Abstract
The mechanical behaviour of solid biological tissues has long been described using models based on classical continuum mechanics. However, the classical continuum theories of elasticity and viscoelasticity cannot easily capture the continual remodelling and associated structural changes in biological tissues. Furthermore, models drawn from plasticity theory are difficult to apply and interpret in this context, where there is no equivalent of a yield stress or flow rule. In this work, we describe a novel one-dimensional mathematical model of tissue remodelling based on the multiplicative decomposition of the deformation gradient. We express the mechanical effects of remodelling as an evolution equation for the effective strain, a measure of the difference between the current state and a hypothetical mechanically relaxed state of the tissue. This morphoelastic model combines the simplicity and interpretability of classical viscoelastic models with the versatility of plasticity theory. A novel feature of our model is that while most models describe growth as a continuous quantity, here we begin with discrete cells and develop a continuum representation of lattice remodelling based on an appropriate limit of the behaviour of discrete cells. To demonstrate the utility of our approach, we use this framework to capture qualitative aspects of the continual remodelling observed in fibroblast-populated collagen lattices, in particular its contraction and its subsequent sudden re-expansion when remodelling is interrupted.







Similar content being viewed by others
References
Amadeu TP, Coulomb B, Desmoulière A, Costa AMA (2003) Cutaneous wound healing: myofibroblastic differentiation and in vitro models. Int J Low Extrem Wounds 2(2):60–68. doi:10.1177/1534734603256155
Ambrosi D, Mollica F (2004) The role of stress in the growth of a multicell spheroid. J Math Biol 48(5):477–499. doi:10.1007/s00285-003-0238-2
Ambrosi D, Guana F (2007) Stress modulated growth. Math Mech Solids 12(3):319–343. doi:10.1177/1081286505059739
Ambrosi D, Guillou A (2007) Growth and dissipation in biological tissues. Continuum Mech Therm 19(5):245–251. doi:10.1007/s00161-007-0052-y
Ambrosi D, Ateshian GA, Arruda EM, Cowin SC, Dumais J, Goriely A, Holzapfel GA, Humphrey JD, Kemkemer R, Kuhl E, Olberding JE, Taber LA, Garikipati K (2011) Perspectives on biological growth and remodeling. J Mech Phys Solids 59(4):863–883. doi:10.1016/j.jmps.2010.12.011
Arora PD, Narani N, McCulloch CAG (1999) The compliance of collagen gels regulates transforming growth factor-\(\beta \) induction of \(\alpha \)-smooth muscle actin in fibroblasts. Am J Pathol 154(3):871–882. doi:10.1016/S0002-9440(10)65334-5
Ascione F, Vasaturo A, Caserta S, D’Esposito V, Formisano P, Guido S (2016) Comparison between fibroblast wound healing and cell random migration assays in vitro. Exp Cell Res 347(1):123–132. doi:10.1016/j.yexcr.2016.07.015
Augusteyn RC (2010) On the growth and internal structure of the human lens. Exp Eye Res 90(6):643–654. doi:10.1016/j.exer.2010.01.013
Barocas VH, Tranquillo RT (1994) Biphasic theory and in vitro assays of cell-fibril mechanical interactions in tissue-equivalent collagen gels. In: Mow VC, Guilak F, Tran-Son-Tay R, Hochmuth RM (eds) Cell Mechanics and Cellular Engineering. Springer, Berlin, pp 185–209
Barocas VH, Tranquillo RT (1997) An anisotropic biphasic theory of tissue-equivalent mechanics: the interplay among cell traction, fibril alignment and cell contact guidance. J Biomech Eng 119:137–145. doi:10.1115/1.2796072
Barocas VH, Moon AG, Tranquillo RT (1995) The fibroblast-populated collagen microsphere assay of cell traction force—Part 2: measurement of the cell traction parameter. J Biomech Eng 117(2):161–170. doi:10.1115/1.2795998
Bell E, Ivarsson B, Merrill C (1979) Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA 76(3):1274–1278
Bellows CG, Melcher AH, Aubin JE (1981) Contraction and organization of collagen gels by cells cultured from periodontal ligament, gingiva and bone suggest functional differences between cell types. J Cell Sci 50(1):299–314
