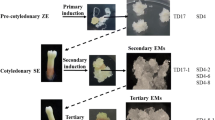
Abstract
The wild grass species Brachypodium distachyon (L.) has been proposed as a new model for temperate grasses. Among the biotechnological tools already developed for the species, an efficient induction protocol of somatic embryogenesis (SE) using immature zygotic embryos has provided the basis for genetic transformation studies. However, a systematic work to better understanding the basic cellular and molecular mechanisms that underlie the SE process of this grass species is still missing. Here, we present new insights at the morpho-histological, histochemical, and molecular aspects of B. distachyon SE pathway. Somatic embryos arose from embryogenic callus formed by cells derived from the protodermal-dividing cells of the scutellum. These protodermal cells showed typical meristematic features and high protein accumulation which were interpreted as the first observable steps towards the acquisition of a competent state. Starch content decreased along embryogenic callus differentiation supporting the idea that carbohydrate reserves are essential to morphogenetic processes. Interestingly, starch accumulation was also observed at late stages of SE process. Searches in databanks revealed three sequences available annotated as BdSERK, being two copies corresponding to SERK1 and one showing greater identity to SERK2. In silico analysis confirmed the presence of characteristic domains in a B. distachyon Somatic Embryogenesis Receptor Kinase genes candidates (BdSERKs), which suggests SERK functions are conserved in B. distachyon. In situ hybridization demonstrated the presence of transcripts of BdSERK1 in all development since globular until scutellar stages. The results reported in this study convey important information about the morphogenetic events in the embryogenic pathway which has been lacking in B. distachyon. This study also demonstrates that B. distachyon provides a useful model system for investigating the genetic regulation of SE in grass species.








Similar content being viewed by others
References
aan den Toorn M, Albrecht C, de Vries S (2015) On the origin of SERKs: bioinformatics analysis of the somatic embryogenesis receptor kinases. Mol Plant 8:762–782
Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N (2010) Consurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res 38:529--533
Baudino S, Hansen S, Brettschneider R, Hecht VEG, Dresselhaus T, Lorz H, Dumas C, Rogowsky PM (2001) Molecular characterization of two novel maize LRR receptor-like kinases, which belong to the SERK gene family. Planta 213:1–10
Betekhtin A, Rojek M, Milewska-Hendel A, Gawecki R, Karcz J, Kurczyńska E, Hasterok R (2016) Spatial distribution of selected chemical cell wall components in the embryogenic callus of Brachypodium distachyon. PLoS One 11(11):e0167426
Bewley JD, Black M (1994) Seeds: physiology of development and germination. Plenum, London
Brisibe EA, Nishioka D, Miyake H, Taniguchi T, Maeda E (1993) Developmental electron microscopy and histochemistry of somatic embryo differentiation in sugarcane. Plant Sci 89:85–92
Brkljacic J, Grotewold E, Scholl R, Mockler T, Garvin DF, Vain P, Brutnell T, Sibout R, Bevan M, Budak H, Caicedo AL, Gao C, Gu Y, Hazen SP, Holt BF III, Hong SY, Jordan M, Manzaneda AJ, Mitchell-Olds T, Mochida K, Mur LAJ, Park CM, Sedbrook J, Watt M, Zheng SJ, Vogel JP (2011) Brachypodium as a model for the grasses: today and the future. Plant Physiol 157:3–13
Cabral GB, Carneiro VTC, Rossi ML, Silva JP, Martinelli AP, Dusi DMA (2015) Plant regeneration from embryogenic callus and cell suspensions of Brachiaria brizantha. In Vitro Cell Dev Biol—Plant 51:369–377
Cangahuala-Inocente GC, Steiner N, Santos M, Guerra MP (2004) Morphological analysis and histochemistry of Feijoa sellowiana somatic embryogenesis. Protoplasma 224:33–40
Cangahuala-Inocente GC, Steiner N, Maldonado SB, Guerra MP (2009) Patterns of protein and carbohydrate accumulation during somatic embryogenesis of Acca sellowiana. Pesq Agropec Bras 44:217–224
