Abstract
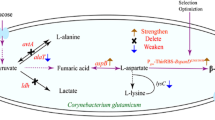
Corynebacterium glutamicum ATCC13032 and Brevibacterium flavum JV16 were engineered for l-valine production by over-expressing ilvEBN r C genes at 31 °C in 72 h fermentation. Different strategies were carried out to reduce the by-products’ accumulation in l-valine fermentation and also to increase the availability of precursor for l-valine biosynthesis. The native promoter of ilvA of C. glutamicum was replaced with a weak promoter MPilvA (P-ilvAM1CG) to reduce the biosynthetic rate of l-isoleucine. Effect of different relative dissolved oxygen on l-valine production and by-products’ formation was recorded, indicating that 15 % saturation may be the most appropriate relative dissolved oxygen for l-valine fermentation with almost no l-lactic acid and l-glutamate formed. To minimize l-alanine accumulation, alaT and/or avtA was inactivated in C. glutamicum and B. flavum, respectively. Compared to high concentration of l-alanine accumulated by alaT inactivated strains harboring ilvEBN r C genes, l-alanine concentration was reduced to 0.18 g/L by C. glutamicum ATCC13032MPilvA△avtA pDXW-8-ilvEBN r C, and 0.22 g/L by B. flavum JV16avtA::Cm pDXW-8-ilvEBN r C. Meanwhile, l-valine production and conversion efficiency were enhanced to 31.15 g/L and 0.173 g/g by C. glutamicum ATCC13032MPilvA△avtA pDXW-8-ilvEBN r C, 38.82 g/L and 0.252 g/g by B. flavum JV16avtA::Cm pDXW-8-ilvEBN r C. This study provides combined strategies to improve l-valine yield by minimization of by-products’ production.





Similar content being viewed by others
References
Almaas E, Kovacs B, Vicsek T, Oltvai ZN, Barabasi AL (2004) Global organization of metabolic fluxes in the bacterium Escherichia coli. Nature 427(6977):839–843. doi:10.1038/nature02289
Ausubel FM, Brent R, Klingston RE, Moore DD, Deidman JD, Smith JA, Struhl K (2005) Recombineering: genetic engineering in bacteria using homologous recombination. In: Thomason L, Court DL, Bubunenko M, Constantino N, Wilson H, Datta S, Oppenheim A (eds) Current protocols in molecular biology. John Wiley & Sons, New York, p 1.16.11–1.16.21
Becker J, Zelder O, Hafner S, Schroder H, Wittmann C (2011) From zero to hero–design-based systems metabolic engineering of Corynebacterium glutamicum for l-lysine production. Metab Eng 13(2):159–168. doi:10.1016/j.ymben.2011.01.003
Blombach B, Schreiner ME, Holatko J, Bartek T, Oldiges M, Eikmanns BJ (2007) l-Valine production with pyruvate dehydrogenase complex-deficient Corynebacterium glutamicum. Appl Environ Microbiol 73(7):2079–2084. doi:10.1128/AEM.02826-06
Blombach B, Schreiner ME, Bartek T, Oldiges M, Eikmanns BJ (2008) Corynebacterium glutamicum tailored for high-yield l-valine production. Appl Microbiol Biotechnol 79(3):471–479. doi:10.1007/s00253-008-1444-z
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97(12):6640–6645. doi:10.1073/pnas.120163297
Eggeling L (2001) Amino acids. In: Ratledge C, Kristiansen B (eds) Basic biotechnology. Cambridge University Press, London, pp 281–303
Eggeling L, Bott M (2005) Handbook of Corynebacterium glutamicum. CRC, Boca Raton
Elisakova V, Patek M, Holatko J, Nesvera J, Leyval D, Goergen JL, Delaunay S (2005) Feedback-resistant acetohydroxy acid synthase increases valine production in Corynebacterium glutamicum. Appl Environ Microbiol 71(1):207–213. doi:10.1128/AEM.71.1.207-213.2005
Hammer K, Mijakovic I, Jensen PR (2006) Synthetic promoter libraries–tuning of gene expression. Trends Biotechnol 24(2):53–55. doi:10.1016/j.tibtech.2005.12.003
Hermann T (2003) Industrial production of amino acids by coryneform bacteria. J Biotechnol 104(1–3):155–172. doi:10.1016/S0168-1656(03)00149-4
Holatko J, Elisakova V, Prouza M, Sobotka M, Nesvera J, Patek M (2009) Metabolic engineering of the l-valine biosynthesis pathway in Corynebacterium glutamicum using promoter activity modulation. J Biotechnol 139(3):203–210. doi:10.1016/j.jbiotec.2008.12.005
