedron 57:385–392
Kudanga T, Nugroho Prasetyo E, Sipila J, Eberl A, Nyanhongo GS, Guebitz G (2009) Coupling of aromatic amines onto syringylglycerol-guaiacylether using Bacillus SF spore laccase: a model for functionalisation of lignin-based materials. J Mol Catal B: Enzym 61:143–149
Kudanga T, Nugroho Prasetyo E, Sipila J, Guebitz GM, Nyanhongo GS (2010a) Reactivity of long chain alkylamines to lignin moieties: implications on hydrophobicity of lignocellulose materials. J Biotechnol 149:81–87
Kudanga T, Nugroho Prasetyo E, Sipila J, Nyanhongo GS, Guebitz G (2010b) Enzymatic grafting of functional molecules to the lignin model dibenzodioxocin and lignocellulose material. Enzyme Microb Technol 46:272–280
Kudanga T, Nugroho Prasetyo E, Widsten P, Kandelbauer A, Jury S, Heathcote C, Sipilä J, Weber H, Nyanhongo GS, Guebitz GM (2010c) Laccase-catalyzed covalent coupling of fluorophenols increases lignocellulose surface hydrophobicity. Bioresour Technol 101:2793–2799
Kudanga T, Burton SG, Nyanhongo GS, Guebitz GM (2011a) Versatility of oxidoreductases in the remediation of environmental pollutants. Front Biosci (Elite Ed) 4:1127–1149
Kudanga T, Nyanhongo GS, Guebitz GM, Burton SG (2011b) Potential applications of laccase-mediated coupling and grafting reactions: a review. Enzyme Microb Technol 48:195–208
Kuhad RC, Mehta G, Gupta R, Sharma KK (2010) Fed batch enzymatic saccharification of newspaper cellulosics improves the sugar content in the hydrolysates and eventually the ethanol fermentation by Saccharomyces cerevisiae. Biomass Bioenergy 34:1189–1194
Kumar L, Arantes V, Chandra R, Saddler J (2012) The lignin present in steam pretreated softwood binds enzymes and limits cellulose accessibility. Bioresour Technol 103:201–208
Kunamneni A, Camarero S, Garcia-Burgos C, Plou FJ, Ballesteros A, Alcalde M (2008a) Engineering and applications of fungal laccases for organic synthesis. Microb Cell Fact 7:32–48
Kunamneni A, Plou FJ, Ballesteros A, Alcalde M (2008b) Laccases and their applications: a patent review. Recent Pat Biotechnol 2:10–24
Larsson S, Cassland P, Jönsson LJ (2001) Development of a Saccharomyces cerevisiae strain with enhanced resistance to phenolic fermentation inhibitors in lignocellulose hydrolysates by heterologous expression of laccase. Appl Environ Microbiol 67:1163–1170
Lee J-W, Koo B-W, Choi J-W, Choi D-H, Choi I-G (2008) Evaluation of waste mushroom logs as a potential biomass resource for the production of bioethanol. Bioresour Technol 99:2736–2741
Lee K-M, Kalyani D, Tiwari MK, Kim T-S, Dhiman SS, Lee J-K, Kim I-W (2012) Enhanced enzymatic hydrolysis of rice straw by removal of phenolic compounds using a novel laccase from yeast Yarrowia lipolytica. Bioresour Technol 123:636–645
Leontievsky AA, Myasodeva NM, Pozdnyakova NN, Golovleva LA (1997a) Yellow laccase of Panus tigrinus oxidizes non-phenolic substrates without electron-transfer mediators. FEBS Lett 413:446–448
Leontievsky AA, Vares T, Lankinen P, Shergill JK, Pozdnyakova NN, Myasodeva NM, Kalkkinen N, Golovleva LA, Cammack R, Thurston CF, Hatakka A (1997b) Blue and yellow laccases of ligninolytic fungi. FEMS Microbiol Lett 156:9–14
Liu J, Wang ML, Tonnis B, Habteselassie M, Liao X, Huang Q (2013) Fungal pretreatment of switchgrass for improved saccharification and simultaneous enzyme production. Bioresour Technol 135:39–45
Lu C, Wang H, Luo Y, Guo L (2010) An efficient system for pre-delignification of gramineous biofuel feedstock in vitro: Application of a laccase from Pycnoporus sanguineus H275. Process Biochem 45:1141–1147
Ludwig D, Amann M, Hirth T, Rupp S, Zibek S (2013) Development and optimization of single and combined detoxification processes to improve the fermentability of lignocellulose hydrolysates. Bioresour Technol 133:455–461
