Abstract
Migraine is an extremely disabling, common neurological disorder characterized by a complex neurobiology, involving a series of central and peripheral nervous system areas and networks. A growing increase in the understanding of migraine pathophysiology in recent years has facilitated translation of that knowledge into novel treatments, which are currently becoming available to patients in many parts of the world and are substantially changing the clinical approach to the disease. In the first part of this review, we will provide an up to date overview of migraine pathophysiology by analyzing the anatomy and function of the main regions involved in the disease, focusing on how these give rise to the plethora of symptoms characterizing the attacks and overall disease. The second part of the paper will discuss the novel therapeutic agents that have emerged for the treatment of migraine, including molecules targeting calcitonin gene-related peptide (gepants and monoclonal antibodies), serotonin 5-HT1F receptor agonists (ditans) and non-invasive neuromodulation, as well as providing a brief overview of new evidence for classic migraine treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is currently listed as the sixth most disabling disorder globally, with the highest ranking among all neurological disorders [1]. The biology of migraine is complex, multifactorial and still, for certain aspects, unsolved. The underlying feature seems to be a, probably complex, genetic predisposition combined with behavioral and environmental conditions that causes an alteration of sensory brain processing, resulting in increased sensory susceptibility. This in turn results in otherwise normal sensory inputs being perceived as bothersome in migraineurs [2].
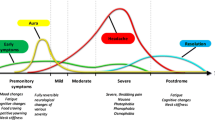
Over the years, our knowledge around migraine has improved considerably, largely thanks to basic science and imaging studies allowing us to better understand the complex models that are needed to explain the plethora of migraine symptoms. In fact, pain, the cardinal symptom of the disorder, is not necessarily the most bothersome for all patients at all times [3, 4]. Migraine is characterized by a succession of key phases that often overlap: the premonitory (prodromal), aura, pain and postdromal phases [5, 6]. Better recognition of these events has allowed us to conceptualize migraine as a network disorder involving multiple cortical, subcortical and brainstem regions, generating a wide constellation of signs and symptoms [7]. These areas, which we will analyze in detail in the following sections, have altered function and structure in individuals with migraine and in animal models of the disease.
The current review follows a translational and anatomical approach, beginning with an outline of the mechanisms and regions that are known to be a part of migraine biology, before moving on to current acute and preventive treatments, providing updated references and insights with respect to our previous review of 5 years ago [8].
Migraine: functional anatomy and pathophysiology
The trigeminovascular system and brainstem nuclei
The trigeminovascular system consists of peripheral axons from the trigeminal ganglion that innervate the meninges and intracranial blood vessels peripherally, and converge centrally in the trigeminocervical complex (TCC), composed of the spinal trigeminal nucleus caudalis and upper cervical spinal cord [9, 10]. Second-order neurons ascend from the TCC to thalamocortical neurons and further project to key brain nuclei in the diencephalon and brainstem, such as the locus coeruleus (LC), periaqueductal gray (PAG) and hypothalamus [11, 12]. Activation of the trigeminovascular pain pathways is thought to mediate part of the qualities of migraine pain by release of neuropeptides, such as calcitonin gene-related peptide (CGRP) and pituitary adenylate cyclase activating polypeptide (PACAP), at the level of the dura mater [13,14,15]. CGRP is widely expressed in both peripheral and central neurons and has potent dilatator qualities. It also shows a regulatory action on second- and third-order neurons, which seems to underlie its modulatory role in central pain mechanisms. CGRP elevation in migraineurs has been linked to a decrease in descending inhibitory mechanisms, which in turn might lead to migraine susceptibility through sensitization of multiple central neuronal circuits [13].
Neurogenic inflammation in the periphery was initially proposed to be the generator of migraine pain [16], although this role has been revisited largely due to the fact that blockers of plasma protein extravasation have failed to treat migraine in clinical trials [17, 18]. While trigeminal activation with associated neurogenic inflammation continues to be discussed [19], direct evidence for a dural inflammatory component in migraine is lacking. As described above, migraine is associated with a spectrum of sensory dysfunctions with cycling behavior, during which the headache phase represents the plateau of trigeminal nociceptive activation [20]. One hypothesis is that peripheral trigeminovascular neurons are sensitized, and thereafter sensitize second-order neurons in the trigeminal nucleus caudalis and upper cervical spinal cord, and project rostrally to thalamic nuclei and key medullary, brainstem and diencephalic regions [21]. Studies have reliably provided evidence for early brainstem involvement in the nociceptive migraine phase; however, it is becoming clearer that the initiation of a migraine attack is linked to intrinsic brain dysfunction in more central areas such as the hypothalamus, and possibly to external factors as well [22]. Whether the central dysfunction, very clearly demonstrated in the premonitory phase, facilitates central sensitization remains an intriguing area of study.