Canhoto JM, Cruz GS (1996) Histodifferentiation of somatic embryos in cotyledons of pineapple guava (Feijoa sellowiana Berg.). Protoplasma 19:34–45
Chandler JW (2008) Cotyledon organogenesis. J Exp Bot 59:2917–2931
De Fillipis LF (2014) Crop improvement through tissue culture. In: Ahmad P, Wani MR, Azooz MM, Tran LSP (eds) Improvement of crops in the era of climate changes, 1st edn. Springer, New York, pp 289–346
Delporte F, Pretova A, du Jardin P, Watillon B (2014) Morpho-histology and genotype dependence of in vitro morphogenesis in mature embryo cultures of wheat. Protoplasma 251:1455–1470
Draper J, Mur LAJ, Jenkins G, Ghosh-Biswas GC, Bablak P, Hasterok R, Routledge APM (2001) Brachypodium distachyon. A new model system for functional genomics in grasses. Plant Physiol 127:1539–1555
Feder N, O’Brien TP (1968) Plant microtechnique: some principles and new methods. Am J Bot 55:123–142
Fehér A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tiss Organ Cult 74:201–228
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fitzgerald TL, Powell JJ, Schneebeli K, Hsia MM, Gardiner DM, Bragg JN, McIntyre CL, Manners JM, Ayliffe M, Watt M, Vogel JP, Henry RJ, Kazan K (2015) Brachypodium as an emerging model for cereal-pathogen interactions. Ann Bot 115:717–731
Fortes AM, Pais MS (2000) Organogenesis from internode-derived nodules of Humulus lupulus var. Nugget (Cannabinaceae): histological studies and changes in the starch content. Am J Bot 87:971–979
Ge XX, Fan GE, Chai LJ, Guo WW (2010) Cloning, molecular characterization and expression analysis of a SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE gene (CitSERK1-like) in Valencia sweet orange. Acta Physiol Plant 32:1197–1207
Girin T, David LC, Chardin C, Sibout R, Krapp A, Ferrario-Méry S, Daniel-Vedele F (2014) Brachypodium: a promising hub between model species and cereals. J Exp Bot 65(19):5683–5696. doi:10.1093/jxb/eru376
Gruszczyńska A, Rakoczy-Trojanowska M (2011) Expression analysis of somatic embryogenesis-related SERK, LEC1, VP1 and NiR ortologues in rye (Secale cereale L.). J Appl Genet 52:1–8
Guillon F, Bouchet B, Jamme F, Robert P, Quemener B, Barron C, Larre C, Dumas P, Saulnier L (2011) Brachypodium distachyon grain: characterization of endosperm cell walls. J Exp Bot 62:1001–1015
Hecht V, Vielle-Calzada JP, Hartog MV, Schmidt ED, Boutilier K, Grossniklaus U (2001) The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol 127:803–816
Hu H, Xiong L, Yang Y (2005) Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection. Planta 222:107–117
Karami O, Aghavaisi B, Pour AM (2009) Molecular aspects of somatic-to-embryogenic transition in plants. J Chem Biol 2:177–190
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137–138
Kellogg EA (2015) Brachypodium distachyon as a genetic model system. Annu Rev Genet 49:1–20
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Kurczyńska EU, Gaj MD, Ujczak A, Mazur E (2007) Histological analysis of direct somatic embryogenesis in Arabidopsis thaliana (L.) Heynh. Planta 226:619–628
Kurczyńska EU, Potocka I, Dobrowolska I, Kulinskalukaszek K, Sala K, Wrobel J (2012) Cellular markers for somatic embryogenesis. In: Sato KI (ed) Embryogenesis. InTech, Rijeka, pp 307–332
Kwaaitaal MACJ, De Vries SC (2007) The SERK1 gene is expressed in procambium and immature vascular cells. J Exp Bot 58:2887–2896
Landau M, Mayrose I, Rosenberg Y, Glaser F, Martz E, Pupko T, Ben-Tal N (2005) Consurf 2005: the projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res 33:299--302
Lenis-Manzano SJ, Araujo ACG, Valle CB, Santana EF, Carneiro VTC (2010) Histologia da embriogênese somática induzida em embriões de sementes maduras de Urochloa brizantha apomítica. Pesq Agropec Bras 45:435–441
Li X, Fang YH, Han JD, Bai SN, Rao GY (2015) Isolation and characterization of a novel SOMATIC EMBRYOGENESIS RECEPTOR KINASE gene expressed in the fern Adiantum capillus-veneris during shoot regeneration in vitro. Plant Mol Biol Rep 33:638–647
