Abstract
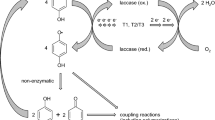
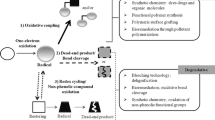
The demand for compounds of therapeutic value is increasing mainly because of new applications of bioactive compounds in medicine, pharmaceutical, agricultural, and food industries. This has necessitated the search for cost-effective methods for producing bioactive compounds and therefore the intensification of the search for enzymatic approaches in organic synthesis. Laccase is one of the enzymes that have shown encouraging potential as biocatalysts in the synthesis of bioactive compounds. Laccases are multicopper oxidases with a diverse range of catalytic activities revolving around synthesis and degradative reactions. They have attracted much attention as potential industrial catalysts in organic synthesis mainly because they are essentially green catalysts with a diverse substrate range. Their reaction only requires molecular oxygen and releases water as the only by-product. Laccase catalysis involves the abstraction of a single electron from their substrates to produce reactive radicals. The free radicals subsequently undergo homo- and hetero-coupling to form dimeric, oligomeric, polymeric, or cross-coupling products which have practical implications in organic synthesis. Consequently, there is a growing body of research focused on the synthetic applications of laccases such as organic synthesis, hair and textile dyeing, polymer synthesis, and grafting processes. This paper reviews the major advances in laccase-mediated synthesis of bioactive compounds, the mechanisms of enzymatic coupling, structure-activity relationships of synthesized compounds, and the challenges that might guide future research directions.






Similar content being viewed by others
References
Abdel-Mohsen HT, Conrad J, Beifuss U (2014) Laccase-catalyzed synthesis of catechol thioethers by reaction of catechols with thiols using air as an oxidant. Green Chem 16:90–95
Abraham I, Joshi R, Pardasani P, Pardasani RT (2011) Recent advances in 1,4-benzoquinone chemistry. J Braz Chem Soc 22:385–421
Adelakun OE, Kudanga T, Parker A, Green IR, le Roes-Hill M, Burton SG (2012a) Laccase-catalyzed dimerization of ferulic acid amplifies antioxidant activity. J Mol Catal B Enzym 74:29–35
Adelakun OE, Kudanga T, Green IR, le Roes-Hill M, Burton SG (2012b) Enzymatic modification of 2,6-dimethoxyphenol for the synthesis of dimers with high antioxidant capacity. Process Biochem 47:1926–1932
Adibi H, Rashidi A, Khodaei MM, Alizadeh A, Majnooni MB, Pakravan N, Abiri R, Nematollahi D (2011) Catechol thioether derivatives: preliminary study of in-vitro antimicrobial and antioxidant activities. Chem Pharm Bull 59:1149–1152
Agematu H, Tsuchida T, Kominato K, Shibamoto N, Yoshioka T, Nishida H, Okamoto R, Shin T, Murao S (1993) Enzymatic dimerization of penicillin X. J Antibiot (Tokyo) 46:141–148
Aljawish A, Chevalot I, Jasniewski J, Paris C, Scher J, Muniglia L (2014a) Laccase-catalysed oxidation of ferulic acid and ethyl ferulate in aqueous medium: a green procedure for the synthesis of new compounds. Food Chem 145:1046–1054
Aljawish A, Chevalot I, Jasniewski J, Revol-Junelles AM, Scher J, Muniglia L (2014b) Laccase-catalysed functionalisation of chitosan by ferulic acid and ethyl ferulate: evaluation of physicochemical and biofunctional properties. Food Chem 161:279–287
Alov P, Tsakovska I, Pajeva I (2015) Computational studies of free radical-scavenging properties of phenolic compounds. Curr Top Med Chem 15:85–104
Aminov RI (2010) A brief history of the antibiotic era: lessons learned and challenges for the future. Front Microbiol 1:134
Anthoni J, Humeau C, Maia E, Chebil L, Engasser JM, Ghoul M (2010) Enzymatic synthesis of oligoesculin: structure and biological activities characterizations. Eur Food Res Technol 231:571–579