The role for brainstem regions, such as the PAG and the dorsolateral pons in migraine is well established thanks to observational [23] and neuroimaging studies [24,25,26], as well as animal models of migraine showing that the brainstem acts as a driver of changes in cortical activity during migraine [27,28,29,30]. Indeed, nuclei such as the LC, rostral ventral medulla, superior salivatory and cuneiform nucleus are key in modulating trigeminovascular pain transmission and autonomic responses in migraine and represent a site of action for triptans [31, 32], ergot derivatives [33, 34] and the novel CGRP receptor antagonists [35, 36]. Further, direct and indirect trigeminal activation of the parabrachial nucleus may explain why head and facial pain is so intense when compared with noncephalic pain, whereas upward trigemino–parabrachial–limbic connections, particularly to the amygdala, can explain affective-motivational aspects of migraine and even appetite and taste abnormalities [12]. Linking the premonitory phase and the onset of pain, neurons in the ventral tegmental parabrachial pigmented (VTAPBP) nucleus can modulate trigeminocervical nociceptive traffic in rat. These effects can be seen with glutamate, naratriptan (5HT1B/1D receptor agonist), PACAP and dopamine D2/3 mediation [37]. Given the role of the VTAPBP in hedonic feeding, and the influence of glucose on trigeminocervical nociceptive transmission [38], the data suggest plausible pathways to explain the much celebrated, yet seldom clinically useful, issues of food triggers [39]. One might re-think some triggers in terms of behaviors arising from central nervous system activation in the premonitory phase [40], with very different conclusions concerning cause and effect. Finally, central sensitization of the trigeminovascular system, especially the trigeminal nucleus caudalis, plays an important role in the development of chronic migraine, possibly influenced by cytokines release and increased astrocytic activation [41, 42]. Interestingly, as shown by neurophysiological [43] and neuroimaging studies [44], brainstem activations seems most prominent in the 24 h preceding the headache onset, and declines during the attack.
The hypothalamus
The theories on migraine as a cyclic sensory threshold disorder have highlighted the importance of the hypothalamus as a central facilitator of pain, and also of the constellation of premonitory symptoms such as yawning, thirst and polyuria [45], which can precede and continue into the pain phase. Functional neuroimaging performed during spontaneous and nitroglycerin-triggered attacks has consolidated the role of the hypothalamus in migraine initiation [46]. Altered hypothalamic-brainstem connectivity with the spinal trigeminal nuclei and the dorsal rostral pons has been shown in the premonitory phase of migraine [44] for up to 48 h preceding pain onset [47]. Positron emission tomography has also previously revealed hypothalamic activation during both spontaneous migraine headache [48] and the premonitory phase [49]. Recent functional imaging studies have even shown that changes in hypothalamic connectivity with the spinal trigeminal nucleus and cortical regions are associated with the development of chronic migraine [50, 51].
The mechanism(s) by which the hypothalamus can become ‘overactive’ in migraine, leading to sensitization of trigeminal nociceptors is still unclear. Anatomically, the hypothalamus has direct and indirect connections to the thalamus [52], the trigeminovascular system [53] and to sympathetic and parasympathetic brainstem neurons [54], influencing nociceptive and autonomic regulation in migraine. Stress, which is said to be a common trigger of migraine, can activate the kappa opioid receptor on tuberoinfundibular dopaminergic neurons and lead to an increase in circulating prolactin causing sensitization of trigeminal afferents, particularly in females [55]. Further, the hypothalamus has chemosensitive neurons that can detect metabolic changes in the brain and periphery. Exogenous stimuli causing a change in homeostasis and this intrinsic biorhythm could thus possibly ‘tip’ the brain towards a migraine attack via activation of the hypothalamus [56].
The thalamus
The thalamus has a critical role in sensory processing, receiving inputs from the extracranial skin and dura mater from second-order trigeminovascular neurons, and projecting to cortical regions involved in autonomic, affective, and cognitive functions—all of which explains in part the complexity of migraine features [57]. Thalamocortical synchronization is affected by a network of neurotransmitters and neuropeptides in the brainstem (glutamate, serotonin, and noradrenaline), reticular thalamic regions (γ-aminobutyric acid—GABA) and hypothalamic nuclei (dopamine, histamine, orexin, and melanin-containing hormone [58]). There is abundant clinical and preclinical evidence showing that the thalamus is crucial for the development of central sensitization, photophobia and allodynia in migraine [59,60,61,62,63,64].