Luna-Acosta A, Rosenfeld E, Amari M, Fruitier-Arnaudin I, Bustamante P, Thomas-Guyon H (2010) First evidence of laccase activity in the Pacific oyster Crassostrea gigas. Fish Shellfish Immunol 28:719–726
Machczynski MC, Vijgenboom E, Sanya B, Canters GW (2004) Characterization of SLAC: a small laccase from Streptomyces coelicolor with the unprecedented activity. Protein Sci 13:2388–2397
Madhavi V, Lele SS (2009) Laccase: properties and applications. BioResources 4:1694–1717
Majeau JA, Brar SK, Tyagi RD (2010) Laccases for removal of recalcitrant and emerging pollutants. Bioresour Technol 101:2331–2350
Martínez ÁT, Ruiz-Dueñas FJ, Martínez MJ, del Río JC, Gutiérrez A (2009) Enzymatic delignification of plant cell wall: from nature to mill. Curr Opin Biotechnol 20:348–357
Martín-Sampedro R, Eugenio ME, Villar JC (2011a) Biobleaching of Eucalyptus globulus kraft pulps: comparison between pulps obtained from exploded and non-exploded chips. Bioresour Technol 102:4530–4535
Martín-Sampedro R, Eugenio ME, Carbajo JM, Villar JC (2011b) Combination of steam explosion and laccase-mediator treatments prior to Eucalyptus globulus kraft pulping. Bioresour Technol 102:7183–7189
Mayer AM, Staples RC (2002) Laccase: new functions for an old enzyme. Phytochemistry 60:551–565
Mikolasch A, Schauer F (2009) Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Appl Microbiol Biotechnol 82:605–624
Minussi RC, Pastore GM, Durán N (2002) Potential applications of laccase in the food industry. Trends Food Sci Technol 13:205–216
Moilanen U, Kellock M, Galkin S, Viikari L (2011) The laccase-catalyzed modification of lignin for enzymatic hydrolysis. Enzyme Microb Technol 49:492–498
Moldes D, Díaz M, Tzanov T, Vidal T (2008) Comparative study of the efficiency of synthetic and natural mediators in laccase-assisted bleaching of eucalyptus kraft pulp. Bioresour Technol 99:7959–7965
Moldes D, Cadena EM, Vidal T (2010) Biobleaching of eucalypt kraft pulp with a two laccase-mediator stages sequence. Bioresour Technol 101:6924–6929
Mood SH, Golfeshan AH, Tabatabaei M, Jouzani GS, Najafi GH, Gholami M, Ardjman M (2013) Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew Sust Energ Rev 27:77–93
Moreno AD, Ibarra D, Fernández JL, Ballesteros M (2012) Different laccase detoxification strategies for ethanol production from lignocellulosic biomass by the thermotolerant yeast Kluyveromyces marxianus CECT 10875. Bioresour Technol 106:101–109
Moreno AD, Ibarra D, Ballesteros I, Fernández JL, Ballesteros M (2013a) Ethanol from laccase-detoxified lignocellulose by the thermotolerant yeast Kluyveromyces marxianus—effects of steam pretreatment conditions, process configurations and substrate loadings. Biochem Eng J 79:94–103
Moreno AD, Ibarra D, Ballesteros I, González A, Ballesteros M (2013b) Comparing cell viability and ethanol fermentation of the thermotolerant yeast Kluyveromyces marxianus and Saccharomyces cerevisiae on steam-exploded biomass treated with laccase. Bioresour Technol 135:239–245
Moreno AD, Tomás-Pejó E, Ibarrac D, Ballesteros M, Olsson L (2013c) In situ laccase treatment enhances the fermentability of steam-exploded wheat straw in SSCF processes at high dry matter consistencies. Bioresour Technol 143:337–343
Morozova OV, Shumakovich GP, Gorbacheva MA, Shleev SV, Yaropolov AI (2007a) “Blue” Laccases. Biochemistry (Mosc) 72:1136–1150
Morozova OV, Shumakovich GP, Shleev SV, Yaropolov YI (2007b) Laccase–mediator systems and their applications: a review. Appl Biochem Microbiol 43:523–535
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686
Mukhopadhyay M, Kuila A, Tuli DK, Banerjee R (2011) Enzymatic depolymerization of Ricinus communis, a potential lignocellulosic for improved saccharification. Biomass Bioenergy 35:3584–3591