Bellows CG, Melcher AH, Bhargava U, Aubin JE (1982) Fibroblasts contracting three-dimensional collagen gels exhibit ultrastructure consistent with either contraction or protein secretion. J Ultrastruct Mol Struct Res 78(2):178–192. doi:10.1016/S0022-5320(82)80022-1
Ben Amar M, Goriely A (2005) Growth and instability in elastic tissues. J Mech Phys Solids 53(10):2284–2319. doi:10.1016/j.jmps.2005.04.008
Bilby BA, Gardner LRT, Stroh AN (1957) Continuous distributions of dislocations and the theory of plasticity. In: Proceedings of the 9th international congress of applied mechanics, vol 8. pp 35–44
Bowden LG, Byrne HM, Maini PK, Moulton DE (2016) A morphoelastic model for dermal wound closure. Biomech Model Mechanobiol 15(3):663–681. doi:10.1007/s10237-015-0716-7
Brown RA, Talas G, Porter RA, McGrouther DA, Eastwood M (1996) Balanced mechanical forces and microtubule contribution to fibroblast contraction. J Cell Physiol 169(3):439–447. doi:10.1002/(SICI)1097-4652(199612)169:3<439::AID-JCP4>3.0.CO;2-P
Carlson MA, Longaker MT (2004) The fibroblast-populated collagen matrix as a model of wound healing: a review of the evidence. Wound Repair Regen 12(2):134–147. doi:10.1111/j.1067-1927.2004.012208.x
Chandran PL, Barocas VH (2004) Microstructural mechanics of collagen gels in confined compression: poroelasticity, viscoelasticity, and collapse. J Biomech Eng 126(2):152–166. doi:10.1115/1.1688774
Chen YC, Hoger A (2000) Constitutive functions of elastic materials in finite growth and deformation. J Elasticity 59(1–3):175–193. doi:10.1023/A:1011061400438
Clement CF (1978) Solutions of the continuity equation. Proc Royal Soc Lond A Math Phys Eng Sci 364(1716):107–119. doi:10.1098/rspa.1978.0190
Comellas E, Gasser TC, Bellomo FJ, Oller S (2016) A homeostatic-driven turnover remodelling constitutive model for healing in soft tissues. J R Soc Interface 13(116):20151081. doi:10.1098/rsif.2015.1081
Cook J (1995) Mathematical models for dermal wound healing: Wound contraction and scar formation. PhD thesis, University of Washington
Dallon JC, Ehrlich HP (2008) A review of fibroblast-populated collagen lattices. Wound Repair Regen 16(4):472–479. doi:10.1111/j.1524-475X.2008.00392.x
Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G (1993) Transforming growth factor-\(\beta \)1 induces \(\alpha \)-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 122(1):103–111. doi:10.1083/jcb.122.1.103
Desmoulière A, Chaponnier C, Gabbiani G (2005) Tissue repair, contraction, and the myofibroblast. Wound Repair Regen 13(1):7–12. doi:10.1111/j.1067-1927.2005.130102.x
Discher DE, Janmey P, Wang YL (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310(5751):1139–1143. doi:10.1126/science.1116995
Eastwood M, Porter R, Khan U, McGrouther G, Brown R (1996) Quantitative analysis of collagen gel contractile forces generated by dermal fibroblasts and the relationship to cell morphology. J Cell Physiol 166(1):33–42. doi:10.1002/(SICI)1097-4652(199601)166:1<33::AID-JCP4>3.0.CO;2-H
Ehrlich HP (2003) The fibroblast-populated collagen lattice: a model of fibroblast collagen interactions in repair. In: DiPietro LA, Burns AL (eds) Wound healing. Springer, Berlin, pp 277–291
Ehrlich HP, Rajaratnam JBM (1990) Cell locomotion forces versus cell contraction forces for collagen lattice contraction: an in vitro model of wound contraction. Tissue Cell 22(4):407–417. doi:10.1016/0040-8166(90)90070-P
Ehrlich HP, Moyer KE (2013) Cell-populated collagen lattice contraction model for the investigation of fibroblast collagen interactions. In: Gourdie RG, Myers TA (eds) Wound regeneration and repair: methods and protocols, methods in molecular biology, vol 1037. Springer, New York, pp 45–58. doi:10.1007/978-1-62703-505-7_3
Elsdale T, Bard J (1972) Collagen substrata for studies on cell behavior. J Cell Biol 54(3):626–637. doi:10.1083/jcb.54.3.626
Enoch S, Leaper DJ (2005) Basic science of wound healing. Surgery 23(2):37–42. doi:10.1016/j.mpsur.2007.11.005