Structural neuroimaging studies have shown differences in volume of thalamic nuclei with microstructural abnormalities [65,66,67]. However, such changes were not seen in a recent large study involving female patients with aura [68]. Functional MRI studies have also shown important changes in the thalamus, both within and outside of attacks. In migraine without aura, connectivity between the thalamus and pain modulating areas seems to be affected during the ictal phase [69]. Abnormal low-frequency oscillations in dynamic thalamocortical networks are implicated in the interictal phase [70], with changes in pulvinar activity allowing differentiation between migraineurs and controls [71]. Another recent study showed that both episodic and chronic migraine patients have greater activation of ascending trigeminal somatosensory pathways and lower activation of top-down pain modulatory circuits. This could indicate interictal dysfunction of the descending pain modulatory system and amplification of nociceptive processing in migraineurs, mediated by the thalamus and possibly contributing to central sensitization [72].
The processing of trigeminovascular nociceptive information in the thalamus can represent a target for management, and indeed several migraine treatments including triptans [31, 73], preventives [74,75,76,77] and non-invasive neuromodulation have been shown to modulate thalamocortical activity [78, 79].
The cortex
The role of the cerebral cortex in migraine was initially linked to the aura phenomenon and its peculiar symptoms [80, 81]. Aura is thought to be generated by cortical spreading depression (CSD) [82], which has been indirectly evidenced in humans through functional neuroimaging [83]. Although CSD can activate the trigeminovascular system in animals [84, 85] possibly through CGRP-mediated mechanisms [86], it is unlikely to contribute to the headache and possibly constitutes an epiphenomenon of migraine [87, 88].
Regardless of CSD, the cortex has been increasingly implicated in migraine genesis, and in fact many changes in the structure and function of key cortical areas associated with pain processing have been reported in patients, both in the ictal and interictal period [89]. During the headache phase, cortical networks including the salience, sensorimotor, default mode, executive and attentional networks, show functional changes; this reflects the cognitive, painful and emotional symptoms of migraine [90]. Studies in patents with aura have consistently shown differences in brain structure [91, 92], functional connectivity [92], cortical excitability [93,94,95] and pain modulation in the visual pathways [96]. Occipital cortex involvement in particular can explain the plethora of visual symptoms associated with migraine, from light sensitivity to visual aura and visual snow [97]. Menstrual migraine has recently been linked to structural and functional connectivity changes in the right anterior cingulum [98] an area involved in the cognitive processing of pain and previously associated with migraine biology [99]. However, evidence from neuroimaging studies has been inconclusive at times [100], with meta-regression analyses failing to pinpoint alterations that are specific to migraine [101], showing that further research on the topic is needed.
Of note, the association of white matter hyperintensity (WMH) in migraine has long been debated [102], particularly in migraine with aura [103, 104]. Recently, an association was identified between the presence of juxtacortical WMHs within the frontal lobe with patient age and duration of disease [105]. WMHs have also been associated with nausea, vomiting, dizziness and pain intensity during attacks, [106].
Dysfunctional cortical mechanisms and in particular thalamocortical dysrhythmia have also been implicated in the mechanism underlying the lack of habituation typical of migraine [107]; in this, repeated sensory stimuli cause an incremental, instead of a reduction, increase in the amplitudes of sensory responses [108, 109]. Lack of habituation, measured for different sensory modalities, usually occurs during the pain-free period and reverts during the ictal phase or when attacks become more frequent [110].
Finally, large genome-wide association studies (GWAS) have identified susceptibility gene variants in migraine patients, mostly involved in glutamatergic neurotransmission, which could lead to abnormal cortical excitability and altered plasticity [111], as evidenced by numerous magnetic resonance spectroscopy studies performed over the years [112]. Readers interested in the genetics are referred to this recent article [113]. Given the complexity that has emerged from GWAS work, in which each change has such a modest effect on the overall phenotype, one might reflect on whether clinically useful genetic changes will emerge in the near term that will have an impact on management and treatment.
Novel therapies in migraine
The last few years have represented an exciting and promising time in the field of migraine, thanks to the introduction of several new medications in clinical practice, and with other therapeutic targets, such as glutamate, amylin, adrenomedullin, orexins and pituitary adenylate cyclase activating polypeptide, currently all in the therapeutic pipeline [114, 115]. The new novel treatments have rapidly changed the paradigm of migraine management, particularly challenging the dichotomous division between acute and preventive medication, which we will however, follow in this review for simplicity. Further, patient-reported outcomes such as interictal burden and time lost due to an attack are becoming more relevant in the consideration of efficacy and tolerability of these drugs [116, 117], allowing for significant advances in the management of this condition [118].
