Abstract
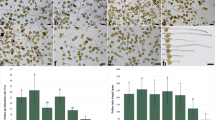
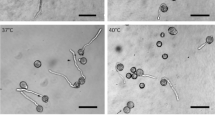
The cell wall of pollen tubes is organized in both spatial and temporal order to allow the pollen tube to grow according to external conditions. The deposition of methyl-esterified and acid pectins in addition to callose/cellulose occurs according to a series of temporally succeeding events. In this work, we attempted to determine how the composition of the external growth medium (in terms of osmolarity) could affect the deposition of cell wall components. Pollen tubes of tobacco were grown in a hypotonic medium and then analyzed for the distribution of pectins and callose/cellulose [as well as for the distribution of the enzyme callose synthase (CALS)]. The data indicate that pollen tubes grown in a hypotonic medium show changes of the initial growth rate followed by modification of the deposition of acid pectins and, to a lesser extent, of CALS. These observations indicate that, under the osmolarity determined by the growth medium, pollen tubes adapt their cell wall to the changing conditions of growth.








Similar content being viewed by others
References
Anderson JR, Barnes WS, Bedinger P (2002) 2,6-Dichlorobenzonitrile, a cellulose biosynthesis inhibitor, affects morphology and structural integrity of petunia and lily pollen tubes. J Plant Physiol 159:61–67
Aouar L, Chebli Y, Geitmann A (2010) Morphogenesis of complex plant cell shapes: the mechanical role of crystalline cellulose in growing pollen tubes. Sex Plant Reprod 23:15–27
Benkert R, Obermeyer G, Bentrup F-W (1997) The turgor pressure of growing lily pollen tubes. Protoplasma 198:1–8
Bosch M, Hepler PK (2005) Pectin methylesterases and pectin dynamics in pollen tubes. Plant Cell 17:3219–3226
Bosch M, Hepler PK (2006) Silencing of the tobacco pollen pectin methylesterase NtPPME1 results in retarded in vivo pollen tube growth. Planta 223:736–745
Bosch M, Cheung AY, Hepler PK (2005) Pectin methylesterase, a regulator of pollen tube growth. Plant Physiol 138:1334–1346
Bougourd S, Marrison J, Haseloff J (2000) An aniline blue staining procedure for confocal microscopy and 3D imaging of normal and perturbed cellular phenotypes in mature Arabidopsis embryos. Plant J 24:543–550
Boyer JS (2009) Evans Review: cell wall biosynthesis and the molecular mechanism of plant enlargement. Funct Plant Biol 36:383–394
Brewbaker JL, Kwack BH (1963) The essential role of calcium ion in pollen germination and pollen tube growth. Amer J Bot 50:859–865
Brownfield L, Ford K, Doblin MS, Newbigin E, Read S, Bacic A (2007) Proteomic and biochemical evidence links the callose synthase in Nicotiana alata pollen tubes to the product of the NaGSL1 gene. Plant J 52:147–156
Brownfield L, Wilson S, Newbigin E, Bacic A, Read S (2008) Molecular control of the glucan synthase-like protein NaGSL1 and callose synthesis during growth of Nicotiana alata pollen tubes. Biochem J 414:43–52
Cai G, Romagnoli S, Moscatelli A, Ovidi E, Gambellini G, Tiezzi A, Cresti M (2000) Identification and characterization of a novel microtubule-based motor associated with membranous organelles in tobacco pollen tubes. Plant Cell 12:1719–1736
Cai G, Faleri C, Del Casino C, Emons AMC, Cresti M (2011) Distribution of callose synthase, cellulose synthase and sucrose synthase in tobacco pollen tube is controlled in dissimilar ways by actin filaments and microtubules. Plant Physiol 155:1169–1190
Cardenas L, Lovy-Wheeler A, Kunkel JG, Hepler PK (2008) Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiol 146:1611–1621
Chebli Y, Kaneda M, Zerzour R, Geitmann A (2012) The cell wall of the Arabidopsis pollen tube—spatial distribution, recycling, and network formation of polysaccharides. Plant Physiol 160:1940–1955
Chen LQ, Ye D (2007) Roles of pectin methylesterases in pollen-tube growth. J Int Plant Biol 49:94–98