Evans MC, Barocas VH (2009) The modulus of fibroblast-populated collagen gels is not determined by final collagen and cell concentration: experiments and an inclusion-based model. J Biomech Eng 131(10):101014
Farsi JMA, Aubin JE (1984) Microfilament rearrangements during fibroblast-induced contraction of three-dimensional hydrated collagen gels. Cell Motil Cytoskel 4(1):29–40. doi:10.1002/cm.970040105
Feng Z, Yamato M, Akutsu T, Nakamura T, Okano T, Umezu M (2003) Investigation on the mechanical properties of contracted collagen gels as a scaffold for tissue engineering. Artif Organs 27(1):84–91. doi:10.1046/j.1525-1594.2003.07187.x
Ferrenq I, Tranqui L, Vailhé B, Gumery PY, Tracqui P (1997) Modelling biological gel contraction by cells: mechanocellular formulation and cell traction force quantification. Acta Biotheor 45(3–4):267–293. doi:10.1023/A:1000684025534
Fluck J, Querfeld C, Cremer A, Niland S, Krieg T, Sollberg S (1998) Normal human primary fibroblasts undergo apoptosis in three-dimensional contractile collagen gels. J Invest Dermatol 110(2):153–157. doi:10.1046/j.1523-1747.1998.00095.x
Fung YC (1993) Biomechanics: mechanical properties of living tissues. Springer, New York
Gabbiani G (2003) The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 200(4):500–503. doi:10.1002/path.1427
Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR, Majno G (1972) Granulation tissue as a contractile organ: a study of structure and function. J Exp Med 135(4):719–734. doi:10.1084/jem.135.4.719
García-Grajales JA, Jérusalem A, Goriely A (2016) Continuum mechanical modeling of axonal growth. Comput Methods Appl Mech Eng. doi:10.1016/j.cma.2016.07.032
Göktepe S, Kuhl E (2010) Electromechanics of the heart: a unified approach to the strongly coupled excitation–contraction problem. Comput Mech 45(2–3):227–243. doi:10.1007/s00466-009-0434-z
Gonzalez O, Stuart AM (2008) A first course in continuum mechanics. Cambridge University Press, Cambridge
Goriely A, Ben Amar M (2007) On the definition and modeling of incremental, cumulative, and continuous growth laws in morphoelasticity. Biomech Model Mechanobiol 6(5):289–296. doi:10.1007/s10237-006-0065-7
Goriely A, Moulton DE (2011) Morphoelasticity–a theory of elastic growth. In: Ben Amar M, Goriely A, Müller MM, Cugliandolo L (eds) New trends in the physics and mechanics of biological systems. Oxford University Press, Oxford
Goriely A, Robertson-Tessi M, Tabor M, Vandiver R (2008) Elastic growth models. In: Mondaini R, Pardalos PM (eds) Mathematical modelling of biosystems. Springer, Berlin, pp 1–45
Green JEF, Bassom AP, Friedman A (2013) A mathematical model for cell-induced gel compaction in vitro. Math Models Methods Appl Sci 23(1):127–163. doi:10.1142/S0218202512500479
Grinnell F (1994) Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol 124(4):401–404. doi:10.1083/jcb.124.4.401
Grinnell F (2000) Fibroblast-collagen-matrix contraction: growth-factor signalling and mechanical loading. Trends Cell Biol 10(9):362–365. doi:10.1016/S0962-8924(00)01802-X
Grinnell F (2003) Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol 13(5):264–269. doi:10.1016/S0962-8924(03)00057-6
Grinnell F, Lamke C (1984) Reorganization of hydrated collagen lattices by human skin fibroblasts. J Cell Sci 66(1):51–63
Grinnell F, Ho CH, Lin YC, Skuta G (1999) Differences in the regulation of fibroblast contraction of floating versus stressed collagen matrices. J Biol Chem 274(2):918–923. doi:10.1074/jbc.274.2.918
Grinnell F, Ho CH, Tamariz E, Lee DJ, Skuta G (2003) Dendritic fibroblasts in three-dimensional collagen matrices. Mol Biol Cell 14(2):384–395. doi:10.1091/mbc.E02-08-0493
Guidry C, Grinnell F (1985) Studies on the mechanism of hydrated collagen gel reorganization by human skin fibroblasts. J Cell Sci 79(1):67–81
Guidry C, Grinnell F (1986) Contraction of hydrated collagen gels by fibroblasts: evidence for two mechanisms by which collagen fibrils are stabilized. Coll Relat Res 6(6):515–529