Acute treatments
Therapy for migraine attacks includes non-steroidal anti-inflammatory drugs (NSAIDs), combination analgesics, ergotamine preparations and migraine-specific medications. The latter class, which until a few years ago meant triptans, has recently grown to include ditans, serotonin 5HT1F receptor agonists, and gepants, CGRP receptor antagonists. Triptans are full agonists of presynaptic serotonin receptors 5-HT1B and 5-HT1D [119], which inhibit CGRP release [120]. The class includes seven options in different formulations [121], which can be switched to find the optimal combination for efficacy and tolerability in the individual patient and which can be combined with NSAIDs to prolong therapeutic effect and limit rebounds [122, 123]. There are gender-related differences in triptan tolerability, as women seem to present higher adverse event frequency and headache recurrence rates with these drugs [124].
Non-responsiveness to triptans may be categorized into refractory: failure of three triptans, one of which should be subcutaneous sumatriptan; and resistant: failure of at least two triptans [125]. Non-responsiveness can have a significant impact on health-related quality of life and work productivity [126] and has been linked by recent neuroimaging data to changes in hippocampal volume [127]. Importantly, even if clinical practice has not demonstrated strong drug-related cardiovascular risk [128], triptans are still contraindicated in at-risk patients due to their vasoconstrictive qualities [129]. The new classes of ditans and gepants do not present this disadvantage.
Ditans
Lasmiditan is the only ditan currently available; it is a potent and selective 5-HT1F receptor agonist [130], acting in migraine by blocking activation of neurons in the trigeminal nucleus caudalis [131] without affecting the vasculature [130]. Lasmiditan has now been studied in two-phase two studies [132, 133] and three large phase three randomized controlled trials [134,135,136] and shown to have better efficacy compared to placebo on rates of 2-h pain freedom and freedom from most bothersome symptoms, particularly with the 100 and 200 mg doses. Pooled data from the phase 3 studies showed no cardiovascular safety concerns, and in fact, these included patients with coronary artery disease, complicated cardiac arrhythmias and/or hypertension [137]. Across these studies, neurological side effects—particularly dizziness, nausea and somnolence—were common, but mostly mild to moderate and self-limiting [138].
Gepants
Gepants are small-molecule CGRP receptor antagonists developed for use in the acute treatment of migraine. Six gepants were initially developed for acute use in migraine, with two being discontinued due to liver toxicity [139, 140], one due to lack of oral availability [141] and one for commercial reasons [142]. Ubrogepant and rimegepant represent a new generation of oral gepants that have received FDA approval for acute migraine therapy [143, 144] following phase 3 studies: Achieve I [145] and II [146] for ubrogepant, and Study 301 [147], 302 [148] and 303 [149] for rimegepant. Ubrogepant has been approved at 50 and 100 mg doses and Rimegepant is available in an orodispersible (lyophilized) form at a dose of 75 mg. Preliminary evidence also shows effectiveness of the zavegepant nasal spray, a non-oral gepant [150]. Importantly, gepants do not seem to cause medication overuse headache, making them a useful option when managing this complication [151] and can be taken in multiple doses during the attack with good rates of success [152]. The low side effect profile of gepants is appealing; however, caution in the early days of real-world use is merited [153].
Metabotropic and ionotropic glutamate receptors may become important targets in the future acute therapy of migraine, although adverse event issues need to be overcome. Experimental and clinical studies have shown an effect of NMDA, AMPA, iGluR5 and mGluR5 receptor antagonists in migraine, although their efficacy was lower than that of sumatriptan and visual side effects were observed [154,155,156]. Blockers of the metabotropic glutamate receptor 5 in particular, or glurants, have a strong clinical potential for becoming a candidate drug class for migraine, if the relevant issues of hepatoxicity and transient dizziness can be resolved [157]. The NMDA receptor is also relevant for migraine with aura, as evidenced by a small RCT showing the efficacy of ketamine on reducing the severity of auras [158].
