Abstract
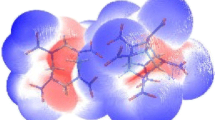
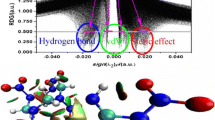
The effects of the molar ratio, temperature, and solvent on the formation of the cocrystal explosive DNP/CL-20 were investigated using molecular dynamics (MD) simulation. The cocrystal structure was predicted through Monte Carlo (MC) simulation and using first-principles methods. The results showed that the DNP/CL-20 cocrystal might be more stable in the molar ratio 1:1 near to 318 K, and the most probable cocrystal crystallizes in the triclinic crystal system with the space group P\( \overline{1} \). Cocrystallization was more likely to occur in methanol and ethanol at 308 K as a result of solvent effects. The optimized structure and the reduced density gradient (RDG) of the DNP/CL-20 complex confirmed that the main driving forces for cocrystallization were a series of hydrogen bonds and van der Waals forces. Analyses of the trigger bonds, the charges on the nitro groups, the electrostatic surface potential (ESP), and the free space per molecule in the cocrystal lattice were carried out to further explore their influences on the sensitivity of CL-20. The results indicated that the DNP/CL-20 complex tended to be more stable and insensitive than pure CL-20. Moreover, an investigation of the detonation performance of the DNP/CL-20 cocrystal indicated that it possesses high power.

DNP/CL-20 cocrystal models with different molar ratios were investigated at different temperatures using molecular dynamics (MD) simulation methods. Binding energies and mechanical properties were probed to determine the stability and performance of each cocrystal model. Solvated DNP/CL-20 models were established by adding solvent molecules to the cocrystal surface. The binding energies of the models in various solvents were calculated in order to identify the most suitable solvent and temperature for preparing the cocrystal explosive DNP/CL-20








Similar content being viewed by others
References
Anslyn EV, Dougherty DA (2004) Modern physical organic chemistry. University Science Books, Sausalito
Lara-Ochoa F, Espinosa-Pérez G (2007) Cocrystals definitions. Supramol Chem 19:553–557
Bolton O, Matzger AJ (2011) Improved stability and smart-material functionality realized in an energetic cocrystal. Angew Chem Int Ed 50:8960–8963
Lin H, Zhu S-G, Li H-Z, Peng X-H (2013) Structure and detonation performance of a novel HMX/LLM-105 cocrystal explosive. J Phys Org Chem 26:898–907
Millar DIA, Maynard-Casely HE, Allan DR, Cumming AS, Lennie AR, Mackay AJ, Oswald IDH, Tang C-C, Pulham CR (2012) Crystal engineering of energetic materials: co-crystals of CL-20. Cryst Eng Comm 14:3742–3749
Yang Z-W, Li H-Z, Huang H, Zhou X-Q, Li J-S, Nie F-D (2013) Preparation and performance of a HNIW/TNT cocrystal explosive. Propell Explos Pyrot 38:495–501
Bolton O, Simke LR, Pagoria PF, Matzger AJ (2012) High power explosive with good sensitivity: a 2:1 cocrystal of CL-20:HMX. Cryst Growth Des 12:4311–4314
Yang Z-W, Li H-Z, Zhou X-Q, Zhang C-Y, Huang H, Li J-S, Nie F-D (2012) Characterization and properties of a novel energetic–energetic cocrystal explosive composed of HNIW and BTF. Cryst Growth Des 12:5155–5158
Wang Y-P, Yang Z-W, Li H-Z, Zhou X-Q, Zhang Q, Wang J-H, Liu Y-C (2014) A novel cocrystal explosive of HNIW with good comprehensive properties. Propell Explos Pyrot 39:590–596
Goncharov TK, Aliev ZG, Aldoshin SM, Dashko DV, Vasil AA, Shishov NI, Milekhin YM (2015) Preparation, structure, and main properties of bimolecular crystals CL-20-DNP and CL-20-DNG. Russ Chem B+ 64:366–374
Xu H-F, Duan X-H, Li H-Z, Pei C-H (2015) A novel high-energetic and good-sensitive cocrystal composed of CL-20 and TATB by a rapid solvent/non-solvent method. RSC Adv. 5:95764–95770