Hall CL (2008) Modelling of some biological materials using continuum mechanics. PhD thesis, Queensland University of Technology
Halliday NL, Tomasek JJ (1995) Mechanical properties of the extracellular matrix influence fibronectin fibril assembly in vitro. Exp Cell Res 217(1):109–117. doi:10.1006/excr.1995.1069
Harris AK, Wild P, Stopak D (1980) Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science 208(4440):177–179. doi:10.1126/science.6987736
Hinz B, Mastrangelo D, Iselin C, Chaponnier C, Gabbiani G (2001) Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol 159(3):1009–1020. doi:10.1016/S0002-9440(10)61776-2
Humphrey JD (2003) Continuum biomechanics of soft biological tissues. Proc Royal Soc Lond A Math Phys Sci 459(2029):3–46. doi:10.1098/rspa.2002.1060
Jones GW, Chapman SJ (2012) Modeling growth in biological materials. SIAM Rev 54(1):52–118. doi:10.1137/080731785
Kelynack KJ (2009) Cell-populated floating collagen lattices: an in vitro model of parenchymal contraction. In: Hewitson TD, Becker GJ (eds) Kidney research. Springer, Berlin, pp 1–11
Knapp DM, Barocas VH, Moon AG, Yoo K, Petzold LR, Tranquillo RT (1997) Rheology of reconstituted type I collagen gel in confined compression. J Rheol 41(5):971–993. doi:10.1122/1.550817
Knapp DM, Tower TT, Tranquillo RT, Barocas VH (1999) Estimation of cell traction and migration in an isometric cell traction assay. AIChE J 45(12):2628–2640. doi:10.1002/aic.690451219
Kolodney MS, Wysolmerski RB (1992) Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J Cell Biol 117(1):73–82. doi:10.1083/jcb.117.1.73
Koppenol DC, Vermolen FJ, Niessen FB, Zuijlen PP, Vuik K (2017a) A biomechanical mathematical model for the collagen bundle distribution-dependent contraction and subsequent retraction of healing dermal wounds. Biomech Model Mechanobiol 16:345–361. doi:10.1007/s10237-016-0821-2
Koppenol DC, Vermolen FJ, Niessen FB, Zuijlen PP, Vuik K (2017b) A mathematical model for the simulation of the formation and the subsequent regression of hypertrophic scar tissue after dermal wounding. Biomech Model Mechanobiol 16:15–32. doi:10.1007/s10237-016-0799-9
Kröner E (1958) Kontinuumstheorie der versetzungen und eigenspannungen. Erg Angew Math 5:1–179
Kröner E (1959) Allgemeine kontinuumstheorie der versetzungen und eigensspannungen. Arch Ration Mech Anal 4(1):273–334
Kuhl E (2014) Growing matter: a review of growth in living systems. J Mech Behavior Biomed Mater 29:529–543. doi:10.1016/j.jmbbm.2013.10.009
Lee EH (1969) Elastic–plastic deformation at finite strains. J Appl Mech 36:1–6. doi:10.1115/1.3564580
Lin YC, Ho CH, Grinnell F (1997) Fibroblasts contracting collagen matrices form transient plasma membrane passages through which the cells take up fluorescein isothiocyanate-dextran and Ca\(^{2+}\). Mol Biol Cell 8(1):59–71
Lubarda VA (2001) Elastoplasticity theory. CRC Press, Boca Raton
Lubarda VA (2004) Constitutive theories based on the multiplicative decomposition of deformation gradient: Thermoelasticity, elastoplasticity, and biomechanics. Appl Mech Rev 57(2):95–109. doi:10.1115/1.1591000
Majno G, Joris I (2004) Cells, tissues and disease: principles of general pathology, 2nd edn. Oxford University Press, New York
Marenzana M, Wilson-Jones N, Mudera V, Brown RA (2006) The origins and regulation of tissue tension: identification of collagen tension–fixation process in vitro. Exp Cell Res 312(4):423–433. doi:10.1016/j.yexcr.2005.11.005
Marquez JP, Genin GM, Zahalak GI, Elson EL (2005) The relationship between cell and tissue strain in three-dimensional bio-artificial tissues. Biophys J 88(2):778–789. doi:10.1529/biophysj.104.041947
Menon SN, Flegg JA, McCue SW, Schugart RC, Dawson RA, McElwain DLS (2012) Modelling the interaction of keratinocytes and fibroblasts during normal and abnormal wound healing processes. Proc Royal Soc Lond B Biol Sci 279(1741):3329–3338. doi:10.1098/rspb.2012.0319
Mochitate K, Pawelek P, Grinnell F (1991) Stress relaxation of contracted collagen gels: disruption of actin filament bundles, release of cell surface fibronectin, and down-regulation of DNA and protein synthesis. Exp Cell Res 193(1):198–207. doi:10.1016/0014-4827(91)90556-A