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–861
Cresti M, van Went JL (1976) Callose deposition and plug formation in Petunia pollen tubes in situ. Planta 133:35–40
Cresti M, Pacini E, Ciampolini F, Sarfatti G (1977) Germination and early tube development in vitro of Lycopersicum peruvianum pollen: ultrastructural features. Planta 136:239–247
Derksen J, Janssen GJ, Wolters-Arts M, Lichtscheidl I, Adlassnig W, Ovecka M, Doris F, Steer M (2011) Wall architecture with high porosity is established at the tip and maintained in growing pollen tubes of Nicotiana tabacum. Plant J 68:495–506
Ferguson C, Teeri TT, Siika-aho M, Read SM, Bacic A (1998) Location of cellulose and callose in pollen tubes and grains of Nicotiana tabacum. Planta 206:452–460
Geitmann A, Ortega JKE (2009) Mechanics and modeling of plant cell growth. Trends Plant Sci 14:467–478
Geitmann A, Li Y-Q, Cresti M (1996) The role of cytoskeleton and dictyosome activity in the pulsatory growth of Nicotiana tabacum and Petunia hybrida pollen tubes. Bot Acta 109:102–109
Hepler PK, Rounds CM, Winship LJ (2013) Control of cell wall extensibility during pollen tube growth. Mol Plant 6:998–1017
Hill AE, Shachar-Hill B, Skepper JN, Powell J, Shachar-Hill Y (2012) An osmotic model of the growing pollen tube. PLoS One 7:e36585
Idilli AI, Morandini P, Onelli E, Rodighiero S, Caccianiga M, Moscatelli A (2013) Microtubule depolymerization affects endocytosis and exocytosis in the tip and influences endosome movement in tobacco pollen tubes. Mol Plant 6:1109–1130
Kroeger J, Geitmann A (2012a) The pollen tube paradigm revisited. Curr Opin Plant Biol 15:618–624
Kroeger JH, Geitmann A (2012b) Pollen tube growth: getting a grip on cell biology through modeling. Mech Res Comm 42:32–39
Kroeger JH, Zerzour R, Geitmann A (2011) Regulator or driving force? The role of turgor pressure in oscillatory plant cell growth. PLoS One 6:e18549. doi:10.1371/journal.pone.0018549
Kroh M, Knuiman B (1982) Ultrastructure of cell wall and plugs of tobacco pollen tubes after chemical extraction of polysaccharides. Planta 154:241–250
Laitiainen E, Nieminen KM, Vihinen H, Raudaskoski M (2002) Movement of generative cell and vegetative nucleus in tobacco pollen tubes is dependent on microtubule cytoskeleton but independent of the synthesis of callose plugs. Sex Plant Reprod 15:195–204
Li Y-Q, Chen F, Linskens HF, Cresti M (1994) Distribution of unesterified and esterified pectins in cell walls of pollen tubes of flowering plants. Sex Plant Reprod 7(145–152):145–152
Li Y-Q, Faleri C, Geitmann A, Zhang HQ, Cresti M (1995) Immunogold localization of arabinogalactan proteins, unesterified and esterified pectins in pollen grains and pollen tubes of Nicotiana tabacum L. Protoplasma 189:26–36
Li Y-Q, Zhang H-Q, Pierson ES, Huang F-Y, Linskens HF, Hepler PK, Cresti M (1996) Enforced growth-rate fluctuation causes pectin ring formation in the cell wall of Lilium longiflorum pollen tubes. Planta 200:41–49
Loguercio LL (2002) Pollen treatment in high osmotic potential: a simple tool for in vitro preservation and manipulation of viability in gametophytic populations. Braz J Plant Physiol 14:65–70
Lopez-Casado G, Matas AJ, Dominguez E, Cuartero J, Heredia A (2007) Biomechanics of isolated tomato (Solanum lycopersicum L.) fruit cuticles: the role of the cutin matrix and polysaccharides. J Exp Bot 58:3875–3883
McKenna ST, Kunkel JG, Bosch M, Rounds CM, Vidali L, Winship LJ, Hepler PK (2009) Exocytosis precedes and predicts the increase in growth in oscillating pollen tubes. Plant Cell 21:3026–3040
Meng D, Gu Z, Yuan H, Wang A, Li W, Yang Q, Zhu Y, Li T (2014) The microtubule cytoskeleton and pollen tube Golgi-vesicle system are required for in vitro S-RNase internalization and gametic self incompatibility in apple. Plant Cell Physiol. doi:10.1093/pcp/pcu031