Liu K, Zhang G, Luan J-Y, Chen Z-Q, Su P-F, Shu Y-J (2016) Crystal structure, spectrum character and explosive property of a new cocrystal CL-20/DNT. J Mol Struct 1110:91–96
Gao H, Jiang W, Liu J, Hao G-Z, Xiao L, Ke X, Chen T (2017) Synthesis and characterization of a new co-crystal explosive with high energy and good sensitivity. J Energ Mater 35:490–498
Ding X, Gou R-J, Ren F-D, Liu F, Zhang S-H, Gao H-F (2016) Molecular dynamics simulation and density functional theory insight into the cocrystal explosive of hexaazaisowurtzitane/nitroguanidine. Int J Quantum Chem 116:88–96
Gao H-F, Zhang S-H, Ren F-D, Liu F, Gou R-J, Ding X (2015) Theoretical insight into the co-crystal explosive of 2,4,6,8,10,12-hexanitrohexaazaisowurtzitane (CL-20)/1,1-diamino-2,2-dinitroethylene (FOX-7). Comput Mater Sci 107:33–41
Feng R-Z, Zhang S-H, Ren F-D, Gou R-J, Gao L (2016) Theoretical insight into the binding energy and detonation performance of ε-, γ-, β-CL-20 cocrystals with β-HMX, FOX-7, and DMF in different molar ratios, as well as electrostatic potential. J Mol Model 22:1–14
Wei X-F, Zhang A-B, Ma Y, Xue X-G, Zhou J-H, Zhu Y-Q, Zhang C-Y (2015) Toward low-sensitive and high-energetic cocrystal III: thermodynamics of energetic–energetic cocrystal formation. Cryst Eng Comm 17:9037–9047
Gao H-F, Zhang S-H, Ren F-D, Gou R-J, Wu C-L (2016) Theoretical insight into the temperature-dependent acetonitrile (ACN) solvent effect on the diacetone diperoxide (DADP)/1,3,5-tribromo-2,4,6-trinitrobenzene (TBTNB) cocrystallization. Comput Mater Sci 121:232–239
Han G, Gou R-J, Ren F-D, Zhang S-H, Zhu S-F (2017) Theoretical investigation into the influence of molar ratio on binding energy, mechanical property and detonation performance of 1,3,5,7-tetranitro 1,3,5,7-tetrazacyclo octane (HMX)/1-methyl-4,5-dinitroimidazole (MDNI) cocrystal explosive. Comput Theor Chem 1109:27–35
Ravi P, Badgujar DM, Gore GM, Tewari SP, Sikder AK (2011) Review on melt cast explosive. Prop Explos Pyrotech 36:393–403
Nielsen AT, Christian SL, Moore DW, Nadler MP, Nissan RA, Vanderah DJ, Gilardi RD, George CF, Flippen-Anderson JL, Chafin AP (1998) Synthesis of polyazapolycyclic caged polynitramines. Tetrahedron 54:11793–11812
Chen L-Z, Song L, Cao D-L, Wang J-L (2016) Crystal structure of 3,4-dinitropyrazole, C3H2N4O4. Z Kristallogr—New Cryst Struct 231:1099–1100
Sun H (1998) COMPASS: an ab initio force-field optimized for condensed-phase applications—overview with details on alkane and benzene compounds. J Phys Chem B 102:7338–7364
Wei C-X, Huang H, Duan X-H, Pei C-H (2011) Structures and properties prediction of HMX/TATB co-crystal. Propell Explos Pyrot 36:416–423
Li Y-X, Chen S-S, Ren F-D (2015) Theoretical insights into the structures and mechanical properties of HMX/NQ cocrystal explosives and their complexes, and the influence of molecular ratios on their bonding energies. J Mol Model 21:245
Andersen HC (1980) Molecular dynamics simulations at constant pressure and/or temperature. J Chem Phys 72:2384–2393
Allan DR, Clark SJ, Brugmans MJP, Ackland GJ, Vos WL (1998) Structure of crystalline methanol at high pressure. Phys Rev B 58:R11809
Accelrys Software Inc. (2013) Materials Studio release notes, release 7.0. Accelrys Software Inc., San Diego
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chim Acta 120:215–241
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A.01. Gaussian, Inc., Wallingford
Lu T, Chen FW (2012) Multiwfn: A multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Bondi A (1964) van der Waals volumes and radii. J Phys Chem 68:441–451
Razas I (2007) On the nature of hydrogen bonds: an overview on computational studies and a word about patterns. Phys Chem Chem Phys 9:2782–2790