Liu B, Su S, Wu Y, Li Y, Shan X, Li S, Liu H, Dong H, Ding M, Han J, Yuan Y (2015) Histological and transcript analyses of intact somatic embryos in an elite maize (Zea mays L.) inbred line Y423. Plant Physiol Biochem 92:81–91
Mariani TS, Miyake H, Takeoka Y (1998) Changes in surface structure during direct somatic embryogenesis in rice scutellum observed by scanning electron microscopy. Plant Prod Sci 1:223–231
Martin AB, Cuadrado Y, Guerra H, Gallego P, Hita O, Martin L, Dorado A, Villalobos N (2000) Differences in the contents of total sugars, reducing sugars, starch and sucrose in embryogenic and non-embryogenic calli from Medicago arborea L. Plant Sci 29:143–151
Moura EF, Ventrella MC, Motoike SY (2010) Anatomy, histochemistry and ultrastructure of seed and somatic embryo of Acrocomia aculeata (Arecaceae). Sci Agric 67:399–407
Moura EF, Ventrella MC, Motoike SY, Sá Júnior AQ, Carvalho M, Manfio CE (2008) Histological study of somatic embryogenesis induction on zygotic embryos of macaw palm (Acrocomia aculeata (Jacq.) Lodd. ex Martius). Plant Cell Tiss Organ Cult 95:175-184
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Namasivayam P (2007) Acquisition of embryogenic competence during somatic embryogenesis. Plant Cell Tiss Organ Cult 90:1–8
Nolan KE, Irwanto RR, Rose RJ (2003) Auxin upregulates MtSERK1 expression in both Medicago truncatula root-forming and embryogenic cultures. Plant Physiol 133:218–230
Nolan KE, Kurdyukov S, Rose RJ (2009) Expression of the SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 (SERK1) gene is associated with developmental change in the life cycle of the model legume Medicago truncatula. J Exp Bot 60:1759–1771
Nolan KE, Kurdyukov S, Rose RJ (2011) Characterisation of the legume SERK-NIK gene superfamily including splice variants: Implication for development and defence. BMC Plant Biol 11:44
Nonohay JS, Mariath JEA, Winge H (1999) Histological analysis of somatic embryogenesis in Brazilian cultivars of barley, Hordeum vulgare vulgare, Poaceae. Plant Cell Rep 18:929–934
O’Brien TP, McCully ME (1981) The study of plant structure principles and selected methods. Termarcarphi Pty, Melbourne
Opanowicz M, Vain P, Draper J, Parker D, Doonan JHS (2008) Brachypodium distachyon: making hay with a wild grass. Trends Plant Sci 13:172–177
Opanowicz M, Hands P, Betts D, Parker ML, Toole GA, Mills EN, Doonan JH, Drea S (2011) Endosperm development in Brachypodium distachyon. J Exp Bot 62:735–748
Ozias-Akins P, Vasil IK (1982) Plant regeneration from cultured immature embryos and inflorescences of Triticum aestivum L. (wheat): evidence for somatic embryogenesis. Protoplasma 110:95–105
Pan X, Yang X, Lin G, Zou R, Chen H, Samaj J, Xu C (2011) Ultrastructural changes and the distribution of arabinogalactan proteins during somatic embryogenesis of banana (Musa spp. AAA cv. ‘Yueyoukang 1’). Physiol Plant 142:372–389
Pearse AGE (1980) Histochemistry theoretical and applied. Churchill Livingston, Edinburgh
Pérez-Nuñéz MT, Souza R, Sáenz L, Chan JL, Zúñiga-Aguilar JJ, Oropeza C (2009) Detection of a SERK-like gene in coconut and analysis of its expression during the formation of embryogenic callus and somatic embryos. Plant Cell Rep 28:11–19
Petersen TN, Brunak S, Von Heijne G, Nielsen H (2011) Signal P 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786
Pilarska M, Malec P, Salaj J, Bartnicki F, Konieczny R (2016) High expression of SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE coincides with initiation of varius developmental pathways in vitro culture of Trifolium nigrescens. Protoplasma 253:345–355
Pinto G, Silva S, Araújo C, Neves L, Santos C (2010) Histocytological changes and reserves accumulation during somatic embryogenesis in Eucalyptus globulus. Trees 24:763–769
Quiroz-Figueroa FR, Fuentes-Cerda CFJ, Rojas-Herrera R, Loyola-Vargas VM (2002) Histological studies on the developmental stages and differentiation of two different somatic embryogenesis systems of Coffea arabica. Plant Cell Rep 20:1141–1149
