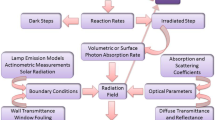
Abstract
Anodic oxidation is a promising method for removing organic pollutants from water due to its high nonselectivity and effectiveness. Nevertheless, its widespread application is limited due to its low current efficiency, high energy consumption and low treatment rates. These problems may be overcome by the optimization of the process parameters, reactor design and electrode geometry, by coupling the experimental investigations with mathematical modeling. Here we review the modeling of anodic oxidation with focus on basics of this process, the competition phenomenon in real wastewater, flow cells and batch cells, historical aspects, general modeling equations, modeling with plate electrodes, modeling with porous 3-dimension electrodes and the density functional theory. Mathematical modeling can provide current, voltage and concentration distributions in the system. Mathematical modeling can also determine the effects on the performance of parameters such as diffusion layer thickness, flow velocity, applied current density, solution treatment time, initial concentration and diffusion coefficients of organic pollutants, electrode surface area, and oxidation reaction rate constant. Mathematical models allow to determine whether the limiting factor of the process is kinetics or diffusion, and to study the impact of competition of phenomena. The density functional theory provides information on probable reaction pathways and by-products.














Similar content being viewed by others
Availability of data and material
Not applicable.
Code availability
Not applicable.
Abbreviations
- Blue-TiO2 :
-
Blue-titanium dioxide
- C6H6 :
-
Benzene
- CH3COCH3 :
-
Acetone
- (C2H5)3N :
-
Triethylamine
- CH3OH :
-
Methanol
- Cl− :
-
Chloride ions
- ClCH꞊CCl2 :
-
Trichloroethylene
- ClO3 − :
-
Chlorate
- CO2 :
-
Carbon dioxide
- H2 :
-
Hydrogen
- H2CO3 :
-
Carbonic acid
- H2O :
-
Water
- IrO2-Ta2O5 :
-
Iridium dioxide-tantalum pentoxide electrode
- NaClO4 :
-
Sodium perchlorate
- NO2 :
-
Nitrogen dioxide
- O3 :
-
Ozone
- O3/H2O2 :
-
Ozone/hydrogen peroxide
- PO4 3− :
-
Phosphate
- RuO2-TiO2 :
-
Ruthenium oxide-titania electrode
- S2O8 2− :
-
Peroxodisulfate
- Ti4O7 :
-
Sub-stoichiometric titanium oxide
- Ti/Pt:
-
Titanium covered by platinum electrode
- B:
-
Boron
- Carbon/Graphite:
-
Carbon-coated graphite electrode
- CH3CH3 :
-
Ethane
- CH3COOH:
-
Acetic acid
- C6H5NH2 :
-
Aniline
- C6H5OH:
-
Phenol
- Cl2 :
-
Chlorine
- ClO− :
-
Hypochlorite
- ClO4 − :
-
Perchlorate
- C2O6 2− :
-
Peroxodicarbonate
- HCO3 − :
-
Hydrogen carbonate
- HClO:
-
Hypochlorous acid
- HCOOH:
-
Formic acid
- NaCl:
-
Sodium chloride
- Na2SO4 :
-
Sodium sulfate
- O2 :
-
Oxygen
- OCH3 :
-
Methoxy groups
- ·OH:
-
Hydroxyl radicals ()
- P2O8 4− :
-
Peroxodiphosphate
- SO4 2− :
-
Sulfate
- TinO2 n −1 :
-
Magnéli phases of sub-stoichiometric titanium oxides
- Ti/PbO2 :
-
Titanium-coated lead dioxide electrode
- Ti/SnO2 :
-
Titanium-coated tin dioxide electrode
- A :
-
Electrode area (m2)
- c :
-
Concentration (mol m−3)
- c s :
-
Concentration at the electrode surface (mol m−3)
- C R :
-
Concentration of anodic reactants (mol m−3)
- c tp :
-
Tracer particles concentration (mol m−3)
- COD :
-
Chemical oxygen demand (mol O2 m−3)
- D :
-
Diffusion coefficient of the compound (m2 s−1)
- d reac :
-
Reaction zone thickness (m)
- \(\overline{f}\) :
-
Body force (N kg−1)
- \(\overline{i}\) :
-
Current density (A cm−2)
- i lim :
-
Limiting current density (A m−2)
- i lim,ne − :
-
Limiting current density of DET(A m−2)
- i 0 :
-
Exchange current density(A cm−2)
- \(\overline{j}\) :
-
Flux density (mol m−2 s−1)
- k · O H :
-
·OH recombination rate constant (m3 mol−1 s−1)
- P :
-
Given loading (mol COD s−1)
- Pe :
-
Peclet number
- R :
-
Reactive term (mol m−3 s−1)
- R c :
-
Electrolyte ohmic resistance (Ω)
- r i :
-
Oxidation rate of each compound in the reaction zone (mol m−2 s−1)
- Sh :
-
Sherwood number
- t :
-
Time (s)
- t s :
-
Special time (s)
- \(\overline{u}\) :
-
Linear fluid velocity (m s−1)
- V R :
-
Reservoir volume (m3)
- ΔV work :
-
Cell potential (V)
- X :
-
COD conversion (%)
- X cr :
-
Critical conversion
- Α :
-
Electron transfer coefficient
- A req :
-
Required electrode area (m2)
- c b :
-
Bulk concentration (mol m−3)
- C 0 :
-
Concentration of cathodic reactants (mol m−3)
- C :
-
Dimensionless tracer particles concentration
- c tp 0 :
-
Initial tracer particles concentration (mol m−3)
- COD0 :
-
Initial chemical oxygen demand (mol O2 m−3)
- E sp :
-
Specific energy consumption (kW h kg COD−1)
- F :
-
Faraday’s constant(C mol−1)
- i :
-
Current intensity (A)
- i appl :
-
Applied current density (A m−2)
- i lim 0 :
-
Initial limiting current density (A m−2)
- i · OH :
-
Initial limiting current density, corresponding to the total mineralization of organic compounds (A m−2)
- J :
-
Flux (mol s−1)
- k m :
-
Mass transfer coefficient (m s−1)
- L x :
-
Axial length (m)
- p :
-
Applied pressure (Pa)
- Q cr :
-
Critical specific charge (Ah m−3)
- \(\overline{R}\) :
-
Universal gas constant (J mol−1 K −1)
- Re :
-
Reynolds number
- Sc :
-
Smidt number
- T :
-
Temperature (K)
- t cr :
-
Critical time (s)
- u int :
-
Interstitial liquid velocity (m s−1)
- V :
-
Volume of electrolyte (dm3)
- ΔV i :
-
Oxidation potential of each process (V)
- X l :
-
Dimensionless axial length (m)
- x :
-
Axis coordinate along the distance (m)
- z :
-
Charge
- δ [delta] :
-
Diffusion layer thickness (m)
- ε [epsilon] :
-
Effectiveness factor
- ε Cl − [epsilon Cl] :
-
Faradic yield as a function of chloride (Cl−) concentration
- η a [eta a] :
-
Overpotential of anodic reaction (V)
- θ [theta] :
-
Dimensionless time
- μ [mu] :
-
Dynamic viscosity (Pa s)
- τ [tau] :
-
Electrolysis time (s)
- φ [phi, small letter] :
-
Electric potential (V)
- α i [alpha i] :
-
Proportion of electrons involved in a particular electrochemical process corresponds to each process i
- α ·OH [alpha OH] :
-
Term accounts for the fraction of current directed toward ·OH production
- δ(exp) [delta exp] :
-
Diffusion layer thickness obtained experimentally (m)
- ε i [epsilon i] :
-
Faradaic yield
- η c [eta c] :
-
Overpotential of cathodic reaction (V)
- θ i [theta i] :
-
Parameter represents the oxidation efficiency
- ρ[rho] :
-
Liquid density (kg m−3)
- ϕ [phi] :
-
Dimensionless parameter expressing the ratio between the chemical reaction rate and the mass transfer coefficient
- φ [phi, small bold letter] :
-
Normalized current efficiency
References
Adams B, Tian M, Chen A (2009) Design and electrochemical study of SnO2-based mixed oxide electrodes. Electrochim Acta 54:1491–1498. https://doi.org/10.1016/j.electacta.2008.09.034
Ahmed F, Lalia BS, Kochkodan V et al (2016) Electrically conductive polymeric membranes for fouling prevention and detection: a review. Desalination 391:1–15. https://doi.org/10.1016/j.desal.2016.01.030
Alighardashi A, Aghta RS, Ebrahimzadeh H (2018) Improvement of carbamazepine degradation by a three-dimensional electrochemical (3-EC) process. Int J Environ Res 12:451–458. https://doi.org/10.1007/s41742-018-0102-2
Andersson S, Collén B, Kuylenstierna U et al (1957) Phase analysis studies on the titanium-oxygen system. Acta Chem Scand 11:1641–1652. https://doi.org/10.3891/acta.chem.scand.11-1641
Angulo A, van der Linde P, Gardeniers H et al (2020) Influence of bubbles on the energy conversion efficiency of electrochemical reactors. Joule 4:555–579. https://doi.org/10.1016/j.joule.2020.01.005