Anyanwutaku IO, Petroski RJ, Rosazza JPN (1994) Oxidative coupling of mithramycin and hydroquinone catalyzed by copper oxidases and benzoquinone. Implications for the mechanism of action of aureolic acid antibiotics. Bioorg Med Chem 2:543–551
Balasundram N, Sundram K, Samman S (2006) Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem 99:191–203
Baldelli E, Danieli B, Fontana G, Riva S (2009) Process for the preparation of bisindole alkaloid derivatives. Google Patents
Barclay LRC, Xi F, Norris JQ (1997) Antioxidant properties of phenolic lignin model compounds. J Wood Chem Technol 17:73–90
Bendary E, Francis RR, Ali HMG, Sarwat MI, El Hady S (2013) Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann Agric Sci 58:173–181
Bhattacharya A, Sood P, Citovsky V (2010) The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol Plant Pathol 11:705–719
Boeriu CG, Bravo D, Gosselink RJA, van Dam JEG (2004) Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind Crop Prod 20:205–218
Božič M, Gorgieva S, Kokol V (2012a) Homogeneous and heterogeneous methods for laccase-mediated functionalization of chitosan by tannic acid and quercetin. Carbohydr Polym 89:854–864
Božič M, Gorgieva S, Kokol V (2012b) Laccase-mediated functionalization of chitosan by caffeic and gallic acids for modulating antioxidant and antimicrobial properties. Carbohydr Polym 87:2388–2398
Božič M, Štrancar J, Kokol V (2013) Laccase-initiated reaction between phenolic acids and chitosan. React Funct Polym 73:1377–1383
Bozic T, Novakovic I, Gasic MJ, Juranic Z, Stanojkovic T, Tufegdzic S, Kljajić Z, Sladić D (2010) Synthesis and biological activity of derivatives of the marine quinone avarone. Eur J Med Chem 45:923–929
Burton SG, Davids LM (2012) Hydroxytyrosol compounds. Google Patents
Cañas AI, Camarero S (2010) Laccases and their natural mediators: biotechnological tools for sustainable eco-friendly processes. Biotechnol Adv 28:694–705
Cannatelli MD, Ragauskas AJ (2015a) Laccase-catalyzed synthesis of 2,3-ethylenedithio-1,4-quinones. J Mol Catal B Enzym 119:85–89
Cannatelli MD, Ragauskas AJ (2015b) Laccase-catalyzed α-arylation of benzoylacetonitrile with substituted hydroquinones. Chem Eng Res Des 97:128–134
Chakroun H, Bouaziz M, Yangui T, Blibech I, Dhouib A, Sayadi S (2013) Enzymatic transformation of tyrosol by Trametes trogii laccases: identification of the product and study of its biological activities. J Mol Catal B Enzym 87:11–17
Chandra AK, Uchimaru T (2002) The O-H bond dissociation energies of substituted phenols and proton affinities of substituted phenoxide ions: a DFT study. Int J Mol Sci 3:407–422
Chioccara F, Poli S, Rindone B, Pilati T, Brunow G, Pietikäinen P, Setala H (1993) Regio- and diastereo-selective synthesis of dimeric lignans using oxidative coupling. Acta Chem Scand 47:610–616
Chirivì C, Fontana G, Monti D, Ottolina G, Riva S, Danieli B (2012) The quest for new mild and selective modifications of natural structures: laccase-catalysed oxidation of ergot alkaloids leads to unexpected stereoselective C-4 hydroxylation. Chem Eur J 18:10355–10361
Christopher LP, Yao B, Ji Y (2014) Lignin biodegradation with laccase-mediator systems. Front Energy Res 2:12
Chung J, Kurisawa M, Uyama H, Kobayashi S (2003) Enzymatic synthesis and antioxidant property of gelatin-catechin conjugates. Biotechnol Lett 25:1993–1997
Claus H (2004) Laccases: structure, reactions, distribution. Micron 35:93–96
Constable DJC, Dunn PJ, Hayler JD, Humphrey GR, Leazer JL Jr, Linderman RJ, Lorenz K, Manley J, Pearlman BA, Wells A, Zaks A, Zhang TY (2007) Key green chemistry research areas—a perspective from pharmaceutical manufacturers. Green Chem 9:411–420
Constantin MA, Conrad J, Beifuss U (2012a) Laccase-catalyzed oxidative phenolic coupling of vanillidene derivatives. Green Chem 14:2375–2379