Quiroz-Figueroa FR, Rojas-Herrera R, Galaz-Avalos RM, Loyola-Vargas VM (2006) Embryo production through somatic embryogenesis can be used to study cell differentiation in plants. Plant Cell Tissue Organ Cult 86:285–301
Reynolds ES (1963) The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol 17:208–212
Rocha DI, Dornelas MC (2013) Molecular overview on plant somatic embryogenesis. CAB Rev 8:1–17
Rocha DI, Vieira LM, Tanaka FAO, Silva LC, Otoni WC (2012) Somatic embryogenesis of a wild passion fruit species Passiflora cincinnata Masters: histocytological and histochemical evidences. Protoplasma 249:747–758
Rocha DI, Pinto DLP, Vieira LM, Tanaka FAO, Dornelas MC, Otoni WC (2016) Cellular and molecular changes associated with competence acquisition during passion fruit somatic embryogenesis: ultrastructural characterization and analysis of SERK gene expression. Protoplasma 253:595–609
Saitou N, Nei M (1987) The neighbour joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406--425
Santa-Catarina C, Hanai LR, Dornelas MC, Viana AM, Floh EIS (2004) SERK gene homolog expression, polyamines and amino acids associated with somatic embryogenic competence of Ocotea catharinensis Mez. (Lauraceae). Plant Cell Tiss Organ Cult 79:53–61
Santiago J, Henzler C, Hothorn M (2013) Molecular mechanism for plant steroid receptor activation by somatic embryogenesis co-receptor kinases. Science 341:889-892
Savona M, Mattioli R, Nigro S, Falasca G, Della Rovere F, Costantino P, De Vries S, Ruffoni B, Trovato M, Altamura MM (2012) Two SERK genes are markers of pluripotency in Cyclamen persicum Mill. J Exp Bot 63:471–488
Schmidt ED, Guzzo F, Toonen MA, Vries SC (1997) A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 124:2049–2062
Schwarz R, Dayhoff M (1979) Matrices for detecting distant relationships. In Dayhoff M (ed) Atlas of protein sequences, National Biomedical Research Foundation. pp 353--358
Sharma SK, Millam S, Hein I, Bryan GJ (2008) Cloning and molecular characterization of a potato SERK gene transcriptionally induced during initiation of somatic embryogenesis. Planta 228:319–330
Silva GM, Cruz ACF, Otoni WC, Pereira TNS, Rocha DI, Silva ML (2015) Histochemical evaluation of induction of somatic embryogenesis in Passiflora edulis Sims (Passifloraceae). In Vitro Cell Dev Biol-Plant 51:539–545
Singla B, Khurana JP, Khurana P (2008) Characterization of three somatic embryogenesis receptor kinase genes from wheat, Triticum aestivum. Plant Cell Rep 27:833–843
Smertenko A, Bozhkov PV (2014) Somatic embryogenesis: life and death processes during apical-basal patterning. J Exp Bot 65:1343–1460
Somleva MN, Schmidt EDL, De Vries SC (2000) Embryogenic cells in Dactylis glomerata L. (Poaceae) explants identified by cell tracking and by SERK expression. Plant Cell Rep 19:718–726
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Steiner N, Santa-Catarina C, Guerra MP, Cutri L, Dornelas MC, Floh EIS (2012) A gymnosperm homolog of SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE-1 (SERK1) is expressed during somatic embryogenesis. Plant Cell Tiss Organ Cult 109:41–50
Su YH, Zhao XY, Liu YB, Zhang CL, O’Neill SD, Zhang XS (2009) Auxin-induced WUS expression is essential for embryogenic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J 59:448–460
Taylor MG, Vasil IK (1996) The ultrastructure of somatic embryo development in pearl millet (Pennisetum glaucum; Poaceae). Am J Bot 83:28–44
Tomlinson K, Denyer K, Callow JA (2003) Starch synthesis in cereal grains. In: Callow JA (ed) Advances in botanical research. Academic, London, pp 1–61
Vain P (2011) Brachypodium as a model system for grass research. J Cereal Sci 54:1–7
Vasil V, Lu C, Vasil IK (1985) Histology of somatic embryogenesis in cultured immature embryos of maize (Zea mays L.). Protoplasma 127:1–8