Messerli MA, Robinson KR (2003) Ionic and osmotic disruptions of the lily pollen tube oscillator: testing proposed models. Planta 217:147–157
Parre E, Geitmann A (2005a) More than a leak sealant. The mechanical properties of callose in pollen tubes. Plant Physiol 137:274–286
Parre E, Geitmann A (2005b) Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta 220:582–592
Peng L, Zhang L, Cheng X, Fan LS, Hao HQ (2013) Disruption of cellulose synthesis by 2,6-dichlorobenzonitrile affects the structure of the cytoskeleton and cell wall construction in Arabidopsis. Plant Biol 15:405–414
Persia D, Cai G, Del Casino C, Faleri C, Willemse MTM, Cresti M (2008) Sucrose synthase is associated with the cell wall of tobacco pollen tubes. Plant Physiol 147:1603–1618
Picton JM, Steer MW (1981) Determination of secretory vesicle production rates by dictyosomes in pollen tubes of Tradescantia using cytochalasin D. J Cell Sci 49:261–272
Qin Y, Chen D, Zhao J (2007) Localization of arabinogalactan proteins in anther, pollen, and pollen tube of Nicotiana tabacum L. Protoplasma 231:43–53
Rockel N, Wolf S, Kost B, Rausch T, Greiner S (2008) Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis, and reflects the distribution of esterified and de-esterified pectins. Plant J 53:133–143
Schopfer P (2006) Biomechanics of plant growth. Am J Bot 93:1415–1425
Stepka M, Ciampolini F, Charzynska M, Cresti M (2000) Localization of pectins in the pollen tube wall of Ornithogalum virens L. Does the pattern of pectin distribution depend on the growth rate of the pollen tube? Planta 210:630–635
Taiz L (1984) Plant cell expansion: regulation of cell wall mechanical properties. Ann Rev Plant Physiol 35:585–657
Taylor LP, Hepler PK (1997) Pollen germination and tube growth. Ann Rev Plant Physiol Plant Mol Biol 48:461–491
Tian GW, Chen MH, Zaltsman A, Citovsky V (2006) Pollen-specific pectin methylesterase involved in pollen tube growth. Dev Biol 294:83–91
Turner A, Bacic A, Harris PJ, Read SM (1998) Membrane fractionation and enrichment of callose synthase from pollen tubes of Nicotiana alata Link et Otto. Planta 205:380–388
Wang Q, Kong L, Hao H, Wang X, Lin J, Samaj J, Baluska F (2005) Effects of Brefeldin A on pollen germination and tube growth. Antagonistic effects on endocytosis and secretion. Plant Physiol 139:1692–1703
Wang W, Wang L, Chen C, Xiong G, Tan XY, Yang KZ, Wang ZC, Zhou Y, Ye D, Chen LQ (2011) Arabidopsis CSLD1 and CSLD4 are required for cellulose deposition and normal growth of pollen tubes. J Exp Bot 62:5161–5177
Wang H, Zhuang X, Cai Y, Cheung AY, Jiang L (2013) Apical F-actin regulated exocytic targeting of NtPPME1 is essential for pollen tube cell wall construction and rigidity. Plant J 76:367–379
Winship LJ, Obermeyer G, Geitmann A, Hepler PK (2010) Under pressure, cell walls set the pace. Trends Plant Sci 15:363–369
Wolf S, Greiner S (2012) Growth control by cell wall pectins. Protoplasma 249:169–175
Zerzour R, Kroeger J, Geitmann A (2009) Polar growth in pollen tubes is associated with spatially confined dynamic changes in cell mechanical properties. Dev Biol 334:437–446
Zhang G, Feng J, Wu J, Wang X (2010) BoPMEI1, a pollen-specific pectin methylesterase inhibitor, has an essential role in pollen tube growth. Planta 231:1323–1334
Acknowledgments
We want to sincerely thank Mr. Massimo Guarnieri (Department of Life Sciences, University of Siena) for his help in the analysis of sucrose by HPLC. We also thank the staff of the Botanical Garden of Siena University for cultivating tobacco plants.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Scott D. Russell.
Rights and permissions
About this article
Cite this article
Biagini, G., Faleri, C., Cresti, M. et al. Sucrose concentration in the growth medium affects the cell wall composition of tobacco pollen tubes. Plant Reprod 27, 129–144 (2014). https://doi.org/10.1007/s00497-014-0246-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-014-0246-y