Nakanishi A, Kuroda K, Ueda M (2012) Direct fermentation of newspaper after laccase-treatment using yeast codisplaying endoglucanase, cellobiohydrolase, and β-glucosidase. Renew Energy 44:199–205
Nyanhongo GS, Gübitz G, Sukyai P, Leitner C, Haltrich D, Ludwig R (2007) Oxidoreductases from Trametes spp. in Biotechnology: a wealth of catalytic activity. Food Technol Biotechnol 45:250–268
Okuda N, Soneura M, Ninomiya K, Katakura Y, Shioya S (2008) Biological detoxification of waste house wood hydrolysate using Ureibacillus thermosphaericus for bioethanol production. J Biosci Bioeng 106:128–133
Osma JF, Toca-Herrera JL, Rodríguez-Couto S (2010) Uses of laccases in the food industry. Enzym Res 2010:1–8
Palonen H, Viikari L (2004) Role of oxidative enzymatic treatments on enzymatic hydrolysis of softwood. Biotechnol Bioeng 86:550–557
Palonen H, Tjerneld F, Zacchi G, Tenkanen M (2004) Adsorption of Trichoderma reesei CBH I and EG II and their catalytic domains on steam pretreated softwood and isolated lignin. J Biotechnol 107:65–72
Parawira W, Tekere M (2011) Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: review. Crit Rev Biotechnol 31:20–31
Pérez J, Muñoz-Dorado J, de la Rubia T, Martínez J (2002) Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int Microbiol 5:53–63
Plácido J, Imam T, Capareda S (2013) Evaluation of ligninolytic enzymes, ultrasonication and liquid hot water as pretreatments for bioethanol production from cotton gin trash. Bioresour Technol 139:203–208
Pollard DJ, Woodley JM (2007) Biocatalysis for pharmaceutical intermediates: the future is now. Trends Biotechnol 25:66–73
Pu Y, Hu F, Huang F, Davison BH, Ragauskas AJ (2013) Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol Biofuels 6:15
Qiu W, Chen H (2012) Enhanced the enzymatic hydrolysis efficiency of wheat straw after combined steam explosion and laccase pretreatment. Bioresour Technol 118:8–12
Rana S, Tiwari R, Arora A, Singh S, Kaushik R, Saxena AK, Dutta SC, Nain L (2013) Prospecting Parthenium sp. pretreated with Trametes hirsuta, as a potential bioethanol feedstock. Biocatal Agric Biotechnol 2:152–158
Rico A, Rencoret J, del Río JC, Martínez AT, Gutiérrez A (2014) Pretreatment with laccase and a phenolic mediator degrades lignin and enhances saccharification of Eucalyptus feedstock. Biotechnol Biofuels 7:6
Riva S (2006) Laccases: blue enzymes for green chemistry. Trends Biotechnol 24:219–226
Rodríguez-Couto S, Toca-Herrera JL (2006) Industrial and biotechnological applications of laccases: a review. Biotechnol Adv 24:500–513
Rogalski J, Leonowicz A (2004) Microbial enzymes: Production and applications: Laccases. In: Pandey A (ed) Concise encyclopedia of bioresource technology. The Haworth press, Binghamton, pp 533–542
Sakakibara A (1980) A structural model of softwood lignin. Wood Sci Technol 14:89–100
Salvachúa D, Prieto A, López-Abelairas M, Lu-Chau T, Martínez ÁT, Martínez MJ (2011) Fungal pretreatment: an alternative in second-generation ethanol from wheat straw. Bioresour Technol 102:7500–7506
Saritha M, Arora A, Singh S, Nain L (2013) Streptomyces griseorubens mediated delignification of paddy straw for improved enzymatic saccharification yields. Bioresour Technol 135:12–17
Sirim D, Wagner F, Wang L, Schmid RD, Pleiss J (2011) The Laccase Engineering Database: a classification and analysis system for laccases and related multicopper oxidases. Database 2011:Article ID bar006
Sitarz AK, Mikkelsen JD, Højrup P, Meyer AS (2013) Identification of a laccase from Ganoderma lucidum CBS 229.93 having potential for enhancing cellulase catalyzed lignocellulose degradation. Enzyme Microb Technol 53:378–385
Skálová T, Dohnálek J, Østegaard LH, Østegaard PR, Kolenko P, Duškova J, Stepankova A, Hasek J (2009) The structure of the small laccase from Streptomyces coelicolor reveals a link between laccase and nitrate reductases. J Mol Biol 785:1165–1178
