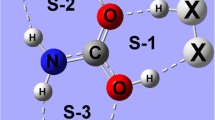
Abstract
Ab initio calculations have been performed to study the structures, binding energies, and bonding properties of the hemi-bonded binary complexes (XH2P···NH2Y)+ with the substituents X and Y being H, F, Cl, Br, NH2, CH3, and OH. The P···N interactions in these open-shelled systems have typical pnicogen bond characteristics but much stronger than the usual pnicogen bonds in closed-shell systems. This P···N bond can be strengthened by an electron-withdrawing substituent X or an electron-donating substituent Y, the bonding energy varies from 17 kcal mol-1 of (CH3H2P···NH2F)+ to 54 kcal mol-1 of (FH2P···NH2CH3)+. A nearly linear X-P···N arrangement is required by the pnicogen bond P···N and results in a strong hyperconjugation and charge transfer from the N lone pair to the X-P σ* antibond orbital for α spin, the P···N interaction is described as a single-electron σ bond of β spin. The AIM and NBO analyses revealed that the P···N bonds in the majority of the hemi-bonded complexes are partly covalent in nature.

The P···N interactions in the open-shelled systems (XH2P···NH2Y)+ (X, Y=H, F, Cl, Br, NH2, CH3, OH) with bonding energy of 17~54 kcal mol-1 have typical pnicogen bond characteristics but much stronger than the usual pnicogen bonds in closed-shell systems. This P···N bond can be strengthened by an electron-withdrawing substituent X or an electron-donating substituent Y




Similar content being viewed by others
References
Hobza P, Zahradník R (1980) Weak intermolecular interactions in chemistry and biology, vol 200. Elsevier, Amsterdam
Kaplan G (1986) Theory of molecular interactions. Elsevier, Amsterdam
Hobza P, Zahradnik R (1988) Intermolecular complexes. Elsevier, Amsterdam
Müller-Dethlefs K, Hobza P (2000) Noncovalent interactions: a challenge for experiment and theory. Chem Rev 100:143–168
Stone AJ (2002) The theory of intermolecular forces. Oxford University Press, Oxford
Hobza P, Zahradník R, Müller-Dethlefs K (2006) The world of noncovalent interactions. Collect Czechoslov Chem Commun 71:443–531
Hobza P, Müller-Dethlefs K (2010) Non-covalent interactions: theory and experiment. Royal Society of Chemistry, Cambridge
Guerra CF, Bickelhaupt FM, Snijders JG, Baerends EJ (2000) Hydrogen bonding in DNA base pairs: reconciliation of theory and experiment. J Am Chem Soc 122(17):4117–4128
Li AY (2007) Chemical origin of blue-shifted and red-shifted H-bonds: intramolecular hyperconjugation and its coupling with intermolecular hyper-conjugation. J Chem Phys 126:154102–154111
Li AY, Yan XH (2007) Electronic properties of multifurcated bent hydrogen bonds CH3···Y and CH2···Y. Phys Chem Chem Phys 9:6263–6271
Arunan E, Desiraju GR, Klein RA, Sadlej J, Scheiner S, Alkorta I, Clary DC, Crabtree RH, Dannenberg JJ, Hobza P et al (2011) Definition of the hydrogen bond (IUPAC recommendations 2011). Pure Appl Chem 83:1637–1641
Metrangolo P, Resnati G (2008) Halogen bonding: fundamentals and applications. Structure and bonding, vol 126. Springer, Berlin
Legon AC (2010) The halogen bond: an interim perspective. Phys Chem Chem Phys 12:7736–7747
Desiraju GR, Ho PS, Kloo L, Legon AC et al (2013) Definition of the halogen bond (IUPAC recommendations 2013). Pure Appl Chem 85:1711–1713
Politzer P, Murray JS (2013) Halogen bonding: an interim discussion. Chem Phys Chem 14:278–294
Nagao Y, Hirata T, Goto S, Sano S, Kakehi A, Iizuka K, Shiro M (1998) Intramolecular nonbonded S···O interaction recognized in (acylimino) thiadiazoline derivatives as angiotensin II receptor antagonists and related compounds. J Am Chem Soc 120:3104–3110
Werz DB, Gleiter R, Rominger F (2002) Nanotube formation favored by chalcogen−chalcogen interactions. J Am Chem Soc 124:10638–10639
Bleiholder C, Werz DB, Köppel H, Gleiter R (2006) Theoretical investigations on chalcogen−chalcogen interactions: what makes these nonbonded interactions bonding? J Am Chem Soc 128:2666–2674