Preventive treatments
Preventive therapy is recommended in patients who are affected by migraine on at least 2 days per month, when there is medication overuse and/or when quality of life is impaired [159]. The application of this guidance will be governed by clinical judgment in the individual case. Classic prevention includes different drug categories, such as β blockers, anticonvulsants, tricyclic antidepressants and calcium channel modulators, which, however, often lead to tolerability issues and poor compliance [160]. In recent years, monoclonal antibodies (mABs) against the CGRP peptide (galcanezumab, fremanezumab, eptinezumab) or its canonical receptor (erenunmab) have been widely introduced in clinical practice and treatment guidelines [161]. These treatments have persistently confirmed their efficacy in phase 3 trials [162,163,164,165,166,167,168,169,170,171,172,173,174], with convenient dosing, faster onset of efficacy and mild to moderate adverse events [175, 176]. Further, real-world studies have shown improvement with mABs and worsening of migraine frequency following discontinuation, with most patients resuming treatment as soon as possible following breaks due to regulatory restrictions [177,178,179,180]. The European Headache Federation currently recommends CGRP mABs as a first-line option for migraine prevention, with treatment to be continued as long as needed [181], although in most jurisdictions, this is not possible to operationalize easily.
Two gepants, atogepant and rimegepant, have recently been introduced into the market after proving effective and well tolerated for the preventive treatment of migraine [182,183,184]. Both have a similar short half-life of around 11 h [185, 186], which facilitates their preventive indication. They bring the advantage of fewer adverse events and increased safety, particularly in women who have unplanned pregnancies given the difference in half-life compared to monoclonal antibodies [151]. Atogepant was directly designed as a preventive agent, and has been FDA-approved for episodic migraine [187]. The most common side effects in the phase 2b and 3 studies were constipation and nausea, each at 10% at the 60 mg daily dose. The currently available data suggests safety on cardiac repolarization even with supratherapeutic doses and, in contrast with the first gepants, no elevation of serum alanine aminotransferase [188, 189]. A further study for the preventive treatment in chronic migraine has been reported in abstract form [190]. It is being studied in combination with onabotulinumtoxinA (NCT05216263) and for long-term safety and tolerability in another trial (NCT04686136).
Rimegepant, initially trialed for acute use, can prevent episodic migraine in adults when taken every other day [184] with the additional benefit of it being used concomitantly during a migraine attack. It is well tolerated, with nausea occurring in 2% of cases and this is the most common side effect.
New evidence on efficacy and tolerability has also emerged for well-known migraine preventives. A meta-analysis has documented the efficacy in chronic migraine of OnabotulinumtoxinA, which allows for a reduction of over 50% in migraine days after 24 weeks of treatment [191]. Several open-label studies have also shown a benefit of combining onabotulinumtoxinA with mAbs to CGRP for CM [192,193,194]. A recent head-to-head trial for chronic migraine showed non-inferiority between propranolol and topiramate, with no significant difference in adverse events incidence between the two [195]. Another prospective randomized trial in chronic migraine compared flunarizine 10 mg with topiramate 50 mg daily, showing both drugs had a similar safety profile, with flunarizine being overall more effective [196]. Recent retrospective studies have confirmed the usefulness of candesartan as a first-line migraine preventive, even in patients who failed numerous previous drugs [197, 198]. Finally, meta-analyses have been conducted on melatonin [199, 200] and memantine [201], both showing favorable side effect profiles and good efficacy in migraine.
Neuromodulation
Non-invasive neuromodulation is an evolving field and is of particular clinical interest for migraine management as it offers the option of being used both as an acute and preventive treatment. It also presents near to no systemic side effects and can thus be offered to patients that present tolerability issues or who need to avoid medication interaction [202].
The devices used in migraine target the nervous system through a transcutaneous approach, either centrally (single-pulse transcranial magnetic stimulation, or sTMS) or in the periphery (non-invasive vagus nerve stimulation or nVNS, supraorbital nerve stimulation or SNS and transcranial direct current stimulation or tDCS).
A handheld sTMS device is now approved in the USA and Europe for the acute and preventive treatment of migraine, following positive results as an acute migraine treatment in a RCT involving 164 migraineurs with aura [203] and subsequent post-marketing survey [204] and open-label study [205] demonstrating an effect on headache day reduction and 50% responder rate. The efficacy of sTMS in migraine prevention has also been shown in difficult-to-treat patients [206].
External trigeminal (supraorbital) nerve stimulation has also shown promise with supporting evidence for the treatment of migraine, with one RCT showing higher efficacy and tolerability than sham in 109 patients after 1 h of acute treatment [207]. For prevention, its effect seems greater in episodic migraine [208] than in refractory [209] or chronic migraine patients [210].
Regarding non-invasive vagus nerve stimulation (nVNS), it has shown evidence of efficacy in a RCT for the acute treatment of migraine [211], but not for prevention [212,213,214]. From a mechanistic perspective, this approach at the bench can suppress cortical spreading depression [215] and inhibit trigeminocervical neurons responding to durovascular nociceptive activation [216].