Strong PJ, Claus H (2011) Laccase: a review of its past and its future in bioremediation. Crit Rev Environ Sci Technol 41:373–434
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11
Tabka MG, Herpoël-Gimbert I, Monod F, Asther M, Sigoillot JC (2006) Enzymatic saccharification of wheat straw for bioethanol production by a combined cellulase xylanase and feruloyl esterase treatment. Enzyme Microb Technol 39:897–902
Talebnia F, Karakashev D, Angelidaki I (2010) Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Bioresour Technol 101:4744–4753
Thurston CF (1994) The structure and function of fungal laccases. Microbiology 140:19–26
Torres-Duarte C, Roman R, Tinoco R, Vazquez-Duhalt R (2009) Halogenated pesticide transformation by a laccase-mediator system. Chemosphere 77:687–692
Trovaslet-Leroy M, Jolivalt C, Froment M-T, Brasme B, Lefebvre B, Daveloose D, Nachon F, Masson P (2010) Application of laccase-mediator system (LMS) for the degradation of organophosphorous compounds. Chem Biol Interact 187:393–396
Uthandi S, Saad B, Humbard MA, Maupin–Furlow JA (2010) LccA, an archaeal laccase secreted as a highly stable glycoprotein into the extracellular medium by Haloferax volcanii. Appl Environ Microbiol 76:733–743
Vila C, Bameto AG, Fillat A, Vidal T, Ariza J (2011) Use of thermogravimetric analysis to monitor the effects of natural laccase mediators on flax pulp. Bioresour Technol 102:6554–6561
Westermark U, Eriksson KE (1974) Carbohydrate-dependent enzymatic quinone reduction during lignin degradation. Acta Chem Scand B 28:204–208
Widsten P, Kandelbauer A (2008) Laccase applications in the forest products industry: a review. Enzyme Microb Technol 42:293–307
Widsten P, Heathcote C, Kandelbauer A, Guebitz G, Nyanhongo GS, Nugroho Prasetyo E, Kudanga T (2010) Enzymatic surface functionalisation of lignocellulosic materials with tannins for enhancing antibacterial properties. Process Biochem 45:1072–1081
Witayakran S, Ragauskas AJ (2009) Synthetic applications of laccase in green chemistry. Adv Synth Catal 351:1187–1209
Woolridge EM (2014) Mixed enzyme systems for delignification of lignocellulosic biomass. Catalysts 4:1–35
Xing M-N, Zhang X-Z, Huang H (2012) Application of metagenomic techniques in mining enzymes from microbial communities for biofuel synthesis. Biotechnol Adv 30:920–929
Yamagishi K, Kimura T, Watanabe T (2011) Treatment of rice straw with selected Cyathus stercoreus strains to improve enzymatic saccharification. Bioresour Technol 102:6937–6943
Yoshida H (1883) Chemistry of lacquer (Urishi) part 1. J Chem Soc (Tokyo) 43:472–486
Yu J, Zhang J, He J, Liu Z, Yu Z (2009) Combinations of mild physical or chemical pretreatment with biological pretreatment for enzymatic hydrolysis of rice hull. Bioresour Technol 100:903–908
Zhang J, Qu Y, Xiao P, Wang X, Wang T, He F (2012) Improved biomass saccharification by Trichoderma reesei through heterologous expression of lacA gene from Trametes sp. AH28-2. J Biosci Bioeng 113:697–703
Acknowledgments
Financial support from the National Research Foundation (NRF)—South Africa and the Cape Peninsula University of Technology Research Funding (URF) is gratefully acknowledged. Any opinion, findings and conclusions or recommendations expressed in this material are those of the author(s) and therefore the NRF does not accept any liability in regard thereto.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kudanga, T., Le Roes-Hill, M. Laccase applications in biofuels production: current status and future prospects. Appl Microbiol Biotechnol 98, 6525–6542 (2014). https://doi.org/10.1007/s00253-014-5810-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5810-8