Armijos-Alcocer KG, Espinoza-Montero PJ, Frontana-Uribe BA et al (2017) Electrochemical degradation of nonylphenol ethoxylate-7 (NP7EO) using a DiaClean® cell equipped with boron-doped diamond electrodes (BDD). Water Air Soil Pollut 228:289. https://doi.org/10.1007/s11270-017-3471-9
Ateya BG, El-Anadouli BE (1991) Effects of gas bubbles on the polarization behavior of porous flow through electrodes. J Electrochem Soc 138:1331–1336. https://doi.org/10.1149/1.2085781
Azizi O, Hubler D, Schrader G et al (2011) Mechanism of perchlorate formation on boron-doped diamond film anodes. Environ Sci Technol 45:10582–10590. https://doi.org/10.1021/es202534w
Barbosa AD, da Silva LF, de Paula HM et al (2018) Combined use of coagulation (M. oleifera) and electrochemical techniques in the treatment of industrial paint wastewater for reuse and/or disposal. Water Res 145:153–161. https://doi.org/10.1016/j.watres.2018.08.022
Bard AJ, Faulkner LR (2001) Electrochemical methods: fundamentals and applications, 2nd edn. Wiley, New York
Beck F, Krohn H, Kaiser W et al (1998) Boron doped diamond/titanium composite electrodes for electrochemical gas generation from aqueous electrolytes. Electrochim Acta 44:525–532. https://doi.org/10.1016/S0013-4686(98)00115-7
Bengoa C, Montillet A, Legentilhomme P, Legrand J (2000) Characterization and modeling of the hydrodynamic behavior in the filter-press-type FM01-LC electrochemical cell by direct flow visualization and residence time distribution. Ind Eng Chem Res 39:2199–2206. https://doi.org/10.1021/ie9907730
Bisquert J (2000) Influence of the boundaries in the impedance of porous film electrodes. Phys Chem Chem Phys 2:4185–4192. https://doi.org/10.1039/b001708f
Bockris JO (1972) Electrochemistry of cleaner environments. Springer, US, Boston, MA
Bonfatti F, De Battisti A, Ferro S et al (2000) Anodic mineralization of organic substrates in chloride-containing aqueous media. Electrochim Acta 46:305–314. https://doi.org/10.1016/S0013-4686(00)00586-7
Borrás C, Laredo T, Mostany J, Scharifker BR (2004) Study of the oxidation of solutions of p-chlorophenol and p-nitrophenol on Bi-doped PbO2 electrodes by UV-Vis and FTIR in situ spectroscopy. Electrochim Acta 49:641–648. https://doi.org/10.1016/j.electacta.2003.09.019
Brillas E, Martínez-Huitle CA (2015) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl Catal B Environ 166–167:603–643. https://doi.org/10.1016/j.apcatb.2014.11.016
Brillas E, Sirés I, Oturan MA (2009) Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem Rev 109:6570–6631. https://doi.org/10.1021/cr900136g
Brito CN, Ferreira MB, de Moura Santos ECM et al (2018) Electrochemical degradation of azo-dye Acid Violet 7 using BDD anode: effect of flow reactor configuration on cell hydrodynamics and dye removal efficiency. J Appl Electrochem 48:1321–1330. https://doi.org/10.1007/s10800-018-1257-4
Brosler P, Girão AV, Silva RF et al (2023) Electrochemical advanced oxidation processes using diamond technology: a critical review. Environments 10:10020015. https://doi.org/10.3390/environments10020015
Brunet R, Dore M (1984) Oxidation of organic compounds through the combination ozone-hydrogen peroxide. Ozone Sci Eng 6:163–183. https://doi.org/10.1080/01919518408551019
Butrón E, Juárez ME, Solis M et al (2007) Electrochemical incineration of indigo textile dye in filter-press-type FM01-LC electrochemical cell using BDD electrodes. Electrochim Acta 52:6888–6894. https://doi.org/10.1016/j.electacta.2007.04.108
Cai J, Zhou M, Pan Y et al (2019) Extremely efficient electrochemical degradation of organic pollutants with co-generation of hydroxyl and sulfate radicals on Blue-TiO2 nanotubes anode. Appl Catal B Environ 257:117902. https://doi.org/10.1016/j.apcatb.2019.117902
Cañizares P, Domínguez JA, Rodrigo MA et al (1999) Effect of the current intensity in the electrochemical oxidation of aqueous phenol wastes at an activated carbon and steel anode. Ind Eng Chem Res 38:3779–3785. https://doi.org/10.1021/ie9901574
Cañizares P, Díaz M, Domínguez JA et al (2002) Electrochemical oxidation of aqueous phenol wastes on synthetic diamond thin-film electrodes. Ind Eng Chem Res 41:4187–4194. https://doi.org/10.1021/ie0105526
Cañizares P, García-Gómez J, Lobato J, Rodrigo MA (2003) Electrochemical oxidation of aqueous carboxylic acid wastes using diamond thin-film electrodes. Ind Eng Chem Res 42:956–962. https://doi.org/10.1021/ie020594+
Cañizares P, García-Gómez J, Lobato J, Rodrigo MA (2004) Modeling of wastewater electro-oxidation processes part I. General description and application to inactive electrodes. Ind Eng Chem Res 43:1915–1922. https://doi.org/10.1021/ie0341294
Cañizares P, García-Gómez J, Fernández de Marcos I et al (2006) Measurement of mass-transfer coefficients by an electrochemical technique. J Chem Educ 83:1204. https://doi.org/10.1021/ed083p1204
Cano A, Barrera C, Cotillas S et al (2016) Use of DiaCell modules for the electro-disinfection of secondary-treated wastewater with diamond anodes. Chem Eng J 306:433–440. https://doi.org/10.1016/j.cej.2016.07.090
Carrillo-Abad J, Mora-Gómez J, García-Gabaldón M et al (2020) Comparison between an electrochemical reactor with and without membrane for the nor oxidation using novel ceramic electrodes. J Environ Manage 268:110710. https://doi.org/10.1016/j.jenvman.2020.110710
Catañeda LF, Rivera FF, Pérez T, Nava JL (2019) Mathematical modeling and simulation of the reaction environment in electrochemical reactors. Curr Opin Electrochem 16:75–82. https://doi.org/10.1016/j.coelec.2019.04.025
Chaplin BP (2014) Critical review of electrochemical advanced oxidation processes for water treatment applications. Environ Sci Process Impacts 16:1182–1203. https://doi.org/10.1039/c3em00679d
Chatzisymeon E, Xekoukoulotakis NP, Diamadopoulos E et al (2009) Boron-doped diamond anodic treatment of olive mill wastewaters: statistical analysis, kinetic modeling and biodegradability. Water Res 43:3999–4009. https://doi.org/10.1016/j.watres.2009.04.007
Chen G, Betterton EA, Arnold RG (1999) Electrolytic oxidation of trichloroethylene using a ceramic anode. J Appl Electrochem 29:961–970. https://doi.org/10.1023/A:1003541706456
Chettiar M, Watkinson AP (1983) Anodic oxidation of phenolics found in coal conversion effluents. Can J Chem Eng 61:568–574. https://doi.org/10.1002/cjce.5450610411
Chung CM, Hong SW, Cho K, Hoffmann MR (2018) Degradation of organic compounds in wastewater matrix by electrochemically generated reactive chlorine species: kinetics and selectivity. Catal Today 313:189–195. https://doi.org/10.1016/j.cattod.2017.10.027
Clematis D, Panizza M (2021) Electrochemical oxidation of organic pollutants in low conductive solutions. Curr Opin Electrochem 26:100665. https://doi.org/10.1016/j.coelec.2020.100665
Comninellis C (1994) Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochim Acta 39:1857–1862. https://doi.org/10.1016/0013-4686(94)85175-1
Comninellis C, Pulgarin C (1991) Anodic oxidation of phenol for waste water treatment. J Appl Electrochem 21:703–708. https://doi.org/10.1007/BF01034049
Comninellis C, Pulgarin C (1993) Electrochemical oxidation of phenol for wastewater treatment using SnO2 anodes. J Appl Electrochem 23:108–112. https://doi.org/10.1007/BF00246946
Cornejo OM, Murrieta MF, Castañeda LF, Nava JL (2020) Characterization of the reaction environment in flow reactors fitted with BDD electrodes for use in electrochemical advanced oxidation processes: a critical review. Electrochim Acta 331:135373. https://doi.org/10.1016/j.electacta.2019.135373