Constantin MA, Conrad J, Beifuss U (2012b) An unprecedented oxidative trimerization of sesamol catalyzed by laccases. Tetrahedron Lett 53:3254–3258
D’Alfonso C, Lanzalunga O, Lapi A, Vadalà R (2014) Comparing the catalytic efficiency of ring substituted 1-hydroxybenzotriazoles as laccase mediators. Tetrahedron 70:3049–3055
Davin LB, Lewis NG (2000) Dirigent proteins and dirigent sites explain the mystery of specificity of radical precursor coupling in lignan and lignin biosynthesis. Plant Physiol 123:453–462
Davin LB, Lewis NG (2005) Dirigent phenoxy radical coupling: advances and challenges. Curr Opin Biotechnol 16:398–406
Davin LB, Wang HB, Crowell AL, Bedgar DL, Martin DM, Sarkanen S, Lewis NG (1997) Stereoselective bimolecular phenoxy radical coupling by an auxiliary (dirigent) protein without an active center. Science 275:362–367
Davis-Searles PR, Nakanishi Y, Kim NC, Graf TN, Oberlies NH, Wani MC, Wall ME, Agarwal R, Kroll DJ (2005) Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res 65:4448–4457
Debbab A, Aly AH, Lin WH, Proksch P (2010) Bioactive compounds from marine bacteria and fungi. Microb Biotechnol 3:544–563
de Regil R, Sandoval G (2013) Biocatalysis for biobased chemicals. Biomolecules 3:812–847
Decker EA (2008) Antioxidant mechanisms. In: Akoh CC, Min DB (eds) Food lipids: chemistry, nutrition, and biotechnology, 3rd edn. CRC Press, Boca Raton, pp. 475–492
dos Santos RMB, Simoes JAM (1998) Energetics of the O-H bond in phenol and substituted phenols: a critical evaluation of litrature data. J Phys Chem Ref Data 27:707–737
During A, Debouche C, Raas T, Larondelle Y (2012) Among plant lignans, pinoresinol has the strongest antiinflammatory properties in human intestinal Caco-2 cells. J Nutr 142:1798–1805
Eggert C (1997) Laccase-catalyzed formation of cinnabarinic acid is responsible for antibacterial activity of Pycnoporus cinnabarinus. Microbiol Res 152:315–318
Eggert C, Temp U, Dean JF, Eriksson KE (1995) Laccase-mediated formation of the phenoxazinone derivative, cinnabarinic acid. FEBS Lett 376:202–206
El Mansouri NE, Salvadó J (2007) Analytical methods for determining functional groups in various technical lignins. Ind Crop Prod 26:116–124
Elmaci BS, Gulgor G, Tokatli M, Erten H, Isci A, Ozcelik F (2015) Effectiveness of chitosan against wine-related microorganisms. Antonie Van Leeuwenhoek 107:675–686
Emirdağ-Öztürk S, Hajdok S, Conrad J, Beifuss U (2013) Laccase-catalyzed reaction of 3-tert-butyl-1H-pyrazol-5(4H)-one with substituted catechols using air as an oxidant. Tetrahedron 69:3664–3668
Erwin ME, Jones RN, Barrett MS, Briggs BM, Johnson DM (1991) In vitro evaluation of GR69153, a novel catechol-substituted cephalosporin. Antimicrob Agents Chemother 35:929–937
Eskin M, Przybylski R (2000) Antioxidants and shelf life of foods. In: Eskin NAM, Robinson DS (eds) Food shelf life stability: chemical, biochemical, and microbiological changes. CRC Press, Boca Raton, p. 194
Fabbrini M, Galli C, Gentili P (2002) Comparing the catalytic efficiency of some mediators of laccase. J Mol Catal B Enzym 16:231–240
Fini L, Hotchkiss E, Fogliano V, Graziani G, Romano M, De Vol EB, Qin H, Selgrad M, Boland CR, Ricciardiello L (2008) Chemopreventive properties of pinoresinol-rich olive oil involve a selective activation of the ATM-p53 cascade in colon cancer cell lines. Carcinogenesis 29:139–146
Flora SJS (2009) Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxidative Med Cell Longev 2:191–206
France S, Weatherwax A, Taggi AE, Lectka T (2004) Advances in the catalytic, asymmetric synthesis of β-lactams. Acc Chem Res 37:592–600
Fung-Tomc J, Bush K, Minassian B, Kolek B, Flamm R, Gradelski E, Bonner D (1997) Antibacterial activity of BMS-180680, a new catechol-containing monobactam. Antimicrob Agents Chemother 41:1010–1016