Murray JS, Lane P, Clark T, Politzer P (2007) σ-hole bonding: molecules containing group VI atoms. J Mol Model 13:1033–1038
Adhikari U, Scheiner S (2012) Sensitivity of pnicogen, chalcogen, halogen and H-bonds to angular distortions. Chem Phys Lett 532:31–35
Wang W, Ji B, Zhang Y (2009) Chalcogen bond: a sister noncovalent bond to halogen bond. J Phys Chem A 113:8132–8135
Manna D, Mugesh G (2012) Regioselective deiodination of thyroxine by iodothyronine deiodinase mimics: an unusual mechanistic pathway involving cooperative chalcogen and halogen bonding. J Am Chem Soc 134:4269–4279
Murray JS, Lane P, Politzer P (2007) A predicted new type of directional noncovalent interaction. Int J Quantum Chem 107:2286–2292
Zahn S, Frank R, Hey-Hawkins E, Kirchner B (2011) Pnicogen bonds: a new molecular linker? Chem Eur J 17:6034–6038
Scheiner S (2011) A new noncovalent force: comparison of P···N interaction with hydrogen and halogen bonds. J Chem Phys 134:094315–094324
Scheiner S (2013) The pnicogen bond: its relation to hydrogen, halogen, and other noncovalent bonds. Acc Chem Res 46:280–288
Scheiner S (2011) Effects of substituents upon the P···N noncovalent interaction: the limits of its strength. J Phys Chem A 115:11202–11209
Del Bene JE, Alkorta I, Sanchez-Sanz G, Elguero J (2011) Structures, energies, bonding, and NMR properties of pnicogen complexes H2XP:NXH2 (X = H, CH3, NH2, OH, F, Cl). J Phys Chem A 115:13724–13731
Del Bene JE, Alkorta I, Elguero J (2015) Substituent effects on the properties of pnicogen-bonded complexes H2XP:PYH2, for X, Y = F, Cl, OH, NC, CCH, CH3, CN, and H. J Phys Chem A 119:224–233
Murray JS, Lane P, Politzer P (2009) Expansion of the σ-hole concept. J Mol Model 15:723–729
Mani D, Arunan E (2013) The X−C···Y (X = O/F, Y = O/S/F/Cl/Br/N/P) ‘carbon bond’ and hydrophobic interactions. Phys Chem Chem Phys 15:14377–14383
Mani D, Arunan E (2014) The X−C···π (X = F, Cl, Br, CN) carbon bond. J Phys Chem A 118:10081–10089
Thomas SP, Pavan MS, Row TG (2014) Experimental evidence for ‘carbon bonding’ in the solid state from charge density analysis. Chem Commun (Cambridge, UK) 50:49–51
Varadwaj PR, Varadwaj A, Jin B (2014) Significant evidence of C···O and C···C long-range contacts in several heterodimeric complexes of CO with CH3−X, should one refer to them as carbon and dicarbon bonds! Phys Chem Chem Phys 16:17238–17252
Gill PMW, Radom L (1988) Structures and stabilities of singly charged three-electron hemibonded systems and their hydrogen bonded isomers. J Am Chem Soc 110:4931–4941
Humbel S, Côte I, Hoffmann N, Bouquant J (1999) Three-electron binding between carbonyl-like compounds and ammonia radical cation. Comparison with the hydrogen bonded complex. J Am Chem Soc 121:5507–5512
Grüning M, Gritsenko OV, van Gisbergen SJA, Baerends EJ (2001) The failure of generalized gradient approximations (GGAs) and meta-GGAs for the two-center three-electron bonds in He2 +, (H2O)2 +, and (NH3)2 +. J Phys Chem A 105(40):9211–9218
Ghanty TK, Ghosh SK (2002) Simple density functional approach to polarizability, hardness, and covalent radius of atomic systems. J Phys Chem A 106(48):11815–11821
Maity DK (2002) Sigma bonded radical cation complexes: a theoretical study. J Phys Chem A 106(23):5716–5721
Ghanty TK, Ghosh SK (2002) Hardness and polarizability profiles for intramolecular proton transfer in water dimer radical cation. J Phys Chem A 106(16):4200–4204
Joshi R, Ghanty TK, Naumov S, Mukherjee T (2007) Structural investigation of asymmetrical dimer radical cation system (H2O−H2S)+: proton-transferred or hemi-bonded? J Phys Chem A 111:2362–2367
Joshi R, Ghanty TK, Naumov S, Mukherjee T (2007) Ionized state of hydroperoxy radical−water hydrogen-bonded complex: (HO2−H2O)+. J Phys Chem A 111(51):13590–13594
Lee HM, Kim KS (2009) Water dimer cation: density functional theory vs Ab initio theory. J Chem Theory Comput 5(4):976–981