Another approach has focused on the application of repeated cathodal or anodal transcranial direct current stimulation over the cortex, although data on its therapeutic effect in migraineurs has been conflicting [217, 218]. This may be due to methodological differences regarding the techniques, the targeted brain regions and stimulation types [219], warranting further investigation.
Novel options for neuromodulation include the remote noncephalic electrical neurostimulation of the upper arm skin. The device works non-invasively through conditioned pain modulation and [220] has been evaluated in a RCT involving 253 patients. Participants reported clinically meaningful relief from migraine pain and pain freedom after 2 h of treatment compared to sham, with a low incidence of device-related adverse events [221]. A recent open-label study showed preliminary evidence supporting its use in chronic migraine [222]. Finally, repetitive peripheral magnetic stimulation (rPMS) targeting the muscles in the neck and shoulder muscles has shown efficacy in the prevention of episodic migraine, particularly in patients with a high level of muscular involvement [223, 224].
Although these techniques are promising for the management of a disabling condition with often little treatment options, further evidence is needed to evaluate the scope of their effect in migraine, including novel mechanisms and targets [225, 226].
Conclusions
The last 2 decades have been an incredibly exciting period for clinicians and researchers interested in migraine, as they have seen a rapid increase in studies that have led to a greater knowledge and understanding of the neurobiology of the disorder. From suffering with a condition that was often overlooked and under-managed, migraineurs are now being offered novel treatments that are more and more tailored to their needs, and that are fundamentally re-shaping our approach to the disease.
More research and progress is needed and is expected in the coming years, and hopefully this will continue to raise awareness around a complex phenomenon affecting millions of people all over the world.
References
Global Burden of Disease Study 2013 Collaborators (2013) Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 386(9995):743–800
Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S (2017) Pathophysiology of migraine—a disorder of sensory processing. Physiol Rev 97(2):553–622
Lipton RB, Dodick DW, Ailani J, McGill L, Hirman J, Cady R (2021) Patient-identified most bothersome symptom in preventive migraine treatment with eptinezumab: a novel patient-centered outcome. Headache 61(5):766–776
Lampl C, Thomas H, Stovner LJ, Tassorelli C, Katsarava Z, Laínez JM et al (2016) Interictal burden attributable to episodic headache: findings from the Eurolight project. J Headache Pain 17:9
Dodick DW (2018) A Phase-by-phase review of migraine pathophysiology. Headache 58(Suppl 1):4–16
Karsan N, Goadsby PJ (2018) Biological insights from the premonitory symptoms of migraine. Nat Rev Neurol 14(12):699–710
Charles A (2013) Migraine: a brain state. Curr Opin Neurol 26(3):235–239
Puledda F, Messina R, Goadsby PJ (2017) An update on migraine: current understanding and future directions. J Neurol 264(9):2031–2039
Goadsby PJ, Hoskin KL (1997) The distribution of trigeminovascular afferents in the nonhuman primate brain Macaca nemestrina: a c-fos immunocytochemical study. J Anat 190(Pt 3):367–375
Hoskin KL, Zagami A, Goadsby PJ (1999) Stimulation of the middle meningeal artery leads to Fos expression in the trigeminocervical nucleus: a comparative study of monkey and cat. J Anat 194:579–588
Akerman S, Holland PR, Goadsby PJ (2011) Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci 12(10):570–584
Goadsby PJ, Holland PR (2019) An update: pathophysiology of migraine. Neurol Clin 37(4):651–671
Ho TW, Edvinsson L, Goadsby PJ (2010) CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol 6(10):573–582
Iyengar S, Johnson KW, Ossipov MH, Aurora SK (2019) CGRP and the trigeminal system in migraine. Headache 59(5):659–681
Zagami AS, Edvinsson L, Goadsby PJ (2014) Pituitary adenylate cyclase activating polypeptide and migraine. Ann Clin Transl Neurol 1(12):1036–1040
Markowitz S, Saito K, Moskowitz MA (1987) Neurogenically mediated leakage of plasma proteins occurs from blood vessels in dura mater but not brain. J Neurosci 7:4129–4136