Gavezzotti P, Vavříková E, Valentová K, Fronza G, Kudanga T, Kuzma M, Riva S, Biedermann D, Kren V (2014) Enzymatic oxidative dimerization of silymarin flavonolignans. J Mol Catal B Enzym 109:24–30
Gažák R, Purchartová K, Marhol P, Živná L, Sedmera P, Valentová K, Kato N, Matsumura H, Kaihatsu K, Kren V (2010) Antioxidant and antiviral activities of silybin fatty acid conjugates. Eur J Med Chem 45:1059–1067
Gažák R, Sedmera P, Marzorati M, Riva S, Křen V (2008) Laccase-mediated dimerization of the flavonolignan silybin. J Mol Catal B Enzym 50:87–92
Gažák R, Svobodová A, Psotová J, Sedmera P, Přikrylová V, Walterová D, Kren V (2004) Oxidised derivatives of silybin and their antiradical and antioxidant activity. Bioorg Med Chem 12:5677–5687
Gerhards N, Neubauer L, Tudzynski P, Li SM (2014) Biosynthetic pathways of ergot alkaloids. Toxins 6:3281–3295
Gil-Chávez JG, Villa JA, Ayala-Zavala FJ, Heredia JB, Sepulveda D, Yahia EM, González-Aguilar GA (2013) Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: an overview. Compr Rev Food Sci F 12:5–23
Girol GC, Fisch KM, Heinekamp T, Gunther S, Huttel W, Piel J, Brakhage AA, Müller M (2012) Regio- and stereoselective oxidative phenol coupling in Aspergillus niger. Angew Chem Int Ed Engl 51:9788–9791
Greenway F, Liu Z, Yu Y, Gupta A (2011) A clinical trial testing the safety and efficacy of a standardized Eucommia ulmoides Oliver bark extract to treat hypertension. Altern Med Rev 16:338–347
Guaadaoui A, Benaicha S, Elmajdoub N, Bellaoui M, Hamal A (2014) What is a bioactive compound? A combined definition for a preliminary consensus. Int J Food Sci Nutr 3:174–179
Hahn V, Mikolasch A, Manda K, Gordes D, Thurow K, Schauer F (2009a) Laccase-catalyzed carbon-nitrogen bond formation: coupling and derivatization of unprotected L-phenylalanine with different para-hydroquinones. Amino Acids 37:315–321
Hahn V, Mikolasch A, Wende K, Bartrow H, Lindequist U, Schauer F (2009b) Synthesis of model morpholine derivatives with biological activities by laccase-catalysed reactions. Biotechnol Appl Biochem 54:187–195
Halls SC, Davin LB, Kramer DM, Lewis NG (2004) Kinetic study of coniferyl alcohol radical binding to the (+)-pinoresinol forming dirigent protein. Biochemistry 43:2587–2595
Infiniti Research Limited (2014) Global antioxidants market 2014–2018. Sandler Research. http://www.sandlerresearch.org/global-antioxidants-market-2014-2018.html.2014. Accessed 28 June 2015
Ishige K, Schubert D, Sagara Y (2001) Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med 30:433–446
Jadhav SB, Singhal RS (2014) Laccase–gum Arabic conjugate for preparation of water-soluble oligomer of catechin with enhanced antioxidant activity. Food Chem 150:9–16
Jasuja R, Passam FH, Kennedy DR, Kim SH, van Hessem L, Lin L, Bowley SR, Joshi SS, Dilks JR, Furie B, Furie BC, Flaumenhaft R (2012) Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. J Clin Invest 122:2104–2113
Jeon JR, Baldrian P, Murugesan K, Chang YS (2012) Laccase-catalysed oxidations of naturally occurring phenols: from in vivo biosynthetic pathways to green synthetic applications. Microb Biotechnol 5:318–332
Ji Z, Su Y (2008) Study on antimicrobial activities of extracts from Eucommia ulmoides Oliv. leaves. J Chem Ind Forest Prod 2:16
Jones F, Ricke S (2003) Observations on the history of the development of antimicrobials and their use in poultry feeds. Poult Sci 82:613–617
Jung HW, Mahesh R, Lee JG, Lee SH, Kim YS, Park YK (2010) Pinoresinol from the fruits of Forsythia koreana inhibits inflammatory responses in LPS-activated microglia. Neurosci Lett 480:215–220
Kazenwadel C, Klebensberger J, Richter S, Pfannstiel J, Gerken U, Pickel B, Schaller A, Hauer B (2013) Optimized expression of the dirigent protein AtDIR6 in Pichia pastoris and impact of glycosylation on protein structure and function. Appl Microbiol Biotechnol 97:7215–7227