Kim Hand Lee HM (2009) Ammonia−water cation and ammonia dimer cation. J Phys Chem A 113(25):6859–6864
Pan PR, Lin YS, Tsai MK, Kuo JL, Chai JD (2012) Assessment of density functional approximations for the hemibonded structure of the water dimer radical cation. Phys Chem Chem Phys 14:10705–10712
Joshi R, Ghanty TK, Mukherjee T, Naumov S (2012) Hydrogen bonding in neutral and cation dimers of H2Se with H2O, H2S, and H2Se. J Phys Chem A 116(48):11965–11972
Do Hand Besley NA (2013) Proton transfer or hemibonding? The structure and stability of radical cation clusters. Phys Chem Chem Phys 15(38):16214–16219
Tentscher PR, Arey JS (2013) On the nature of interactions of radicals with polar molecules. J Phys Chem A 117(47):12560–12568
Ji LF, Li AY, Li ZZ (2015) Structures and stabilities of asymmetrical dimer radical cation systems (AH3–H2O)+ (A=N, P, As). Struct Chem 26:109–119
Marín-Luna M, Alkorta I, Elguero J (2015) A computational study on [(PH2X)2]·+ homodimers involving intermolecular two-center three-electron bonds. Struct Chem. doi:10.1007/s11224-015-0617-5
Clark T, Hennemann M, Murray JS, Politzer P (2007) Halogen bonding: the σ-hole. J Mol Model 13:291–296
Mohajeri A, Pakirai AH, Bagheri N (2009) Theoretical studies on the nature of bonding in σ-hole complexes. Chem Phys Lett 467:393–397
Politzer P, Murray JS, Clark T (2013) Halogen bonding and other σ-hole interactions: a perspective. Phys Chem Chem Phys 15:11178–11189
Ji LF, Li AY, Li ZZ (2015) Structures and stabilities of hemi-bonded vs proton-transferred isomers of dimer radical cation systems (XH3-YH3)+(X, Y = N, P, As). Chem Phys Lett 619:115–121
Frisch MJ, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, CaricatoM, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW,Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A.02. Gaussian, Inc, Wallingford
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Weinhold F, Landis C (2005) Valency and bonding. A natural bond orbital donor–acceptor perspective. Cambridge University Press
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Glendening ED et al (2001) NBO 5.0. Theoretical Chemistry Institute, University of Wisconsin: Madison
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735–746
Wiberg KB (1968) Tetrahedron 24:1083–1096
Reed AE, Weinhold F (1985) Natural localized molecular orbitals. J Chem Phys 83:1736–1740
Bader RFW (1990) Atoms in molecules—a quantum theory. Oxford University Press, Oxford
Ziołkowski M, Grabowski SJ, Leszczynski J (2006) Cooperativity in hydrogen-bonded interactions: ab initio and “atoms in molecules” analyses. J Phys Chem A 110(20):6514–6521
Cremer D, Kraka E (1984) Angew Chem Int Ed Engl 23:627–628
Jenkins S, Morrison I (2000) The chemical character of the intermolecular bonds of seven phases of ice as revealed by ab initio calculation of electron densities. Chem Phys Lett 317:97–102
Keith TA (2011) AIMAll (Versio Version 13.10.19). TK Gristmill software, Overland Park (aim.tkgristmill.com)
Alkorta I, Barrios L, Rozas I, Elguero J (2000) Comparison of models to correlate electron density at the bond critical point and bond distance. J Mol Struct THEOCHEM 496:131–137
Madzhidov TI, Chmutova GA (2013) The nature of the interaction of dimethylselenide with IIIA group element compounds. J Phys Chem A 117:4011–4024
Politzer P, Murray JS, Clark T (2015) Mathematical modeling and physical reality in noncovalent interactions. J Mol Model 21:52
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 85546 kb)
Rights and permissions
About this article
Cite this article
Ji, L.F., Li, A.Y., Li, Z.Z. et al. Substituent effects on the properties of the hemi-bonded complexes (XH2P···NH2Y)+ (X, Y=H, F, Cl, Br, NH2, CH3, OH). J Mol Model 22, 1 (2016). https://doi.org/10.1007/s00894-015-2876-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2876-x