Costa CR, Montilla F, Morallón E, Olivi P (2009) Electrochemical oxidation of acid black 210 dye on the boron-doped diamond electrode in the presence of phosphate ions: effect of current density, pH, and chloride ions. Electrochim Acta 54:7048–7055. https://doi.org/10.1016/j.electacta.2009.07.027
Cruz-Díaz M, Rivera FF, Rivero EP, González I (2012) The FM01-LC reactor modeling using axial dispersion model with a reaction term coupled with a continuous stirred tank (CST). Electrochim Acta 63:47–54. https://doi.org/10.1016/j.electacta.2011.12.038
Cruz-Díaz MR, Rivero EP, Almazán-Ruiz FJ et al (2014) Design of a new FM01-LC reactor in parallel plate configuration using numerical simulation and experimental validation with residence time distribution (RTD). Chem Eng Process Process Intensif 85:145–154. https://doi.org/10.1016/j.cep.2014.07.010
Cruz-Díaz MR, Rivero EP, Rodríguez FA, Domínguez-Bautista R (2018) Experimental study and mathematical modeling of the electrochemical degradation of dyeing wastewaters in presence of chloride ion with dimensional stable anodes (DSA) of expanded meshes in a FM01-LC reactor. Electrochim Acta 260:726–737. https://doi.org/10.1016/j.electacta.2017.12.025
Cuerda-Correa EM, Alexandre-Franco MF, Fernández-González C (2019) Advanced oxidation processes for the removal of antibiotics from water. An overview. Water 12:102. https://doi.org/10.3390/w12010102
Cui L, Zhang Y, He K et al (2022) Ti4O7 reactive electrochemical membrane for humic acid removal: insights of electrosorption and electrooxidation. Sep Purif Technol 293:121112. https://doi.org/10.1016/j.seppur.2022.121112
da Silva SW, Welter JB, Albornoz LL et al (2021) Advanced electrochemical oxidation processes in the treatment of pharmaceutical containing water and wastewater: a review. Curr Pollut Reports 7:146–159. https://doi.org/10.1007/s40726-021-00176-6
Dapo RF, Mann CK (1963) Anodic oxidation of triethylamine. Anal Chem 35:677–680. https://doi.org/10.1021/ac60199a054
de Moura DC, de Araújo CKC, Zanta CLPS et al (2014) Active chlorine species electrogenerated on Ti/Ru0.3Ti0.7O2 surface: electrochemical behavior, concentration determination and their application. J Electroanal Chem 731:145–152. https://doi.org/10.1016/j.jelechem.2014.08.008
de Oliveira GR, Fernandes NS, de Melo JV et al (2011) Electrocatalytic properties of Ti-supported Pt for decolorizing and removing dye from synthetic textile wastewaters. Chem Eng J 168:208–214. https://doi.org/10.1016/j.cej.2010.12.070
de OS Santos G, Gonzaga IMD, Dória AR et al (2020) Testing and scaling-up of a novel Ti/Ru0.7Ti0.3O2 mesh anode in a microfluidic flow-through reactor. Chem Eng J 398:125568. https://doi.org/10.1016/j.cej.2020.125568
de Paiva Barreto JP, de Freitas Araujo KC, de Araujo DM, Martinez-Huitle CA (2015) Effect of sp3/sp2 ratio on boron doped diamond films for producing persulfate. ECS Electrochem Lett 4:E9–E11. https://doi.org/10.1149/2.0061512eel
Degaki AH, Pereira GF, Rocha-Filho RC et al (2014) Effect of specific active chlorine species and temperature on the electrochemical degradation of the reactive blue 19 dye using a boron-doped diamond or DSA anode in a flow reactor. Electrocatalysis 5:8–15. https://doi.org/10.1007/s12678-013-0156-z
Donaghue A, Chaplin BP (2013) Effect of select organic compounds on perchlorate formation at boron-doped diamond film anodes. Environ Sci Technol 47:12391–12399. https://doi.org/10.1021/es4031672
Duan W, Ronen A, Walker S, Jassby D (2016) Polyaniline-coated carbon nanotube ultrafiltration membranes: enhanced anodic stability for in situ cleaning and electro-oxidation processes. ACS Appl Mater Interfaces 8:22574–22584. https://doi.org/10.1021/acsami.6b07196
Duguet JP, Brodard E, Dussert B, Mallevialle J (1985) Improvement in the effectiveness of ozonation of drinking water through the use of hydrogen peroxide. Ozone Sci Eng 7:241–258. https://doi.org/10.1080/01919518508552366
Farinos RM, Ruotolo LAM (2017) Comparison of the electrooxidation performance of three-dimensional RVC/PbO2 and boron-doped diamond electrodes. Electrochim Acta 224:32–39. https://doi.org/10.1016/j.electacta.2016.12.025
Fedkiw PS (1981) Ohmic potential drop in flow-through and flow-by porous electrodes. J Electrochem Soc 128:831–838. https://doi.org/10.1149/1.2127513
Fierro S, Ouattara L, Calderon EH et al (2009) Investigation of formic acid oxidation on Ti/IrO2 electrodes. Electrochim Acta 54:2053–2061. https://doi.org/10.1016/j.electacta.2008.06.060
Fisher V, Gandini D, Laufer S et al (1998) Preparation and characterization of Ti/Diamond electrodes. Electrochim Acta 44:521–524. https://doi.org/10.1016/S0013-4686(98)00116-9
Foutch GL, Johannes AH (2003) Reactors in process engineering. Encyclopedia of physical science and technology. Elsevier, UK, pp 23–43
Frías-Ferrer Á, Tudela I, Louisnard O et al (2011) Optimized design of an electrochemical filter-press reactor using CFD methods. Chem Eng J 169:270–281. https://doi.org/10.1016/j.cej.2011.02.053
Fu W, Wang X, Zheng J et al (2019) Antifouling performance and mechanisms in an electrochemical ceramic membrane reactor for wastewater treatment. J Memb Sci 570–571:355–361. https://doi.org/10.1016/j.memsci.2018.10.077
Fu R, Zhang P-S, Jiang Y-X et al (2023) Wastewater treatment by anodic oxidation in electrochemical advanced oxidation process: Advance in mechanism, direct and indirect oxidation detection methods. Chemosphere 311:136993. https://doi.org/10.1016/j.chemosphere.2022.136993
Galus Z, Adams RN (1964) The anodic oxidation of triphenylmethane dyes. J Am Chem Soc 86:1666–1671. https://doi.org/10.1021/ja01063a003
Ganiyu SO, Gamal El-Din M (2020) Insight into in-situ radical and non-radical oxidative degradation of organic compounds in complex real matrix during electrooxidation with boron doped diamond electrode: a case study of oil sands process water treatment. Appl Catal B Environ 279:119366. https://doi.org/10.1016/j.apcatb.2020.119366
Ganiyu SO, Zhou M, Martínez-Huitle CA (2018) Heterogeneous electro-Fenton and photoelectro-Fenton processes: a critical review of fundamental principles and application for water/wastewater treatment. Appl Catal B Environ 235:103–129. https://doi.org/10.1016/j.apcatb.2018.04.044
Ganiyu SO, Oturan N, Raffy S et al (2019) Efficiency of plasma elaborated sub-stoichiometric titanium oxide (Ti4O7) ceramic electrode for advanced electrochemical degradation of paracetamol in different electrolyte media. Sep Purif Technol 208:142–152. https://doi.org/10.1016/j.seppur.2018.03.076
Ganiyu SO, Martínez-Huitle CA, Rodrigo MA (2020) Renewable energies driven electrochemical wastewater/soil decontamination technologies: a critical review of fundamental concepts and applications. Appl Catal B Environ 270:118857. https://doi.org/10.1016/j.apcatb.2020.118857
Ganiyu SO, Martínez-Huitle CA, Oturan MA (2021) Electrochemical advanced oxidation processes for wastewater treatment: advances in formation and detection of reactive species and mechanisms. Curr Opin Electrochem 27:100678. https://doi.org/10.1016/j.coelec.2020.100678
Gao G, Zhang Q, Vecitis CD (2014) CNT-PVDF composite flow-through electrode for single-pass sequential reduction-oxidation. J Mater Chem A 2:6185–6190. https://doi.org/10.1039/c3ta14080f
García O, Isarain-Chávez E, Garcia-Segura S et al (2013) Degradation of 2,4-dichlorophenoxyacetic acid by electro-oxidation and electro-Fenton/BDD processes using a pre-pilot plant. Electrocatalysis 4:224–234. https://doi.org/10.1007/s12678-013-0135-4
Garcia-Rodriguez O, Mousset E, Olvera-Vargas H, Lefebvre O (2022) Electrochemical treatment of highly concentrated wastewater: a review of experimental and modeling approaches from lab- to full-scale. Crit Rev Environ Sci Technol 52:240–309. https://doi.org/10.1080/10643389.2020.1820428