Moon AG, Tranquillo RT (1993) Fibroblast-populated collagen microsphere assay of cell traction force: Part 1. Continuum model. AIChE J 39(1):163–177. doi:10.1002/aic.690390116
Mudera VCi, (2000) Molecular responses of human dermal fibroblasts to dual cues: contact guidance and mechanical load. Cell Motil Cytoskel 45(1):1–9. doi:10.1002/(SICI)1097-0169(200001)45:1<1::AID-CM1>3.0.CO;2-J
Murphy KE, Hall CL, McCue SW, McElwain DLS (2011a) A two-compartment mechanochemical model of the roles of transforming growth factor \(\beta \) and tissue tension in dermal wound healing. J Theor Biol 272(1):145–159. doi:10.1016/j.jtbi.2010.12.011
Murphy KE, McCue SW, McElwain DLS (2011b) Clinical strategies for contractures from a predictive mathematical model of dermal repair. Wound Rep Regen 20(2):104–202. doi:10.1111/j.1524-475X.2012.00775.x
Murphy KE, Hall CL, Maini PK, McCue SW, McElwain DLS (2012) A fibrocontractive mechanochemical model of dermal wound closure incorporating realistic growth factor kinetics. Bull Math Biol 74(5):1143–1170. doi:10.1007/s11538-011-9712-y
Murray JD (2001) Mathematical biology. II spatial models and biomedical applications. In: Interdisciplinary applied mathematics, vol 18. Springer, New York Incorporated
Patwari P, Lee RT (2008) Mechanical control of tissue morphogenesis. Circ Res 103(3):234–243. doi:10.1161/CIRCRESAHA.108.175331
Pryse KM, Nekouzadeh A, Genin GM, Elson EL, Zahalak GI (2003) Incremental mechanics of collagen gels: new experiments and a new viscoelastic model. Ann Biomed Eng 31:1287–1296. doi:10.1114/1.1615571
Rajagopal KR, Srinivasa AR (2004) On the thermomechanics of materials that have multiple natural configurations Part I: viscoelasticity and classical plasticity. Z Angew Math Physik 55(5):861–893. doi:10.1007/s00033-004-4019-6
Ramtani S (2004) Mechanical modelling of cell/ecm and cell/cell interactions during the contraction of a fibroblast-populated collagen microsphere: theory and model simulation. J Biomech 37(11):1709–1718. doi:10.1016/j.jbiomech.2004.01.028
Ramtani S, Fernandes-Morin E, Geiger D (2002) Remodeled-matrix contraction by fibroblasts: numerical investigations. Comput Biol Med 32(4):283–296. doi:10.1016/S0010-4825(02)00018-5
Rausch MK, Dam A, Göktepe S, Abilez OJ, Kuhl E (2011) Computational modeling of growth: systemic and pulmonary hypertension in the heart. Biomech Model Mechanobiol 10(6):799–811. doi:10.1007/s10237-010-0275-x
Rhee S, Grinnell F (2007) Fibroblast mechanics in 3D collagen matrices. Adv Drug Delivery Rev 59(13):1299–1305. doi:10.1016/j.addr.2007.08.006
Roberts AJ (1994) A one-dimensional introduction to continuum mechanics. World Scientific, Singapore
Rodriguez EK, Hoger A, McCulloch AD (1994) Stress-dependent finite growth in soft elastic tissues. J Biomech 27(4):455–467. doi:10.1016/0021-9290(94)90021-3
Roseborough IE, Grevious MA, Lee RC (2004) Prevention and treatment of excessive dermal scarring. J Natl Med Assoc 96(1):108–116
Rosenfeldt H, Grinnell F (2000) Fibroblast quiescence and the disruption of ERK signaling in mechanically unloaded collagen matrices. J Biol Chem 275(5):3088–3092. doi:10.1074/jbc.275.5.3088
Roy P, Petroll WM, Cavanagh HD, Chuong CJ, Jester JV (1997) An in vitro force measurement assay to study the early mechanical interaction between corneal fibroblasts and collagen matrix. Exp Cell Res 232(1):106–117. doi:10.1006/excr.1997.3511
Roy P, Petroll WM, Chuong CJ, Cavanagh HD, Jester JV (1999) Effect of cell migration on the maintenance of tension on a collagen matrix. Ann Biomed Eng 27(6):721–730. doi:10.1114/1.227
Sander LM (2013) Alignment localization in nonlinear biological media. J Biomech Eng 135(7):071006
Schreiber DI, Enever PAJ, Tranquillo RT (2001) Effects of PDGF-BB on rat dermal fibroblast behavior in mechanically stressed and unstressed collagen and fibrin gels. Exp Cell Res 266(1):155–166. doi:10.1006/excr.2001.5208