Hou X, Ge X, Wu D, Qian H, Zhang W (2012) Improvement of l-valine production at high temperature in Brevibacterium flavum by overexpressing ilvEBN r C genes. J Ind Microbiol Biotechnol 39(1):63–72. doi:10.1007/s10295-011-1000-1
Ikeda M (2003) Amino acid production processes. Adv Biochem Eng Biotechnol 79:1–35
Lee KH, Park JH, Kim TY, Kim HU, Lee SY (2007) Systems metabolic engineering of Escherichia coli for l-threonine production. Mol Syst Biol 3:149. doi:10.1038/msb4100196
Leuchtenberger W, Huthmacher K, Drauz K (2005) Biotechnological production of amino acids and derivatives: current status and prospects. Appl Microbiol Biotechnol 69(1):1–8. doi:10.1007/s00253-005-0155-y
Lindroth P, Mopper K (1979) High performance liquid chromatographic determination of subpicomole amounts of amino acids by precolumn fluorescence derivatization with o-phthalaldehyde. Anal Chem 51:1667–1674
Marienhagen J, Eggeling L (2008) Metabolic function of Corynebacterium glutamicum aminotransferases AlaT and AvtA and impact on l-valine production. Appl Environ Microbiol 74(24):7457–7462. doi:10.1128/AEM.01025-08
Marienhagen J, Kennerknecht N, Sahm H, Eggeling L (2005) Functional analysis of all aminotransferase proteins inferred from the genome sequence of Corynebacterium glutamicum. J Bacteriol 187(22):7639–7646. doi:10.1128/Jb.187.22.7639-7646.2005
Park JH, Lee SY (2010) Fermentative production of branched chain amino acids: a focus on metabolic engineering. Appl Microbiol Biotechnol 85(3):491–506. doi:10.1007/s00253-009-2307-y
Park JH, Lee KH, Kim TY, Lee SY (2007) Metabolic engineering of Escherichia coli for the production of l-valine based on transcriptome analysis and in silico gene knockout simulation. Proc Natl Acad Sci U S A 104(19):7797–7802. doi:10.1073/pnas.0702609104
Patek M (2007) Branched-chain amino acid. In: VF Wendisch (ed) Amino acid biosynthesis. Springer, Berlin Heidelberg, p 130–162
Radmacher E, Vaitsikova A, Burger U, Krumbach K, Sahm H, Eggeling L (2002) Linking central metabolism with increased pathway flux: l-valine accumulation by Corynebacterium glutamicum. Appl Environ Microbiol 68(5):2246–2250. doi:10.1128/AEM.68.5.2246-2250.2002
Sahm H, Eggeling L (1999) D-Pantothenate synthesis in Corynebacterium glutamicum and use of panBC and genes encoding l-valine synthesis for d-pantothenate overproduction. Appl Environ Microbiol 65(5):1973–1979
Sambrook J, Russel DV (2001) Molecular cloning, a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Schafer A, Tauch A, Jager W, Kalinowski J, Thierbach G, Puhler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145(1):69–73
Solem C, Koebmann B, Yang F, Jensen PR (2007) The las enzymes control pyruvate metabolism in Lactococcus lactis during growth on maltose. J Bacteriol 189(18):6727–6730. doi:10.1128/JB.00902-07
Xu DQ, Tan YZ, Huan XJ, Hu XQ, Wang XY (2010) Construction of a novel shuttle vector for use in Brevibacterium flavum, an industrial amino acid producer. J Microbiol Meth 80(1):86–92. doi:10.1016/j.mimet.2009.11.003
Yamada K, Kinoshita S, Tsunoda T, Aida K (1972) The microbial production of amino acids. Halsted Press, New York
Zimmermann HF, Anderlei T, Buchs J, Binder M (2006) Oxygen limitation is a pitfall during screening for industrial strains. Appl Microbiol Biotechnol 72(6):1157–1160. doi:10.1007/s00253-006-0414-6
Acknowledgments
This work was financially supported by the Program of Chinese 863 National High-Tech Research and Development Plan Project (No. 2008AA02Z212).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, X., Chen, X., Zhang, Y. et al. l-Valine production with minimization of by-products’ synthesis in Corynebacterium glutamicum and Brevibacterium flavum . Amino Acids 43, 2301–2311 (2012). https://doi.org/10.1007/s00726-012-1308-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-012-1308-9