Garcia-Segura S, Brillas E (2011) Mineralization of the recalcitrant oxalic and oxamic acids by electrochemical advanced oxidation processes using a boron-doped diamond anode. Water Res 45:2975–2984. https://doi.org/10.1016/j.watres.2011.03.017
Garcia-Segura S, Ocon JD, Chong MN (2018) Electrochemical oxidation remediation of real wastewater effluents: a review. Process Saf Environ Prot 113:48–67. https://doi.org/10.1016/j.psep.2017.09.014
Garcia-Segura S, Nienhauser AB, Fajardo AS et al (2020) Disparities between experimental and environmental conditions: research steps toward making electrochemical water treatment a reality. Curr Opin Electrochem 22:9–16. https://doi.org/10.1016/j.coelec.2020.03.001
Gayen P, Chen C, Abiade JT, Chaplin BP (2018) Electrochemical oxidation of atrazine and clothianidin on Bi-doped SnO2: TinO2n–1 electrocatalytic reactive electrochemical membranes. Environ Sci Technol 52:12675–12684. https://doi.org/10.1021/acs.est.8b04103
Ge M, Li Q, Cao C et al (2017) One-dimensional TiO2 nanotube photocatalysts for solar water splitting. Adv Sci 4:1600152. https://doi.org/10.1002/advs.201600152
Geng P, Chen G (2016) Magnéli Ti4O7 modified ceramic membrane for electrically-assisted filtration with antifouling property. J Memb Sci 498:302–314. https://doi.org/10.1016/j.memsci.2015.07.055
Geng P, Chen G (2017) Antifouling ceramic membrane electrode modified by Magnéli Ti4O7 for electro-microfiltration of humic acid. Sep Purif Technol 185:61–71. https://doi.org/10.1016/j.seppur.2017.05.023
Ghazouani M, Akrout H, Jomaa S et al (2016) Enhancing removal of nitrates from highly concentrated synthetic wastewaters using bipolar Si/BDD cell: optimization and mechanism study. J Electroanal Chem 783:28–40. https://doi.org/10.1016/j.jelechem.2016.10.048
Ghazouani M, Akrout H, Jellali S, Bousselmi L (2019) Comparative study of electrochemical hybrid systems for the treatment of real wastewaters from agri-food activities. Sci Total Environ 647:1651–1664. https://doi.org/10.1016/j.scitotenv.2018.08.023
Ghazouani M, Bousselmi L, Akrout H (2020) Combined electrocoagulation and electrochemical treatment on BDD electrodes for simultaneous removal of nitrates and phosphates. J Environ Chem Eng 8:104509. https://doi.org/10.1016/j.jece.2020.104509
Gherardini L, Michaud PA, Panizza M et al (2001) Electrochemical oxidation of 4-chlorophenol for wastewater treatment: definition of normalized current efficiency (φ). J Electrochem Soc 148:D78–D82. https://doi.org/10.1149/1.1368105
Glaze WH, Kang JW, Chapin DH (1987) The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci Eng 9:335–352. https://doi.org/10.1080/01919518708552148
Gomez-Ruiz B, Gómez-Lavín S, Diban N et al (2017) Efficient electrochemical degradation of poly- and perfluoroalkyl substances (PFASs) from the effluents of an industrial wastewater treatment plant. Chem Eng J 322:196–204. https://doi.org/10.1016/j.cej.2017.04.040
Groenen-Serrano K, Weiss-Hortala E, Savall A, Spiteri P (2013) Role of hydroxyl radicals during the competitive electrooxidation of organic compounds on a boron-doped diamond anode. Electrocatalysis 4:346–352. https://doi.org/10.1007/s12678-013-0150-5
Gude VG (2016) Wastewater treatment in microbial fuel cells: an overview. J Clean Prod 122:287–307. https://doi.org/10.1016/j.jclepro.2016.02.022
Hao Y, Ma H, Proietto F et al (2022) Electrochemical treatment of wastewater contaminated by organics and containing chlorides: effect of operative parameters on the abatement of organics and the generation of chlorinated by-products. Electrochim Acta 402:139480. https://doi.org/10.1016/j.electacta.2021.139480
Hayfield PCS (1983) Electrode material, electrode and electrochemical cell. Patent US 4(422):917
He Y, Lin H, Guo Z et al (2019) Recent developments and advances in boron-doped diamond electrodes for electrochemical oxidation of organic pollutants. Sep Purif Technol 212:802–821. https://doi.org/10.1016/j.seppur.2018.11.056
Hu Z, Cai J, Song G et al (2021) Anodic oxidation of organic pollutants: anode fabrication, process hybrid and environmental applications. Curr Opin Electrochem 26:100659. https://doi.org/10.1016/j.coelec.2020.100659
Hulin LP (1897) Process of electrolytic decomposition of solutions. Patent US 586:236
Ibrahim DS, Veerabahu C, Palani R et al (2013) Flow dynamics and mass transfer studies in a tubular electrochemical reactor with a mesh electrode. Comput Fluids 73:97–103. https://doi.org/10.1016/j.compfluid.2012.12.001
Iniesta J, Michaud PA, Panizza M et al (2001a) Electrochemical oxidation of phenol at boron-doped diamond electrode. Electrochim Acta 46:3573–3578. https://doi.org/10.1016/S0013-4686(01)00630-2
Iniesta J, Michaud PA, Panizza M, Comninellis C (2001b) Electrochemical oxidation of 3-methylpyridine at a boron-doped diamond electrode: application to electroorganic synthesis and wastewater treatment. Electrochem Commun 3:346–351. https://doi.org/10.1016/S1388-2481(01)00174-6
Isidro J, Llanos J, Sáez C et al (2018) Can CabECO® technology be used for the disinfection of highly faecal-polluted surface water? Chemosphere 209:346–352. https://doi.org/10.1016/j.chemosphere.2018.06.106
Isidro J, Brackemeyer D, Sáez C et al (2019) Operating the CabECO® membrane electrolytic technology in continuous mode for the direct disinfection of highly fecal-polluted water. Sep Purif Technol 208:110–115. https://doi.org/10.1016/j.seppur.2018.04.070
Jiang H, Lee PS, Li C (2013) 3D carbon based nanostructures for advanced supercapacitors. Energy Environ Sci 6:41–53. https://doi.org/10.1039/c2ee23284g
Jing Y, Chaplin BP (2016) Electrochemical impedance spectroscopy study of membrane fouling characterization at a conductive sub-stoichiometric TiO2 reactive electrochemical membrane: transmission line model development. J Memb Sci 511:238–249. https://doi.org/10.1016/j.memsci.2016.03.032
Jing Y, Chaplin BP (2017) Mechanistic study of the validity of using hydroxyl radical probes to characterize electrochemical advanced oxidation processes. Environ Sci Technol 51:2355–2365. https://doi.org/10.1021/acs.est.6b05513
Jing Y, Guo L, Chaplin BP (2016) Electrochemical impedance spectroscopy study of membrane fouling and electrochemical regeneration at a sub-stoichiometric TiO2 reactive electrochemical membrane. J Memb Sci 510:510–523. https://doi.org/10.1016/j.memsci.2016.03.029
Kapałka A, Fóti G, Comninellis C (2008) Kinetic modelling of the electrochemical mineralization of organic pollutants for wastewater treatment. J Appl Electrochem 38:7–16. https://doi.org/10.1007/s10800-007-9365-6
Kapałka A, Fóti G, Comninellis C (2009) The importance of electrode material in environmental electrochemistry. Formation and reactivity of free hydroxyl radicals on boron-doped diamond electrodes. Electrochim Acta 54:2018–2023. https://doi.org/10.1016/j.electacta.2008.06.045
Khalid YS, Misal SN, Mehraeen S, Chaplin BP (2022) Reactive-transport modeling of electrochemical oxidation of perfluoroalkyl substances in porous flow-through electrodes. ACS ES&T Eng 2:713–725. https://doi.org/10.1021/acsestengg.1c00362
Kiendrebeogo M, Karimi Estahbanati MR, Khosravanipour Mostafazadeh A et al (2021) Treatment of microplastics in water by anodic oxidation: a case study for polystyrene. Environ Pollut 269:116168. https://doi.org/10.1016/j.envpol.2020.116168
Kiendrebeogo M, Karimi Estahbanati MR, Ouarda Y et al (2022) Electrochemical degradation of nanoplastics in water: analysis of the role of reactive oxygen species. Sci Total Environ 808:151897. https://doi.org/10.1016/j.scitotenv.2021.151897