Kermanshai R, McCarry BE, Rosenfeld J, Summers PS, Weretilnyk EA, Sorger GJ (2001) Benzyl isothiocyanate is the chief or sole anthelmintic in papaya seed extracts. Phytochemistry 57:427–435
Kim KW, Moinuddin SGA, Atwell KM, Costa MA, Davin LB, Lewis NG (2012) Opposite stereoselectivities of dirigent proteins in Arabidopsis and Schizandra species. J Biol Chem 287:33957–33972
Klein E, Lukeš V (2006) Study of gas-phase O–H bond dissociation enthalpies and ionization potentials of substituted phenols—applicability of ab initio and DFT/B3LYP methods. J Chem Phys 330:515–525
Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD (2002) Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med 113:71–88
Kudanga T, Burton S, Nyanhongo GS, Guebitz GM (2011a) Versatility of oxidoreductases in the remediation of environmental pollutants. Front Biosci (Elite Ed) 4:1127–1149
Kudanga T, Le Roes-Hill M (2014) Laccase applications in biofuels production: current status and future prospects. Appl Microbiol Biotechnol 98:6525–6542
Kudanga T, Prasetyo EN, Sipilä J, Eberl A, Nyanhongo GS, Guebitz GM (2009) Coupling of aromatic amines onto syringylglycerol β-guaiacylether using Bacillus SF spore laccase: a model for functionalization of lignin-based materials. J Mol Catal B Enzym 61:143–149
Kudanga T, Nyanhongo GS, Guebitz GM, Burton S (2011b) Potential applications of laccase-mediated coupling and grafting reactions: a review. Enzym Microb Technol 48:195–208
Kudanga T, Prasetyo EN, Sipilä J, Guebitz GM, Nyanhongo GS (2010a) Reactivity of long chain alkylamines to lignin moieties: implications on hydrophobicity of lignocellulose materials. J Biotechnol 149:81–87
Kudanga T, Prasetyo EN, Sipilä J, Nousiainen P, Widsten P, Kandelbauer A, Nyanhongo GS (2008) Laccase-mediated wood surface functionalization. Eng Life Sci 8:297–302
Kudanga T, Prasetyo EN, Sipilä J, Nyanhongo GS, Guebitz GM (2010b) Enzymatic grafting of functional molecules to the lignin model dibenzodioxocin and lignocellulose material. Enzym Microb Technol 46:272–280
Kunamneni A, Camarero S, Garcia-Burgos C, Plou F, Ballesteros A, Alcalde M (2008a) Engineering and applications of fungal laccases for organic synthesis. Microb Cell Factories 7:32
Kunamneni A, Plou FJ, Ballesteros A, Alcalde M (2008b) Laccases and their applications: a patent review. Recent Pat Biotechnol 2:10–24
Kurisawa M, Chung JE, Uyama H, Kobayashi S (2003a) Enzymatic synthesis and antioxidant properties of poly(rutin). Biomacromolecules 4:1394–1399
Kurisawa M, Chung JE, Uyama H, Kobayashi S (2003b) Laccase-catalyzed synthesis and antioxidant property of poly (catechin). Macromol Biosci 3:758–764
Leutbecher H, Constantin MA, Mika S, Conrad J, Beifuss U (2011) A new laccase-catalyzed domino process and its application to the efficient synthesis of 2-aryl-1H-benzimidazoles. Tetrahedron Lett 52:605–608
Lewis K (2013) Platforms for antibiotic discovery. Nat Rev Drug Discov 12:371–387
Li CJ, Trost BM (2008) Green chemistry for chemical synthesis. Proc Natl Acad Sci U S A 105:13197–13202
Li F, Yang XW (2012) Analysis of anti-inflammatory dehydrodiisoeugenol and metabolites excreted in rat feces and urine using HPLC-UV. Biomed Chromatogr 26:703–707
Li XJ, Zhang HY (2008) Western-medicine-validated anti-tumor agents and traditional Chinese medicine. Trends Mol Med 14:1–2
Lien EJ, Ren S, Bui HH, Wang R (1999) Quantitative structure-activity relationship analysis of phenolic antioxidants. Free Radic Biol Med 26:285–294
Lu Z, Nie G, Belton PS, Tang H, Zhao B (2006) Structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem Int 48:263–274
Luo X, Ma M, Chen B, Yao S, Wan Z, Yang D, Hang H (2004) Analysis of nine bioactive compounds in Eucommia ulmoides Oliv. and their preparation by HPLC-photodiode array detection and mass spectrometry. J Liq Chromatogr Relat Technol 27:63–81