Schreiber DI, Barocas VH, Tranquillo RT (2003) Temporal variations in cell migration and traction during fibroblast-mediated gel compaction. Biophys J 84(6):4102–4114. doi:10.1016/S0006-3495(03)75135-2
Steinberg BM, Smith K, Colozzo M, Pollack R (1980) Establishment and transformation diminish the ability of fibroblasts to contract a native collagen gel. J Cell Biol 87(1):304–308. doi:10.1083/jcb.87.1.304
Stevenson MD, Sieminski AL, McLeod CM, Byfield FJ, Barocas VH, Gooch KJ (2010) Pericellular conditions regulate extent of cell-mediated compaction of collagen gels. Biophys J 99(1):19–28
Stojanović R, Djurić S, Vujošević L (1964) On finite thermal deformations. Arch Mech Stosow 16:103–108
Stojanović R, Vujosěvić L, Blagojević D (1970) Couple stresses in thermoelasticity. Rev Roum Sci Techn-Meć Appl 15:517–537
Stopak D, Harris AK (1982) Connective tissue morphogenesis by fibroblast traction: I. Tissue culture observations. Dev Biol 90(2):383–398. doi:10.1016/0012-1606(82)90388-8
Taber LA (1995) Biomechanics of growth, remodeling, and morphogenesis. Appl Mech Rev 48(8):487–545. doi:10.1115/1.3005109
Talas G, Adams TST, Eastwood M, Rubio G, Brown RA (1997) Phenytoin reduces the contraction of recessive dystrophic epidermolysis bullosa fibroblast populated collagen gels. Int J Biochem Cell B 29(1):261–270. doi:10.1016/S1357-2725(96)00132-X
Tamariz E, Grinnell F (2002) Modulation of fibroblast morphology and adhesion during collagen matrix remodeling. Mol Biol Cell 13(11):3915–3929. doi:10.1091/mbc.E02050291
Tomasek JJ, Haaksma CJ, Eddy RJ, Vaughan MB (1992) Fibroblast contraction occurs on release of tension in attached collagen lattices: dependency on an organized actin cytoskeleton and serum. Anat Rec 232(3):359–368. doi:10.1002/ar.1092320305
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Bio 3(5):349–363. doi:10.1038/nrm809
Tracqui P, Woodward DE, Cruywagen GC, Cook J, Murray JD (1995) A mechanical model for fibroblast-driven wound healing. J Biol Syst 3(4):1075–1084. doi:10.1142/S0218339095000976
Tranquillo RT, Murray JD (1992) Continuum model of fibroblast-driven wound contraction: inflammation-mediation. J Theor Biol 158(2):135–172. doi:10.1016/S0022-5193(05)80715-5
Valero C, Javierre E, García-Aznar JM, Gómez-Benito MJ (2014) A cell-regulatory mechanism involving feedback between contraction and tissue formation guides wound healing progression. PloS ONE 9(3):e92774. doi:10.1371/journal.pone.0092774
Vandiver R (2009) Morphoelasticity: the mechanics and mathematics of elastic growth. PhD Thesis, University of Arizona
Vandiver R, Goriely A (2009) Differential growth and residual stress in cylindrical elastic structures. Philos Trans Royal Soc Lond A Math Phys Eng Sci 367(1902):3607–3630. doi:10.1098/rsta.2009.0114
Vaughan MB, Howard EW, Tomasek JJ (2000) Transforming growth factor-\(\beta \)1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res 257(1):180–189. doi:10.1006/excr.2000.4869
Wakatsuki T, Kolodney MS, Zahalak GI, Elson EL (2000) Cell mechanics studied by a reconstituted model tissue. Biophys J 79(5):2353–2368. doi:10.1016/S0006-3495(00)76481-2
Xiao H, Bruhns OT, Meyers A (2006) Elastoplasticity beyond small deformations. Acta Mech 182(1–2):31–111. doi:10.1007/s00707-005-0282-7
Yang L, Witten TM, Pidaparti RM (2013) A biomechanical model of wound contraction and scar formation. J Theor Biol 332:228–48. doi:10.1016/j.jtbi.2013.03.013
Yavari A (2010) A geometric theory of growth mechanics. J Nonlinear Sci 20:781–830. doi:10.1007/s00332-010-9073-y
Zahalak GI, Wagenseil JE, Wakatsuki T, Elson EL (2000) A cell-based constitutive relation for bio-artificial tissues. Biophys J 79(5):2369–2381. doi:10.1016/S0006-3495(00)76482-4
Acknowledgements
This research was primarily supported by the Australian Research Council’s Discovery Projects funding scheme (Project number DP0878011). SNM is supported by the IMSc Complex Systems Project (12th Plan). CLH acknowledges the support of the Mathematics Applications Consortium for Science and Industry, funded by the Science Foundation Ireland Grant investigator award 12/IA/1683.