Kim C, Kim S, Choi J et al (2014) Blue TiO2 nanotube array as an oxidant generating novel anode material fabricated by simple cathodic polarization. Electrochim Acta 141:113–119. https://doi.org/10.1016/j.electacta.2014.07.062
Kirk DW, Sharifian H, Foulkes FR (1985) Anodic oxidation of aniline for waste water treatment. J Appl Electrochem 15:285–292. https://doi.org/10.1007/BF00620944
Kislyi A, Moroz I, Guliaeva V et al (2023) Electrochemical oxidation of organic pollutants in aqueous solution using a Ti4O7 particle anode. Membranes (basel) 13:521. https://doi.org/10.3390/membranes13050521
Kiwi J, Lopez A, Nadtochenko V (2000) Mechanism and kinetics of the OH-radical intervention during Fenton oxidation in the presence of a significant amount of radical scavenger (Cl- ). Environ Sci Technol 34:2162–2168. https://doi.org/10.1021/es991406i
Kothari MS, Shah KA (2020) Electrochemical oxidation for decolorization of rhodamine-B dye using mixed metal oxide electrode: modeling and optimization. Water Sci Technol 81:720–731. https://doi.org/10.2166/wst.2020.151
Kötz R, Stucki S, Carcer B (1991) Electrochemical waste water treatment using high overvoltage anodes. Part I: physical and electrochemical properties of SnO2 anodes. J Appl Electrochem 21:14–20. https://doi.org/10.1007/BF01103823
Kuhn AT (1971) Electrolytic decomposition of cyanides, phenols and thiocyanates in effluent streams-a literature review. J Appl Chem Biotechnol 21:29–34. https://doi.org/10.1002/jctb.5020210201
Kumar A, Barbhuiya NH, Singh SP (2022) Magnéli phase titanium sub-oxides synthesis, fabrication and its application for environmental remediation: current status and prospect. Chemosphere 307:135878. https://doi.org/10.1016/j.chemosphere.2022.135878
Lan Y, Coetsier C, Causserand C, Groenen Serrano K (2018) An experimental and modelling study of the electrochemical oxidation of pharmaceuticals using a boron-doped diamond anode. Chem Eng J 333:486–494. https://doi.org/10.1016/j.cej.2017.09.164
Law HD, Perkin FM (1905) Electrolytic oxidation of hydrocarbons of the benzene series. Part I., hydrocarbons, containing the methyl group. Trans Faraday Soc 1:31. https://doi.org/10.1039/tf9050100031
Li D, Tang J, Zhou X et al (2016) Electrochemical degradation of pyridine by Ti/SnO2-Sb tubular porous electrode. Chemosphere 149:49–56. https://doi.org/10.1016/j.chemosphere.2016.01.078
Lin M-H, Bulman DM, Remucal CK, Chaplin BP (2020) Chlorinated byproduct formation during the electrochemical advanced oxidation process at Magnéli phase Ti4O7 electrodes. Environ Sci Technol 54:12673–12683. https://doi.org/10.1021/acs.est.0c03916
Liu H, Vajpayee A, Vecitis CD (2013) Bismuth-doped tin oxide-coated carbon nanotube network: improved anode stability and efficiency for flow-through organic electrooxidation. ACS Appl Mater Interfaces 5:10054–10066. https://doi.org/10.1021/am402621v
Liu J, Ren N, Qu C et al (2022) Recent advances in the reactor design for industrial wastewater treatment by electro-oxidation process. Water 14:3711. https://doi.org/10.3390/w14223711
Łukaszewicz G, Kalita P (2016) Navier-Stokes equations. An Introduction with applications. Springer International Publishing, Switzerland
Ma J, Gao M, Shi H et al (2021) Progress in research and development of particle electrodes for three-dimensional electrochemical treatment of wastewater: a review. Environ Sci Pollut Res 28:47800–47824. https://doi.org/10.1007/s11356-021-13785-x
Ma J, Trellu C, Oturan N et al (2022) Development of Ti/TiOx foams for removal of organic pollutants from water: influence of porous structure of Ti substrate. Appl Catal B Environ 317:121736. https://doi.org/10.1016/j.apcatb.2022.121736
Ma J, Trellu C, Oturan N et al (2023a) Porous Magnéli phase obtained from 3D printing for efficient anodic oxidation process. Chem Eng J 456:141047. https://doi.org/10.1016/j.cej.2022.141047
Ma J, Trellu C, Skolotneva E et al (2023b) Investigating the reactivity of TiOx and BDD anodes for electro-oxidation of organic pollutants by experimental and modeling approaches. Electrochim Acta 439:141513. https://doi.org/10.1016/j.electacta.2022.141513
Magro C, Mateus EP, Paz-Garcia JM, Ribeiro AB (2020) Emerging organic contaminants in wastewater: understanding electrochemical reactors for triclosan and its by-products degradation. Chemosphere 247:125758. https://doi.org/10.1016/j.chemosphere.2019.125758
Mareev S, Skolotneva E, Cretin M, Nikonenko V (2021) Modeling the formation of gas bubbles inside the pores of reactive electrochemical membranes in the process of the anodic oxidation of organic compounds. Int J Mol Sci 22:5477. https://doi.org/10.3390/ijms22115477
Marshall AT, Herritsch A (2018) Understanding how the oxygen evolution reaction kinetics influences electrochemical wastewater oxidation. Electrochim Acta 282:448–458. https://doi.org/10.1016/j.electacta.2018.06.065
Martínez-Huitle CA, Ferro S (2006) Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chem Soc Rev 35:1324–1340. https://doi.org/10.1039/b517632h
Martínez-Huitle CA, Rodrigo MA, Sirés I, Scialdone O (2015) Single and coupled electrochemical processes and reactors for the abatement of organic water pollutants: a critical review. Chem Rev 115:13362–13407. https://doi.org/10.1021/acs.chemrev.5b00361
Martínez-Huitle CA, Scialdone O, Rodrigo MA (2018) Electrochemical water and wastewater treatment. Elsevier Science
Mascia M, Vacca A, Palmas S, Polcaro AM (2007) Kinetics of the electrochemical oxidation of organic compounds at BDD anodes: modelling of surface reactions. J Appl Electrochem 37:71–76. https://doi.org/10.1007/s10800-006-9217-9
Mascia M, Vacca A, Polcaro AM et al (2010) Electrochemical treatment of phenolic waters in presence of chloride with boron-doped diamond (BDD) anodes: experimental study and mathematical model. J Hazard Mater 174:314–322. https://doi.org/10.1016/j.jhazmat.2009.09.053
Mascia M, Vacca A, Palmas S (2012) Fixed bed reactors with three dimensional electrodes for electrochemical treatment of waters for disinfection. Chem Eng J 211–212:479–487. https://doi.org/10.1016/j.cej.2012.09.091
Mascia M, Vacca A, Palmas S (2013) Electrochemical treatment as a pre-oxidative step for algae removal using Chlorella vulgaris as a model organism and BDD anodes. Chem Eng J 219:512–519. https://doi.org/10.1016/j.cej.2012.12.097
Mascia M, Monasterio S, Vacca A, Palmas S (2016) Electrochemical treatment of water containing Microcystis aeruginosa in a fixed bed reactor with three-dimensional conductive diamond anodes. J Hazard Mater 319:111–120. https://doi.org/10.1016/j.jhazmat.2016.03.004
McBeath ST, Wilkinson DP, Graham NJD (2019) Application of boron-doped diamond electrodes for the anodic oxidation of pesticide micropollutants in a water treatment process: a critical review. Environ Sci Water Res Technol 5:2090–2107. https://doi.org/10.1039/C9EW00589G
Mengelizadeh N, Pourzamani H, Saloot MK et al (2019) Electrochemical degradation of reactive black 5 using three-dimensional electrochemical system based on multiwalled carbon nanotubes. J Environ Eng 145:04019021. https://doi.org/10.1061/(ASCE)EE.1943-7870.0001517
Mieluch J, Sadkowski A, Wild J, Zoltowski P (1975) Electrochemical oxidation of phenolic compounds in aqueous solutions [Elektrochemiczne utlenianie zwiazkow fenolowych w roztworze wodnym]. Przem Chem 54:513–516