Madhavi V, Lele SS (2009) Laccase: properties and applications. Bioresources 4:1694–1717
Manda K, Hammer E, Mikolasch A, Gördes D, Thurow K, Schauer F (2006) Laccase-induced derivatization of unprotected amino acid L-tryptophan by coupling with p-hydroquinone 2,5-dihydroxy-N-(2-hydroxyethyl)-benzamide. Amino Acids 31:409–419
Mantegani S, Brambilla E, Varasi M (1999) Ergoline derivatives: receptor affinity and selectivity. Farmaco 54:288–296
Martins S, Mussatto SI, Martínez-Avila G, Montañez-Saenz J, Aguilar CN, Teixeira JA (2011) Bioactive phenolic compounds: production and extraction by solid-state fermentation. A review. Biotechnol Adv 29:365–373
Mate DM, Alcalde M (2015) Laccase engineering: from rational design to directed evolution. Biotechnol Adv 33:25–40
Matern U, Kneusel RE (1988) Phenolic compounds in plant disease resistance. Phytoparasitica 16:153–170
Matsuura T, Ohkatsu Y (2000) Phenolic antioxidants: effect of o-benzyl substituents. Polym Degrad Stab 70:59–63
Maugh TH (1984) Semisynthetic enzymes are new catalysts. Science 223:154–156
Mayer AM, Staples RC (2002) Laccase: new functions for an old enzyme. Phytochemistry 60:551–565
Mikolasch A, Hessel S, Salazar MG, Neumann H, Manda K, Gordes D, Schmidt E, Thurow K, Hammer E, Lindequist U, Beller M, Schauer F (2008a) Synthesis of new N-analogous corollosporine derivatives with antibacterial activity by laccase-catalyzed amination. Chem Pharm Bull 56:781–786
Mikolasch A, Manda K, Schlüter R, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Jülich WD, Lindequist U (2012) Comparative analyses of laccase-catalyzed amination reactions for production of novel β-lactam antibiotics. Biotechnol Appl Biochem 59:295–306
Mikolasch A, Niedermeyer TH, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Gesell M, Hessel S, Jülich WD, Lindequist U (2006) Novel penicillins synthesized by biotransformation using laccase from Trametes species. Chem Pharm Bull 54:632–638
Mikolasch A, Niedermeyer THJ, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Gesell Salazar M, Hessel S, Jülich WD, Lindequist U (2007) Novel cephalosporins synthesized by amination of 2,5-dihydroxybenzoic acid derivatives using fungal laccases II. Chem Pharm Bull 55:412–416
Mikolasch A, Schauer F (2009) Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Appl Microbiol Biotechnol 82:605–624
Mikolasch A, Wurster M, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Jülich WD, Lindequist U (2008b) Novel β-lactam antibiotics synthesized by amination of catechols using fungal laccase. Chem Pharm Bull 56:902–907
Morozova O, Shumakovich G, Gorbacheva M, Shleev S, Yaropolov A (2007a) “Blue” laccases. Biochem Mosc 72:1136–1150
Morozova O, Shumakovich G, Shleev S, Yaropolov YI (2007b) Laccase-mediator systems and their applications: a review. Appl Biochem Microbiol 43:523–535
Muller WEG, Grebenjuk VA, Le Pennec G, Schroder HC, Brummer F, Hentschel U, Müller IM, Breter H (2004) Sustainable production of bioactive compounds by sponge-cell culture and gene cluster approach: a review. Mar Biotechnol 6:105–117
Murakami Y, Ito S, Atsumi T, Fujisawa S (2005a) Theoretical prediction of the relationship between phenol function and COX-2/AP-1 inhibition for ferulic acid-related compounds. In Vivo 19:1039–1044
Murakami Y, Shoji M, Hirata A, Tanaka S, Yokoe I, Fujisawa S (2005b) Dehydrodiisoeugenol, an isoeugenol dimer, inhibits lipopolysaccharide-stimulated nuclear factor kappa B activation and cyclooxygenase-2 expression in macrophages. Arch Biochem Biophys 434:326–332
Najafi M (2014) On the antioxidant activity of the ortho and meta substituted daidzein derivatives in the gas phase and solvent environment. J Mex Chem Soc 58:36–45