Author information
Authors and Affiliations
Corresponding author
Appendices
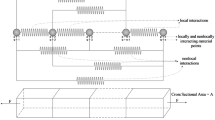
Appendix 1: effective strain and contraction in a 1-D morphoelastic body
1.1 The multiplicative decomposition of the deformation gradient
The idea that an observed deformation gradient tensor \({F}\) could be expressed in terms of elastic and plastic tensor fields through a multiplicative decomposition was first introduced by Bilby et al. (1957) and Kröner (1958, 1959). This was further developed by Stojanović et al. (1964, 1970) in the context of thermoelasticity and Lee (1969) for the description of metal plasticity at large deformations, while Rodriguez et al. (1994) and Cook (1995) were the first to utilise this in the context of biomechanics. The pioneering work of Rodriguez et al. (1994) and Cook (1995) was later extended and expanded by Hoger and coworkers (see especially Chen and Hoger (2000)), Goriely and coworkers (Ben Amar and Goriely 2005; Goriely and Ben Amar 2007; Goriely et al. 2008), Ambrosi and coworkers (Ambrosi and Mollica 2004; Ambrosi and Guana 2007; Ambrosi and Guillou 2007) and by Vandiver (2009). This biological work has developed alongside applications to thermoelasticity and plasticity, and achievements in these areas have informed each other. This is exemplified by the cross-disciplinary work of Lubarda (2001, 2004) and Rajagopal and coworkers (see, for example, Rajagopal and Srinivasa (2004)). For a comprehensive review of the history and applications of the multiplicative decomposition of deformation gradient, see Lubarda (2004).
In a recent comprehensive review of current work on modelling growth and remodelling, Ambrosi et al. (2011) describe a number of applications of the multiplicative decomposition of the deformation gradient, ranging from the remodelling of heart muscle to morphogenesis. The multiplicative decomposition of the deformation gradient is now being used in models of biomechanical phenomena ranging from tissue growth (Ben Amar and Goriely 2005) to the operation of the heart (Göktepe and Kuhl 2010; Rausch et al. 2011), although it is important to note that this approach will only be valid when the tissue behaves elastically on the timescale of remodelling (Jones and Chapman 2012) and that some authors have general reservations about the use of the multiplicative decomposition on theoretical grounds (Xiao et al. 2006). Indeed, Ambrosi et al. (2011) note that there are problems and ambiguities to be resolved when developing appropriate laws to describe the evolution of the growth part of the deformation gradient in response to remodelling. In the context of the present work, it is important to note that many of these difficulties are avoided as we restrict our analysis to the one-dimensional (1-D) Cartesian case, although it is still necessary to ensure that the constitutive relation is appropriate for the type of remodelling under consideration.
An accessible introduction to the use of the multiplicative decomposition in biological applications can be found in Goriely and Moulton (2011), which begins with an analysis of a 1-D growing material that is relevant to the research presented here. It is important to note that a 1-D body can never be residually stressed: it is impossible to encounter the situation in which the zero stress state cannot be achieved without introducing cuts. Moreover, ensuring observer independence of time derivatives is much simpler along a single dimension, as it excludes the possibility of a rotating observer. We now develop a simple mathematical framework for modelling the growth or contraction of a 1-D Cartesian morphoelastic body.
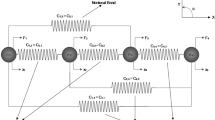
1.2 Strain evolution
The deformation gradient is given by the scalar function
Following the 1-D version of Eq. (1) in Goriely and Moulton (2011), we express this as the product
where the elastic stretch \(\alpha \) is the local size ratio between the current state and the zero stress state, and the growth stretch \(\gamma \) is the local size ratio between the zero stress state and the initial state (the growth stretch).