Misal SN, Lin M-H, Mehraeen S, Chaplin BP (2020) Modeling electrochemical oxidation and reduction of sulfamethoxazole using electrocatalytic reactive electrochemical membranes. J Hazard Mater 384:121420. https://doi.org/10.1016/j.jhazmat.2019.121420
Monteil H, Pechaud Y, Oturan N et al (2021) Pilot scale continuous reactor for water treatment by electrochemical advanced oxidation processes: development of a new hydrodynamic/reactive combined model. Chem Eng J 404:127048. https://doi.org/10.1016/j.cej.2020.127048
Mora-Gómez J, García-Gabaldón M, Carrillo-Abad J et al (2020) Influence of the reactor configuration and the supporting electrolyte concentration on the electrochemical oxidation of Atenolol using BDD and SnO2 ceramic electrodes. Sep Purif Technol 241:116684. https://doi.org/10.1016/j.seppur.2020.116684
Moreira FC, Boaventura RAR, Brillas E, Vilar VJP (2017) Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters. Appl Catal B Environ 202:217–261. https://doi.org/10.1016/j.apcatb.2016.08.037
Mostafa E, Reinsberg P, Garcia-Segura S, Baltruschat H (2018) Chlorine species evolution during electrochlorination on boron-doped diamond anodes: in-situ electrogeneration of Cl2, Cl2O and ClO2. Electrochim Acta 281:831–840. https://doi.org/10.1016/j.electacta.2018.05.099
Mousset E (2022) Interest of micro-reactors for the implementation of advanced electrocatalytic oxidation with boron-doped diamond anode for wastewater treatment. Curr Opin Electrochem 32:100897. https://doi.org/10.1016/j.coelec.2021.100897
Najafinejad MS, Chianese S, Fenti A et al (2023) Application of electrochemical oxidation for water and wastewater treatment: an overview. Molecules 28:4208. https://doi.org/10.3390/molecules28104208
Nakayama S, Esaki K, Namba K et al (1979) Improved ozonation in aqueous systems. Ozone Sci Eng 1:119–131. https://doi.org/10.1080/01919517908550839
Nava JL, Núñez F, González I (2007) Electrochemical incineration of p-cresol and o-cresol in the filter-press-type FM01-LC electrochemical cell using BDD electrodes in sulfate media at pH 0. Electrochim Acta 52:3229–3235. https://doi.org/10.1016/j.electacta.2006.09.072
Nava JL, Recéndiz A, Acosta JC, González I (2008) Electrochemical incineration of vinasse in filter-press-type FM01-LC reactor using 3D BDD electrode. Water Sci Technol 58:2413–2419. https://doi.org/10.2166/wst.2008.558
Nava JL, Sirés I, Brillas E (2014) Electrochemical incineration of indigo. A comparative study between 2D (plate) and 3D (mesh) BDD anodes fitted into a filter-press reactor. Environ Sci Pollut Res 21:8485–8492. https://doi.org/10.1007/s11356-014-2781-3
Neodo S, Rosestolato D, Ferro S, De Battisti A (2012) On the electrolysis of dilute chloride solutions: influence of the electrode material on Faradaic efficiency for active chlorine, chlorate and perchlorate. Electrochim Acta 80:282–291. https://doi.org/10.1016/j.electacta.2012.07.017
Newman J, Tiedemann W (1975) Porous-electrode theory with battery applications. AIChE J 21:25–41. https://doi.org/10.1002/aic.690210103
Nilsson A, Ronlán A, Parker VD (1973) Anodic oxidation of phenolic compounds. Part III. Anodic hydroxylation of phenols. A simple general synthesis of 4-alkyl-4-hydroxycyclo-hexa-2,5-dienones from 4-alkylphenols. J Chem Soc Perkin Trans 1:2337–2345. https://doi.org/10.1039/P19730002337
Oxley JE, Johnson GK, Buzalski BT (1964) The anodic oxidation of methanol: open-circuit transient reactions. Electrochim Acta 9:897–910. https://doi.org/10.1016/0013-4686(64)85040-4
Panizza M, Cerisola G (2009) Direct and mediated anodic oxidation of organic pollutants. Chem Rev 109:6541–6569. https://doi.org/10.1021/cr9001319
Panizza M, Michaud PA, Cerisola G, Comninellis C (2001a) Anodic oxidation of 2-naphthol at boron-doped diamond electrodes. J Electroanal Chem 507:206–214. https://doi.org/10.1016/S0022-0728(01)00398-9
Panizza M, Michaud PA, Cerisola G, Comninellis CH (2001b) Electrochemical treatment of wastewaters containing organic pollutants on boron-doped diamond electrodes: prediction of specific energy consumption and required electrode area. Electrochem Commun 3:336–339. https://doi.org/10.1016/S1388-2481(01)00166-7
Panizza M, Kapałka A, Comninellis C (2008) Oxidation of organic pollutants on BDD anodes using modulated current electrolysis. Electrochim Acta 53:2289–2295. https://doi.org/10.1016/j.electacta.2007.09.044
Papouchado L, Sandford RW, Petrie G, Adams RN (1975) Anodic oxidation pathways of phenolic compounds Part 2. Stepwise electron transfers and coupled hydroxylations. J Electroanal Chem 65:275–284. https://doi.org/10.1016/0368-1874(75)85123-9
Periyasamy S, Lin X, Ganiyu SO et al (2022) Insight into BDD electrochemical oxidation of florfenicol in water: kinetics, reaction mechanism, and toxicity. Chemosphere 288:132433. https://doi.org/10.1016/j.chemosphere.2021.132433
Piersma BJ, Gileadi E (1966) The mechanism of electrochemical oxidation of organic fuels. In: Modern Aspects of Electrochemistry No. 4. Springer US, Boston, MA, pp 47–175
Pillai IMS, Gupta AK (2015) Batch and continuous flow anodic oxidation of 2,4-dinitrophenol: modeling, degradation pathway and toxicity. J Electroanal Chem 756:108–117. https://doi.org/10.1016/j.jelechem.2015.08.020
Polcaro AM, Palmas S (1997) Electrochemical oxidation of chlorophenols. Ind Eng Chem Res 36:1791–1798. https://doi.org/10.1021/ie960557g
Polcaro AM, Palmas S, Renoldi F, Mascia M (1999) On the performance of Ti/SnO2 and Ti/PbO2 anodes in electrochemical degradation of 2-chlorophenol for wastewater treatment. J Appl Electrochem 29:147–151. https://doi.org/10.1023/A:1003411906212
Polcaro AM, Vacca A, Palmas S, Mascia M (2003) Electrochemical treatment of wastewater containing phenolic compounds: oxidation at boron-doped diamond electrodes. J Appl Electrochem 33:885–892. https://doi.org/10.1023/A:1025815828503
Polcaro AM, Vacca A, Mascia M et al (2009) Electrochemical treatment of waters with BDD anodes: kinetics of the reactions involving chlorides. J Appl Electrochem 39:2083–2092. https://doi.org/10.1007/s10800-009-9870-x
Popović ND, Johnson DC (1998) A ring−disk study of the competition between anodic oxygen-transfer and dioxygen-evolution reactions. Anal Chem 70:468–472. https://doi.org/10.1021/ac9707803
Popović NĐ, Cox JA, Johnson DC (1998) A mathematical model for anodic oxygen-transfer reactions at Bi(V)-doped PbO2-film electrodes. J Electroanal Chem 456:203–209. https://doi.org/10.1016/S0022-0728(98)00219-8
Porada S, Zhao R, van der Wal A et al (2013) Review on the science and technology of water desalination by capacitive deionization. Prog Mater Sci 58:1388–1442. https://doi.org/10.1016/j.pmatsci.2013.03.005
Qi G, Wang X, Zhao J et al (2022) Fabrication and characterization of the porous Ti4O7 reactive electrochemical membrane. Front Chem 9:1–11. https://doi.org/10.3389/fchem.2021.833024
Radjenovic J, Duinslaeger N, Avval SS, Chaplin BP (2020) Facing the challenge of poly- and perfluoroalkyl substances in water: Is electrochemical oxidation the answer? Environ Sci Technol 54:14815–14829. https://doi.org/10.1021/acs.est.0c06212
Ricardo IA, Alberto EA, Silva Júnior AH et al (2021) A critical review on microplastics, interaction with organic and inorganic pollutants, impacts and effectiveness of advanced oxidation processes applied for their removal from aqueous matrices. Chem Eng J 424:130282. https://doi.org/10.1016/j.cej.2021.130282
Rivera FF, Cruz-Díaz MR, Rivero EP, González I (2010) Analysis and interpretation of residence time distribution experimental curves in FM01-LC reactor using axial dispersion and plug dispersion exchange models with closed–closed boundary conditions. Electrochim Acta 56:361–371. https://doi.org/10.1016/j.electacta.2010.08.069