Nicotra S, Cramarossa MR, Mucci A, Pagnoni UM, Riva S, Forti L (2004a) Biotransformation of resveratrol: synthesis of trans-dehydrodimers catalyzed by laccases from Myceliophthora thermophila and from Trametes pubescens. Tetrahedron 60:595–600
Nicotra S, Intra A, Ottolina G, Riva S, Danieli B (2004b) Laccase-mediated oxidation of the steroid hormone 17β-estradiol in organic solvents. Tetrahedron Asymmetry 15:2927–2931
Orlandi M, Rindone B, Molteni G, Rummakko P, Brunow G (2001) Asymmetric biomimetic oxidations of phenols: the mechanism of the diastereo-and enantioselective synthesis of dehydrodiconiferyl ferulate (DDF) and dehydrodiconiferyl alcohol (DDA). Tetrahedron 57:371–378
Osiadacz J, Al-Adhami AJH, Bajraszewska D, Fischer P, Peczyñska-Czoch W (1999) On the use of Trametes versicolor laccase for the conversion of 4-methyl-3-hydroxyanthranilic acid to actinocin chromophore. J Biotechnol 72:141–149
Pasha I, Saeed F, Waqas K, Anjum FM, Arshad MU (2013) Nutraceutical and functional scenario of wheat straw. Crit Rev Food Sci Nutr 53:287–295
Pelletier SW (1983) Alkaloids: chemical and biological perspectives. Wiley, New York
Pickel B, Schaller A (2013) Dirigent proteins: molecular characteristics and potential biotechnological applications. Appl Microbiol Biotechnol 97:8427–8438
Pietruszka J, Wang C (2012) Laccase-catalysed [small alpha]-arylation of cyclic [small beta]-dicarbonyl compounds. Green Chem 14:2402–2409
Plíšková M, Vondráček J, Křen V, Gažák R, Sedmera P, Walterová D, Psotová J, Šimánek V, Machala M (2005) Effects of silymarin flavonolignans and synthetic silybin derivatives on estrogen and aryl hydrocarbon receptor activation. Toxicology 215:80–89
Präg A, Grüning BA, Häckh M, Lüdeke S, Wilde M, Luzhetskyy A, Richter M, Luzhetska M, Günther S, Müller M (2014) Regio- and stereoselective intermolecular oxidative phenol coupling in Streptomyces. J Am Chem Soc 136:6195–6198
Prins A, Kleinsmidt L, Khan N, Kirby B, Kudanga T, Vollmer J, Pleiss J, Burton S, Le Roes-Hill M (2015) The effect of mutations near the T1 copper site on the biochemical characteristics of the small laccase from Streptomyces coelicolor A3(2). Enzym Microb Technol 68:23–32
Raafat D, von Bargen K, Haas A, Sahl HG (2008) Insights into the mode of action of chitosan as an antibacterial compound. Appl Environ Microbiol 74:3764–3773
Rakesh KP, Manukumar HM, Gowda DC (2015) Schiff’s bases of quinazolinone derivatives: synthesis and SAR studies of a novel series of potential anti-inflammatory and antioxidants. Bioorg Med Chem Lett 25:1072–1077
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Rich JO, Anderson AM, Berhow MA (2016) Laccase-mediator catalyzed conversion of model lignin compounds. Biocatal Agric Biotechnol 5:111–115
Riva S (2006) Laccases: blue enzymes for green chemistry. Trends Biotechnol 24:219–226
Robert V, Mekmouche Y, Pailley PR, Tron T (2011) Engineering laccases: in search for novel catalysts. Curr Genomics 12:123–129
Roberts MF, Wink M (1998) Alkaloids: biochemistry, ecology, and medicinal applications, 1st edn. Springer Science & Business Media, New York
Rocasalbas G, Francesko A, Touriño S, Fernández-Francos X, Guebitz GM, Tzanov T (2013) Laccase-assisted formation of bioactive chitosan/gelatin hydrogel stabilized with plant polyphenols. Carbohydr Polym 92:989–996
Rodríguez Couto S, Toca Herrera JL (2006) Industrial and biotechnological applications of laccases: a review. Biotechnol Adv 24:500–513
Sagui F, Chirivì C, Fontana G, Nicotra S, Passarella D, Riva S, Danieli B (2009) Laccase-catalyzed coupling of catharanthine and vindoline: an efficient approach to the bisindole alkaloid anhydrovinblastine. Tetrahedron 65:312–317
Sánchez-Moreno C, Larrauri JA, Saura-Calixto F (1998) A procedure to measure the antiradical efficiency of polyphenols. J Sci Food Agric 76:270–276