Verdeil JL, Hocher V, Huet C, Grosdemange F, Escoute J, Ferriere N, Nicole M (2001) Ultrastructural changes in coconut calli associated with the acquisition of embryogenic competence. Ann Bot 88:9–18
Verdeil JL, Alemanno L, Niemenak N, Trambarger TJ (2007) Pluripotent versus totipotent plant stem cells: dependence versus autonomy? Trends Plant Sci 12:245–252
Vernoud V, Hajduch M, Khaled A-S, Depège N, Rogowski P (2005) Maize embryogenesis. Maydica 50:469–483
Vidal BC (1977) Acid glycosaminoglycans and endochondral ossification microespectrophotometric evaluation and macromolecular orientation. Cell Mol Biol 22:45–64
Vogel JP (2016) Plant genetics and genomics: crops and models, v. 18, Genetics and genomics of Brachypodium. Springer International Publishing, Heidelberg
Vogel J, Bragg J (2009) Brachypodium distachyon, a new model for the Triticeae. In: Feuillet C, Muehlbauer GJ (eds) Genetics and genomics of the Triticeae, vol 7, 1st edn. Springer-Verlag, New York, pp 427–449
Von Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L (2002) Developmental pathways of somatic embryogenesis. Plant Cell Tiss Organ Cult 69:233–249
Wrobel J, Barlow PW, Gorka K, Nabialkowska D, Kurczyńska EU (2011) Histology and symplasmic tracer distribution during development of barley androgenic embryos. Planta 233:873–881
Yang X, Zhang X (2010) Regulation of somatic embryogenesis in higher plants. Crit Rev Plant Sci 29:36–57
Ye XG, Tao LL (2008) Research outline on some characteristics of Brachypodium distachyon as a new model plant species. Acta Agron Sin 34:919–925
Zhang S, Liu X, Lin Y, Xie G, Fu F, Liu H, Wang J, Gao S, Lan H, Rong T (2011) Characterization of a ZmSERK gene and its relationship to somatic embryogenesis in a maize culture. Plant Cell Tiss Organ Cult 105:29–37
Zimmerman JL (1993) Somatic embryogenesis: a model for early development in higher plants. Plant Cell 5:1411–1423
Acknowledgments
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Brasília, DF, Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (Brasília, DF, Brazil), and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) (Belo Horizonte, MG, Brazil). Caio G. Otoni is also acknowledged for the English revision.
Authors’ contributions
FTSN and WCO designed the research; EJO established the embryogenic cultures; ACFC, EJO, and LMV performed the light microscopy analysis; DIR and FAOT performed the scanning and transmission electron microscopy analyses; ADK, LMV, MVMP, EMM, and TCRS performed the characterization of sequences and in situ hybridization analysis; and ADK, DIR, EMM, EJO, FTSN, and WCO wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Handling Editor: Peter Nick
Electronic supplementary material

Figure S1
Discrimination of signal peptide from deduced amino acid sequences of BdSERK using SignalP v. 4.1. Server. (GIF 119 kb)
Figure S2
Phylogenetic relationship of SERK proteins. The evolutionary history was inferred using the Neighbor-Joining method (Saitou and Nei, 1987). The optimal tree with the sum of branch length = 7.11556016 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches (Felsenstein 1985). The evolutionary distances were computed using the Dayhoff matrix based method and are in the units of the number of amino acid substitutions per site (Schwarz and Dayhoff 1979). The analysis involved 65 amino acid sequences of SERK proteins and 4 LRRII-RLK non-SERKs, available at Genbank. All positions containing gaps and missing data were eliminated. There were a total of 287 positions in the final dataset. Evolutionary analyses were conducted in MEGA7 (Kumar et al. 2016). (PNG 40 kb)
Rights and permissions
About this article
Cite this article
Oliveira, E.J., Koehler, A.D., Rocha, D.I. et al. Morpho-histological, histochemical, and molecular evidences related to cellular reprogramming during somatic embryogenesis of the model grass Brachypodium distachyon . Protoplasma 254, 2017–2034 (2017). https://doi.org/10.1007/s00709-017-1089-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-017-1089-9