Rivera FF, de León CP, Nava JL, Walsh FC (2015a) The filter-press FM01-LC laboratory flow reactor and its applications. Electrochim Acta 163:338–354. https://doi.org/10.1016/j.electacta.2015.02.179
Rivera FF, de León CP, Walsh FC, Nava JL (2015b) The reaction environment in a filter-press laboratory reactor: the FM01-LC flow cell. Electrochim Acta 161:436–452. https://doi.org/10.1016/j.electacta.2015.02.161
Rivera FF, Pérez T, Castañeda LF, Nava JL (2021) Mathematical modeling and simulation of electrochemical reactors: a critical review. Chem Eng Sci 239:116622. https://doi.org/10.1016/j.ces.2021.116622
Rivero EP, Rivera FF, Cruz-Díaz MR et al (2012) Numerical simulation of mass transport in a filter press type electrochemical reactor FM01-LC: comparison of predicted and experimental mass transfer coefficient. Chem Eng Res Des 90:1969–1978. https://doi.org/10.1016/j.cherd.2012.04.010
Rivero EP, Rodríguez FA, Cruz-Díaz MR, González I (2018) Reactive diffusion migration layer and mass transfer wall function to model active chlorine generation in a filter press type electrochemical reactor for organic pollutant degradation. Chem Eng Res Des 138:533–545. https://doi.org/10.1016/j.cherd.2018.07.010
Rodgers JD, Jedral W, Bunce NJ (1999) Electrochemical oxidation of chlorinated phenols. Environ Sci Technol 33:1453–1457. https://doi.org/10.1021/es9808189
Rodrigo MA, Michaud PA, Duo I et al (2001) Oxidation of 4-chlorophenol at boron-doped diamond electrode for wastewater treatment. J Electrochem Soc 148:60–64. https://doi.org/10.1149/1.1362545
Rodriguez S, Santos A, Romero A, Vicente F (2012) Kinetic of oxidation and mineralization of priority and emerging pollutants by activated persulfate. Chem Eng J 213:225–234. https://doi.org/10.1016/j.cej.2012.09.077
Rosestolato D, Fregoni J, Ferro S, De Battisti A (2014) Influence of the nature of the electrode material and process variables on the kinetics of the chlorine evolution reaction. The case of IrO2-based electrocatalysts. Electrochim Acta 139:180–189. https://doi.org/10.1016/j.electacta.2014.07.037
Roy Ghatak H (2020) Simulated process integration of wastewater electrooxidation with recuperated micro gas turbine for energy recovery. Int J Hydrog Energy 45:31466–31480. https://doi.org/10.1016/j.ijhydene.2020.08.161
Russo D (2021) Kinetic modeling of advanced oxidation processes using microreactors: challenges and opportunities for scale-up. Appl Sci 11:1042. https://doi.org/10.3390/app11031042
Saleh MM (2007) Simulation of oxygen evolution reaction at porous anode from flowing electrolytes. J Solid State Electrochem 11:811–820. https://doi.org/10.1007/s10008-006-0227-7
Saleh MM (2009) Effects of gas bubbles on the concentration profiles and conversion efficiency of three-dimensional packed-bed electrodes. J Solid State Electrochem 13:343–351. https://doi.org/10.1007/s10008-008-0545-z
Saleh MM, Awad MI, Kitamura F, Ohsaka T (2006) Mathematical modeling of gas evolution from flowing electrolytes on stable porous anodes of finite matrix phase conductivity. Electrochim Acta 51:6331–6337. https://doi.org/10.1016/j.electacta.2006.04.017
Salvestrini S, Fenti A, Chianese S et al (2020) Electro-oxidation of humic acids using platinum electrodes: an experimental approach and kinetic modelling. Water 12:2250. https://doi.org/10.3390/w12082250
Sandoval MA, Calzadilla W, Salazar R (2022) Influence of reactor design on the electrochemical oxidation and disinfection of wastewaters using boron-doped diamond electrodes. Curr Opin Electrochem 33:100939. https://doi.org/10.1016/j.coelec.2022.100939
Scialdone O, Proietto F, Galia A (2021) Electrochemical production and use of chlorinated oxidants for the treatment of wastewater contaminated by organic pollutants and disinfection. Curr Opin Electrochem 27:100682. https://doi.org/10.1016/j.coelec.2020.100682
Seibert D, Zorzo CF, Borba FH et al (2020) Occurrence, statutory guideline values and removal of contaminants of emerging concern by electrochemical advanced oxidation processes: a review. Sci Total Environ 748:141527. https://doi.org/10.1016/j.scitotenv.2020.141527
Serrano K, Michaud PA, Comninellis C, Savall A (2002) Electrochemical preparation of peroxodisulfuric acid using boron doped diamond thin film electrodes. Electrochim Acta 48:431–436. https://doi.org/10.1016/S0013-4686(02)00688-6
Shahid MK, Kashif A, Fuwad A, Choi Y (2021) Current advances in treatment technologies for removal of emerging contaminants from water: a critical review. Coord Chem Rev 442:213993. https://doi.org/10.1016/j.ccr.2021.213993
Sharifian H, Kirk DW (1986) Electrochemical oxidation of phenol. J Electrochem Soc 133:921–924. https://doi.org/10.1149/1.2108763
Simond O, Comninellis C (1997) Anodic oxidation of organics on Ti/IrO2 anodes using Nafion® as electrolyte. Electrochim Acta 42:2013–2018. https://doi.org/10.1016/S0013-4686(97)85476-X
Simond O, Schaller V, Comninellis C (1997) Theoretical model for the anodic oxidation of organics on metal oxide electrodes. Electrochim Acta 42:2009–2012. https://doi.org/10.1016/S0013-4686(97)85475-8
Sirés I, Brillas E, Oturan MA et al (2014) Electrochemical advanced oxidation processes: today and tomorrow. A review. Environ Sci Pollut Res 21:8336–8367. https://doi.org/10.1007/s11356-014-2783-1
Skban Ibrahim D, Seethala Devi P, Veerababhu C, Balasubramanian N (2014) Treatment of petroleum effluent using a tubular electrochemical reactor. Pet Sci Technol 32:1932–1939. https://doi.org/10.1080/10916466.2012.701693
Skolotneva E, Trellu C, Cretin M, Mareev S (2020) A 2D convection-diffusion model of anodic oxidation of organic compounds mediated by hydroxyl radicals using porous reactive electrochemical membrane. Membranes (basel) 10:7–10. https://doi.org/10.3390/membranes10050102
Skolotneva E, Cretin M, Mareev S (2021) A simple 1D convection-diffusion model of oxalic acid oxidation using reactive electrochemical membrane. Membranes (basel) 11:1–14. https://doi.org/10.3390/membranes11060431
Smith JR, Walsh FC, Clarke RL (1998) Electrodes based on Magnéli phase titanium oxides: the properties and applications of Ebonex® materials. J Appl Electrochem 28:1021–1033. https://doi.org/10.1023/A:1003469427858
Sowmiya S, Gandhimathi R, Ramesh ST, Nidheesh PV (2016) Granular activated carbon as a particle electrode in three-dimensional electrochemical treatment of Reactive Black B from aqueous solution. Environ Prog Sustain Energy 35:1616–1622. https://doi.org/10.1002/ep.12396
Sun Y-F, Liu S-B, Meng F-L et al (2012) Metal oxide nanostructures and their gas sensing properties: a review. Sensors 12:2610–2631. https://doi.org/10.3390/s120302610
Sun X, Wu J, Chen Z et al (2013) Fouling characteristics and electrochemical recovery of carbon nanotube membranes. Adv Funct Mater 23:1500–1506. https://doi.org/10.1002/adfm.201201265
Taqieddin A, Allshouse MR, Alshawabkeh AN (2018) Editors’ choice—critical review—mathematical formulations of electrochemically gas-evolving systems. J Electrochem Soc 165:E694–E711. https://doi.org/10.1149/2.0791813jes
Titchou FE, Zazou H, Afanga H et al (2021) An overview on the elimination of organic contaminants from aqueous systems using electrochemical advanced oxidation processes. J Water Process Eng 41:102040. https://doi.org/10.1016/j.jwpe.2021.102040
Trainham JA, Newman J (1977) A flow-through porous electrode model: application to metal-ion removal from dilute streams. J Electrochem Soc 124:1528–1540. https://doi.org/10.1149/1.2133106