May A, Goadsby PJ (2001) Substance P receptor antagonists in the therapy of migraine. Expert Opin Investig Drugs 10:1–6
Peroutka SJ (2005) Neurogenic inflammation and migraine: implications for therapeutics. Mol Interv 5:306–313
Ramachandran R (2018) Neurogenic inflammation and its role in migraine. Semin Immunopathol 40(3):301–314
Peng KP, May A, Basedau H (2022) Cycling multisensory changes in migraine: more than a headache. Curr Opin Neurol 35(3):367–372
Harriott AM, Orlova Y (2022) Anatomy and physiology of headache. Semin Neurol 42(4):459–473
Messina R, Gollion C, Christensen RH, Amin FM (2022) Functional MRI in migraine. Curr Opin Neurol 35(3):328–335
Raskin NH, Hosobuchi Y, Lamb S (1987) Headache may arise from perturbation of brain. Headache 27(8):416–420
Weiller C, May A, Limmroth V, Juptner M, Kaube H, Schayck RV et al (1995) Brain stem activation in spontaneous human migraine attacks. Nat Med 1(7):658–660
Bahra A, Matharu MS, Buchel C, Frackowiak RS, Goadsby PJ (2001) Brainstem activation specific to migraine headache. Lancet 357(9261):1016–1017
Stankewitz A, Aderjan D, Eippert F, May A (2011) Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J Neurosci 31(6):1937–1943
Goadsby PJ, Duckworth JW (1989) Low frequency stimulation of the locus coeruleus reduces regional cerebral blood flow in the spinalized cat. Brain Res 476(1):71–77
Vinogradova LV (2015) Comparative potency of sensory-induced brainstem activation to trigger spreading depression and seizures in the cortex of awake rats: implications for the pathophysiology of migraine aura. Cephalalgia 35(11):979–986
Knight YE, Bartsch T, Kaube H, Goadsby PJ (2002) P/Q-type calcium-channel blockade in the periaqueductal gray facilitates trigeminal nociception: a functional genetic link for migraine? J Neurosci 22(5):Rc213
Knight YE, Goadsby PJ (2001) The periaqueductal grey matter modulates trigeminovascular input: a role in migraine? Neuroscience 106(4):793–800
Kroger IL, May A (2015) Triptan-induced disruption of trigemino-cortical connectivity. Neurology 84(21):2124–2131
Goadsby PJ, Hoskin KL (1996) Inhibition of trigeminal neurons by intravenous administration of the serotonin (5HT)1B/D receptor agonist zolmitriptan (311C90): are brain stem sites therapeutic target in migraine? Pain 67(2–3):355–359
Goadsby PJ, Gundlach AL (1991) Localization of 3H-dihydroergotamine-binding sites in the cat central nervous system: relevance to migraine. Ann Neurol 29(1):91–94
Hoskin KL, Kaube H, Goadsby PJ (1996) Central activation of the trigeminovascular pathway in the cat is inhibited by dihydroergotamine. A c-Fos and electrophysiological study. Brain J Neurol 119(Pt 1):249–256
Pozo-Rosich P, Storer RJ, Charbit AR, Goadsby PJ (2015) Periaqueductal gray calcitonin gene-related peptide modulates trigeminovascular neurons. Cephalalgia 35(14):1298–1307
Storer RJ, Akerman S, Goadsby PJ (2004) Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br J Pharmacol 142(7):1171–1181
Martins-Oliveira M, Akerman S, Holland PR, Tavares I, Goadsby PJ (2022) Pharmacological modulation of ventral tegmental area neurons elicits changes in trigeminovascular sensory processing and is accompanied by glycemic changes: Implications for migraine. Cephalalgia Int J Headache 42(13):1359–1374
Martins-Oliveira M, Akerman S, Holland PR, Hoffmann JR, Tavares I, Goadsby PJ (2017) Neuroendocrine signaling modulates specific neural networks relevant to migraine. Neurobiol Dis 101:16–26
Martins-Oliveira M, Tavares I, Goadsby PJ (2021) Was it something I ate? Understanding the bidirectional interaction of migraine and appetite neural circuits. Brain Res 1770:147629
Karsan N, Bose P, Newman J, Goadsby PJ (2021) Are some patient-perceived migraine triggers simply early manifestations of the attack? J Neurol 268(5):1885–1893
Edvinsson L, Haanes KA, Warfvinge K (2019) Does inflammation have a role in migraine? Nat Rev Neurol 15(8):483–490
Zhang L, Lu C, Kang L, Li Y, Tang W, Zhao D et al (2022) Temporal characteristics of astrocytic activation in the TNC in a mice model of pain induced by recurrent dural infusion of inflammatory soup. J Headache Pain 23(1):8
Hsiao F-J, Chen W-T, Pan L-LH, Liu H-Y, Wang Y-F, Chen S-P et al (2022) Dynamic brainstem and somatosensory cortical excitability during migraine cycles. J Headache Pain 23(1):21