Arunan E, Desiraju GR, Klein RA, Sadlej J, Scheiner S, Alkorta I, Clary DC, Crabtree RH, Dannenberg JJ, Hobza P, Kjaergaard HG, Legon AC, Mennucci B, Nesbitt DJ (2011) Definition of the hydrogen bond (IUPAC recommendations 2011). Pure Appl Chem 83:1637–1641
Barckholtz C, Barckholtz TA, Hadad CM (1999) C−H and N−H bond dissociation energies of small aromatic hydrocarbons. J Am Chem Soc 121:491–500
Lu T, Chen F-W (2013) Bond order analysis based on the Laplacian of electron density in fuzzy overlap space. J Phys Chem A 117:3100–3108
Rice BM, Hare JJ (2002) A quantum mechanical investigation of the relation between impact sensitivity and the charge distribution in energetic molecules. J Phys Chem A 106:1770–1783
Lu T, Chen F-W (2012) Atomic dipole moment corrected Hirshfeld population method. Theor Comput Chem 11:163–183
Steiner T (2002) The hydrogen bond in the solid state. Angew Chem Int Ed 41:48–76
Johnson ER, Keinan S, Mori-Sanchez P, Contreras-Garcia J, Cohen AJ, Yang W-T (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91:893–928
Pospíšil M, Vávra P, Concha MC, Murray JS, Politzer P (2010) A possible crystal volume factor in the impact sensitivities of some energetic compounds. J Mol Model 16:895–901
Politzer P, Murray JS (2016) High performance, low sensitivity: conflicting or compatible? Propell Explos Pyrot 41:414–425
Lin H, Zhu S-G, Zhang L, Peng X-H, Chen P-Y, Li H-Z (2013) Intermolecular interactions, thermodynamic properties, crystal structure, and detonation performance of HMX/NTO cocrystal explosive. Int J Quantum Chem 113:1591–1599
Landenberger KB, Matzger AJ (2010) Cocrystal engineering of a prototype energetic material: supramolecular chemistry of 2,4,6-trinitrotoluene. Cryst Growth Des 10:5341–5347
Politzer P, Murray JS (2015) Some molecular/crystalline factors that affect the sensitivities of energetic materials: molecular surface electrostatic potentials, lattice free space and maximum heat of detonation per unit volume. J Mol Model 21:25–35
Politzer P, Murray JS (2014) Detonation performance and sensitivity: a quest for balance. Adv Quantum Chem 69:1–30
Pospíšil M, Vávra P, Concha MC, Murray JS, Politzer P (2011) Sensitivity and the available free space per molecule in the unit cell. J Mol Model 17:2569–2574
Politzer P, Murray JS (2014) Impact sensitivity and crystal lattice compressibility/free space. J Mol Model 20:2223–2230
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396
Wu J-T, Zhang J-G, Li T, Li Z-M, Zhang T-L (2015) A novel cocrystal explosive NTO/TZTN with good comprehensive properties. RSC Adv 5:28354–28359
Wei Y-J, Ren F-D, Shi W-J, Zhao Q (2016) Theoretical insight into the influences of molecular ratios on stabilities and mechanical properties, solvent effect of HMX/FOX-7 cocrystal explosive. J Energ Mater 34:426–439
Sućeska M (2013) EXPLO5 6.0.1. Brodarski Institute, Zagreb
Keshavarz MH, Motamedoshariati H, Moghayadnia R, Nazari HR, Azarniamehraban J (2009) A new computer code to evaluate detonation performance of high explosives and their thermochemical properties, part I. J Hazard Mater 172:1218–1228
Politzer P, Murray JS (2015) Impact sensitivity and the maximum heat of detonation. J Mol Model 21:262–272
Acknowledgments
We gratefully acknowledge the National Key Laboratory of Applied Physics and Chemistry for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 1458 kb)
Rights and permissions
About this article
Cite this article
Zhu, Sf., Zhang, Sh., Gou, Rj. et al. Theoretical investigation of the effects of the molar ratio and solvent on the formation of the pyrazole–nitroamine cocrystal explosive 3,4-dinitropyrazole (DNP)/2,4,6,8,10,12-hexanitrohexaazaisowurtzitane (CL-20). J Mol Model 23, 353 (2017). https://doi.org/10.1007/s00894-017-3516-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3516-4