Trellu C, Péchaud Y, Oturan N et al (2016) Comparative study on the removal of humic acids from drinking water by anodic oxidation and electro-Fenton processes: mineralization efficiency and modelling. Appl Catal B Environ 194:32–41. https://doi.org/10.1016/j.apcatb.2016.04.039
Trellu C, Chaplin BP, Coetsier C et al (2018a) Electro-oxidation of organic pollutants by reactive electrochemical membranes. Chemosphere 208:159–175. https://doi.org/10.1016/j.chemosphere.2018.05.026
Trellu C, Coetsier C, Rouch J-C et al (2018b) Mineralization of organic pollutants by anodic oxidation using reactive electrochemical membrane synthesized from carbothermal reduction of TiO2. Water Res 131:310–319. https://doi.org/10.1016/j.watres.2017.12.070
Trellu C, Olvera Vargas H, Mousset E et al (2021) Electrochemical technologies for the treatment of pesticides. Curr Opin Electrochem 26:100677. https://doi.org/10.1016/j.coelec.2020.100677
Trinidad P, Walsh FC (1996) Hydrodynamic behaviour of the FM01-LC reactor. Electrochim Acta 41:493–502. https://doi.org/10.1016/0013-4686(95)00335-5
Trinidad P, Ponce de León C, Walsh FC (2006) The application of flow dispersion models to the FM01-LC laboratory filter-press reactor. Electrochim Acta 52:604–613. https://doi.org/10.1016/j.electacta.2006.05.040
Vasconcelos VM, Ponce-de-León C, Nava JL, Lanza MRV (2016) Electrochemical degradation of RB-5 dye by anodic oxidation, electro-Fenton and by combining anodic oxidation–electro-Fenton in a filter-press flow cell. J Electroanal Chem 765:179–187. https://doi.org/10.1016/j.jelechem.2015.07.040
Vecitis CD, Gao G, Liu H (2011) Electrochemical carbon nanotube filter for adsorption, desorption, and oxidation of aqueous dyes and anions. J Phys Chem C 115:3621–3629. https://doi.org/10.1021/jp111844j
Velazquez-Peña S, Sáez C, Cañizares P et al (2013) Production of oxidants via electrolysis of carbonate solutions with conductive-diamond anodes. Chem Eng J 230:272–278. https://doi.org/10.1016/j.cej.2013.06.078
Vijayakumar V, Saravanathamizhan R, Balasubramanian N (2016) Electro oxidation of dye effluent in a tubular electrochemical reactor using TiO2/RuO2 anode. J Water Process Eng 9:155–160. https://doi.org/10.1016/j.jwpe.2015.12.006
Vu A, Qian Y, Stein A (2012) Porous electrode materials for lithium-ion batteries-how to prepare them and what makes them special. Adv Energy Mater 2:1056–1085. https://doi.org/10.1002/aenm.201200320
Walcarius A (2012) Electrocatalysis, sensors and biosensors in analytical chemistry based on ordered mesoporous and macroporous carbon-modified electrodes. TrAC Trends Anal Chem 38:79–97. https://doi.org/10.1016/j.trac.2012.05.003
Wang J, Li T, Zhou M et al (2015) Characterization of hydrodynamics and mass transfer in two types of tubular electrochemical reactors. Electrochim Acta 173:698–704. https://doi.org/10.1016/j.electacta.2015.05.135
Wang Z, Li J, He X et al (2019) Organic pollutants removal performance and enhanced mechanism investigation of surface-modified steel slag particle electrode. Environ Prog Sustain Energy 38:7–14. https://doi.org/10.1002/ep.12910
Wang L, Wang L, Shi Y et al (2022) Blue TiO2 nanotube electrocatalytic membrane electrode for efficient electrochemical degradation of organic pollutants. Chemosphere 306:135628. https://doi.org/10.1016/j.chemosphere.2022.135628
Wei X, Wang H, Yin Z et al (2017) Tubular electrocatalytic membrane reactor for alcohol oxidation: CFD simulation and experiment. Chin J Chem Eng 25:18–25. https://doi.org/10.1016/j.cjche.2016.05.020
Wei K, Cui T, Huang F et al (2020) Membrane separation coupled with electrochemical advanced oxidation processes for organic wastewater treatment: a short review. Membranes (basel) 10:1–29. https://doi.org/10.3390/membranes10110337
Wu ZH, Chen HB, Dong YM et al (2008) Cleaning using nanobubbles: defouling by electrochemical generation of bubbles. J Colloid Interface Sci 328:10–14. https://doi.org/10.1016/j.jcis.2008.08.064
Xu SG (2016) Modeling and experimental study of electrochemical oxidation of organics on boron-doped diamond anode. CNL Nucl Rev 7:103–117. https://doi.org/10.12943/CNR.2016.00018
Xu A, Han W, Li J et al (2016) Electrogeneration of hydrogen peroxide using Ti/IrO2–Ta2O5 anode in dual tubular membranes electro-Fenton reactor for the degradation of tricyclazole without aeration. Chem Eng J 295:152–159. https://doi.org/10.1016/j.cej.2016.03.001
Yan L, Ma H, Wang B et al (2011) Electrochemical treatment of petroleum refinery wastewater with three-dimensional multi-phase electrode. Desalination 276:397–402. https://doi.org/10.1016/j.desal.2011.03.083
Yang Y (2020) Recent advances in the electrochemical oxidation water treatment: spotlight on byproduct control. Front Environ Sci Eng 14:85. https://doi.org/10.1007/s11783-020-1264-7
Yin X, Li W, Zhu H et al (2023) Electrochemical oxidation of bio-treated landfill leachate using a novel dynamic reactive electrochemical membrane (DREM). J Hazard Mater 446:130745. https://doi.org/10.1016/J.JHAZMAT.2023.130745
Zaky AM, Chaplin BP (2013) Porous substoichiometric TiO2 anodes as reactive electrochemical membranes for water treatment. Environ Sci Technol 47:6554–6563. https://doi.org/10.1021/es401287e
Zaky AM, Chaplin BP (2014) Mechanism of p-substituted phenol oxidation at a Ti4O7 reactive electrochemical membrane. Environ Sci Technol 48:5857–5867. https://doi.org/10.1021/es5010472
Zhang Y, Wei K, Han W et al (2016) Improved electrochemical oxidation of tricyclazole from aqueous solution by enhancing mass transfer in a tubular porous electrode electrocatalytic reactor. Electrochim Acta 189:1–8. https://doi.org/10.1016/j.electacta.2015.10.119
Zhang L, Ma C, Liu L et al (2019) Fabrication of novel particle electrode γ-Al2O3@ZIF-8 and its application for degradation of Rhodamine B. Water Sci Technol 80:109–116. https://doi.org/10.2166/wst.2019.251
Zhang J, Zhou Y, Yao B et al (2021) Current progress in electrochemical anodic-oxidation of pharmaceuticals: mechanisms, influencing factors, and new technique. J Hazard Mater 418:126313. https://doi.org/10.1016/j.jhazmat.2021.126313
Zhang Y, Ding J, Gao Q et al (2022) Synthesis of low-cost Ti4O7 membrane electrode for electrooxidation of tetracycline under flow-through conditions: performance, kinetics and mechanism. Process Saf Environ Prot 159:931–943. https://doi.org/10.1016/j.psep.2022.01.068
Zhao X, Ren H, Luo L (2019) Gas bubbles in electrochemical gas evolution reactions. Langmuir 35:5392–5408. https://doi.org/10.1021/acs.langmuir.9b00119
Zhou M, Oturan MA, Sirés I (2018) Electro-Fenton process. New trends and scale-up. Springer Singapore
Zhu L, Liu X-Q, Jiang H-L, Sun L-B (2017) Metal–organic frameworks for heterogeneous basic catalysis. Chem Rev 117:8129–8176. https://doi.org/10.1021/acs.chemrev.7b00091
Funding
This research was funded by Russian Science Foundation, project No. 22-79-10177.
Author information
Authors and Affiliations
Contributions
ES, DC, MP and SM contributed to conceptualization and writing—review and editing; ES and SM contributed to methodology; ES, AK and AK contributed to software, project administration, funding, visualization and investigation; SM contributed to resources; ES, AK, AK and SM contributed to writing—original draft preparation; and SM, DC and MP supervised the study. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Skolotneva, E., Kislyi, A., Klevtsova, A. et al. Mathematical modeling of the anodic oxidation of organic pollutants: a review. Environ Chem Lett 22, 1521–1561 (2024). https://doi.org/10.1007/s10311-023-01693-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-023-01693-0