Shahidi F, Arachchi JKV, Jeon YJ (1999) Food applications of chitin and chitosans. Trends Food Sci Tech 10:37–51
Shahidi F, Naczk M (2004) Antioxidant properties of food phenolics. In: Shahidi F, Naczk M (eds) Phenolics in food and nutraceuticals. CRC Press, Boca Raton, pp. 403–436
Silley P, Griffiths JW, Monsey D, Harris AM (1990) Mode of action of GR69153, a novel catechol-substituted cephalosporin, and its interaction with the tonB-dependent iron transport system. Antimicrob Agents Chemother 34:1806–1808
Srinivasan M, Sudheer AR, Menon VP (2007) Ferulic acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr 40:92–100
Szymusiak H, Zielinski R (2003) Bond dissociation enthalpy of phenolic antioxidants. Pol J Food Nutr Sci 53:129–135
Turner NJ (2009) Directed evolution drives the next generation of biocatalysts. Nat Chem Biol 5:567–573
Umezawa T (2003) Diversity in lignan biosynthesis. Phytochem Rev 2:371–390
van der Heijden R, Jacobs DI, Snoeijer W, Hallard D, Verpoorte R (2004) The catharanthus alkaloids: pharmacognosy and biotechnology. Curr Med Chem 11:607–628
Vardanyan R, Hruby V (2006) Synthesis of essential drugs. Elsevier, Amsterdam
Wan Y, Lu R, Akiyama K, Miyakoshi T, Du Y (2007) Enzymatic synthesis of bioactive compounds by Rhus laccase from Chinese Rhus vernicifera. Sci China Ser B 50:179–182
Watanabe NA, Nagasu T, Katsu K, Kitoh K (1987) E-0702, a new cephalosporin, is incorporated into Escherichia coli cells via the tonB-dependent iron transport system. Antimicrob Agents Chemother 31:497–504
Wellington KW, Qwebani-Ogunleye T, Kolesnikova NI, Brady D, de Koning CB (2013) One-pot laccase-catalysed synthesis of 5,6-dihydroxylated benzo[b]furans and catechol derivatives, and their anticancer activity. Arch Pharm Chem Life Sci 346:266–277
Wellington KW, Kolesnikova NI (2012) A laccase-catalysed one-pot synthesis of aminonaphthoquinones and their anticancer activity. Bioorg Med Chem 20:4472–4481
Widsten P, Heathcote C, Kandelbauer A, Guebitz G, Nyanhongo GS, Prasetyo EN, Kudanga T (2010) Enzymatic surface functionalisation of lignocellulosic materials with tannins for enhancing antibacterial properties. Process Biochem 45:1072–1081
Witayakran S, Ragauskas AJ (2009) Synthetic applications of laccase in green chemistry. Adv Synth Catal 351:1187–1209
Yoshida O, Nakamura J, Yamashiro H, Miura K, Hayashi S, Umetsu K, Xu S, Maki H, Arimoto H (2011) New insight into the mode of action of vancomycin dimers in bacterial cell wall synthesis. Med Chem Commun 2:278–282
Zhang Q, Su Y, Zhang J (2013) Seasonal difference in antioxidant capacity and active compounds contents of Eucommia ulmoides Oliver leaf. Molecules 18:1857–1867
Zhu C, Zhang Z, Ding W, Xie J, Chen Y, Wu J, Chen X, Ying H (2014) A mild and highly efficient laccase-mediator system for aerobic oxidation of alcohols. Green Chem 16:1131–1138
Zoia L, Bruschi M, Orlandi M, Tolppa EL, Rindone B (2008) Asymmetric biomimetic oxidations of phenols: the mechanism of the diastereo-and enantioselective synthesis of thomasidioic acid. Molecules 13:129–148
Zwane RE, Parker A, Kudanga T, Davids LM, Burton SG (2012) Novel, biocatalytically produced hydroxytyrosol dimer protects against ultraviolet-induced cell death in human immortalized keratinocytes. J Agric Food Chem 60:11509–11511
Acknowledgements
Financial support from the National Research Foundation (South Africa) is gratefully acknowledged. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the author(s), and therefore, the NRF does not accept any liability in regard thereto.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kudanga, T., Nemadziva, B. & Le Roes-Hill, M. Laccase catalysis for the synthesis of bioactive compounds. Appl Microbiol Biotechnol 101, 13–33 (2017). https://doi.org/10.1007/s00253-016-7987-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7987-5