In Goriely and Moulton (2011), the constitutive relation for 1-D growth is assumed to depend on the rate of growth \(g(x,\,t)\):
While (30) is useful at small deformations (i.e. when \(F \approx 1\)), it leads to inconsistencies if the current state is significantly different from the initial state, as in the case of FPCL contraction. In order to obtain an equivalent of (30) for large deformations, we first note that \(g(x,\,t)\) should satisfy
That is, \(g(x,\,t)\) should be defined with reference to the current configuration, but it should measure the rate of change of the zero stress state of any collection of material particles. It follows from (31) that \(g(x,\,t)\) is related to the material derivative of \(\gamma (X,\,t)\):
Note that this reduces to (30) when \(F \equiv 1\).
Now, we expect that the stress at any point in the body will be related to the difference between the zero stress state and the current state. A plausible constitutive law that relates the stress, \(\sigma \), to the elastic stretch, \(\alpha \) is
where E is the Young’s modulus. This is analogous to Hooke’s law for a linear elastic material, but uses an Eulerian rather than a pseudo-Lagrangian measure of strain, since
where \(\Delta x\) and \(\Delta z\) relate to the changes in the current and zero stress states, respectively. In cases where the current state is close to the zero stress state, and hence, \(\alpha \approx 1\), we see that \(e^{E}\approx e^{L}\) and (33) is equivalent to other plausible constitutive laws, such as those in Goriely and Moulton (2011):
Experimental observations indicate that most of the change in size of a contracting FPCL is due to the permanent rearrangement of fibres by fibroblasts (Guidry and Grinnell 1985). Hence, it is appropriate to use (33) and assume a linear relationship between stress and strain, rather than the nonlinear model (35). Moreover, (33) has an interesting advantage over (34) and (35), namely that the evolution of Eulerian strain in response to growth can neatly be expressed as an advection equation with a source term that is independent of \(e^E\).
In order to see this, we substitute (29) into (32) to obtain
Since \(F^{-1} \, D F/D t = \partial v/\partial x\) where v is the velocity and where \(\partial v/\partial x\) is the velocity gradient, it follows that \(\alpha ^{-1}\) satisfies the equation
and using \(e^{E} \equiv 1 - \alpha ^{-1}\) we thus obtain the mechanical model for a morphoelastic solid with small effective strain
Note that thus far we have made the assumption that the relation between stress and strain is purely elastic
However, as discussed in Sect. 3.1, this can easily be extended to viscoelastic bodies through the use of a Kelvin–Voigt viscoelastic constitutive law. In Sect. 3 of the main text, we use this formulation together with (37) to derive a set of governing equations for the contraction of a 1-D morphoelastic body.
Appendix 2: derivation of the spatial and temporal transformations between coordinate systems
In one spatial dimension, we use X to represent the Lagrangian coordinate and x to represent the Eulerian coordinate. At any given time t, there will be a one-to-one mapping from the initial configuration to the current configuration. Thus, we can always write \(X = X(x,\,t)\) and \(x = x(X,\,t)\); moreover, the fact that particles are not permitted to move through each other implies that \({\partial x}/{\partial X} > 0\).
Now, the Eulerian displacement gradient is the spatial derivative of \(u(x,\,t) =x-X(x,\,t)\), i.e.
Similarly, the Lagrangian displacement gradient is
We thus have the relation
Using the chain rule, the Eulerian spatial derivative is
The derivative \(\partial T/\partial x\) is equal to zero, and using (38) we obtain the expression
Furthermore, using (38) and (39), we obtain the following transformation for the spatial derivative
We similarly use the chain rule to obtain the following expression for the Eulerian temporal derivative
The derivative \(\partial T/\partial t\) is equal to one, and so using (40) we have
Using (15), we have
which, on using (39) and the fact that \(v(0,\,t)=0\), yields
Now, from (10), we have \(v\,(1-w)=\partial {u}/\partial {t}\). Since \(u\equiv U\), using the above expression in conjunction with (39), this yields
where we have used the definitions of V and W. This implies that \(v\equiv V\), and so we obtain the following transformation for the temporal derivative
Now, from (15) we have \(\partial {w}/\partial {t} + \partial {(w\,v)}/\partial {x} = \partial {v}/\partial {x}\). Using (39), (41) and (42), this reduces to the following relation between the Lagrangian displacement gradient and velocity:
We use this expression in Sect. 3.2 of the main text to derive our morphoelastic model of FPCL contraction.
Rights and permissions
About this article
Cite this article
Menon, S.N., Hall, C.L., McCue, S.W. et al. A model for one-dimensional morphoelasticity and its application to fibroblast-populated collagen lattices. Biomech Model Mechanobiol 16, 1743–1763 (2017). https://doi.org/10.1007/s10237-017-0917-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-017-0917-3