Schulte LH, May A (2016) The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain J Neurol 139(Pt 7):1987–1993
Peng KP, May A (2019) Migraine understood as a sensory threshold disease. Pain 160(7):1494–1501
May A, Burstein R (2019) Hypothalamic regulation of headache and migraine. Cephalalgia Int J Headache 39(13):1710–1719
Schulte LH, Mehnert J, May A (2020) Longitudinal neuroimaging over 30 days: temporal characteristics of migraine. Ann Neurol 87(4):646–651
Denuelle M, Fabre N, Payoux P, Chollet F, Geraud G (2007) Hypothalamic activation in spontaneous migraine attacks. Headache 47(10):1418–1426
Maniyar FH, Sprenger T, Monteith T, Schankin C, Goadsby PJ (2014) Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain J Neurol 137(Pt 1):232–241
Lee MJ, Park BY, Cho S, Kim ST, Park H, Chung CS (2019) Increased connectivity of pain matrix in chronic migraine: a resting-state functional MRI study. J Headache Pain 20(1):29
Schulte LH, Allers A, May A (2017) Hypothalamus as a mediator of chronic migraine: evidence from high-resolution fMRI. Neurology 88(21):2011–2016
Kagan R, Kainz V, Burstein R, Noseda R (2013) Hypothalamic and basal ganglia projections to the posterior thalamus: possible role in modulation of migraine headache and photophobia. Neuroscience 248:359–368
Malick A, Burstein R (1998) Cells of origin of the trigeminohypothalamic tract in the rat. J Comp Neurol 400(1):125–144
Abdallah K, Artola A, Monconduit L, Dallel R, Luccarini P (2013) Bilateral descending hypothalamic projections to the spinal trigeminal nucleus caudalis in rats. PLoS One 8(8):e73022
Watanabe M, Kopruszinski CM, Moutal A, Ikegami D, Khanna R, Chen Y et al (2022) Dysregulation of serum prolactin links the hypothalamus with female nociceptors to promote migraine. Brain 145(8):2894–2909
Gross EC, Lisicki M, Fischer D, Sandor PS, Schoenen J (2019) The metabolic face of migraine—from pathophysiology to treatment. Nat Rev Neurol 15(11):627–643
Noseda R, Jakubowski M, Kainz V, Borsook D, Burstein R (2011) Cortical projections of functionally identified thalamic trigeminovascular neurons: implications for migraine headache and its associated symptoms. J Neurosci 31(40):14204–14217
Noseda R, Borsook D, Burstein R (2017) Neuropeptides and neurotransmitters that modulate thalamo-cortical pathways relevant to migraine headache. Headache 57(Suppl 2):97–111
Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R et al (2010) Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol 68(1):81–91
Burstein R, Yamamura H, Malick A, Strassman AM (1998) Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol 79(2):964–982
Strassman AM, Raymond SA, Burstein R (1996) Sensitization of meningeal sensory neurons and the origin of headaches. Nature 384(6609):560–564
Suzuki K, Suzuki S, Shiina T, Kobayashi S, Hirata K (2022) Central sensitization in migraine: a narrative review. J Pain Res 15:2673–2682
Noseda R, Bernstein CA, Nir RR, Lee AJ, Fulton AB, Bertisch SM et al (2016) Migraine photophobia originating in cone-driven retinal pathways. Brain 139(Pt 7):1971–1986
Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K et al (2010) A neural mechanism for exacerbation of headache by light. Nat Neurosci 13(2):239–245
Magon S, May A, Stankewitz A, Goadsby PJ, Tso AR, Ashina M et al (2015) Morphological abnormalities of thalamic subnuclei in migraine: a multicenter MRI study at 3 Tesla. J Neurosci 35(40):13800–13806
Granziera C, Daducci A, Romascano D, Roche A, Helms G, Krueger G et al (2014) Structural abnormalities in the thalamus of migraineurs with aura: a multiparametric study at 3 T. Hum Brain Mapp 35(4):1461–1468
Messina R, Rocca MA, Colombo B, Pagani E, Falini A, Comi G et al (2015) White matter microstructure abnormalities in pediatric migraine patients. Cephalalgia 35(14):1278–1286
Hougaard A, Nielsen SH, Gaist D, Puonti O, Garde E, Reislev NL et al (2020) Migraine with aura in women is not associated with structural thalamic abnormalities. Neuroimage Clin 28:102361
Amin FM, Hougaard A, Magon S, Sprenger T, Wolfram F, Rostrup E et al (2018) Altered thalamic connectivity during spontaneous attacks of migraine without aura: a resting-state fMRI study. Cephalalgia 38(7):1237–1244
Hodkinson DJ, Wilcox SL, Veggeberg R, Noseda R, Burstein R, Borsook D et al (2016) Increased amplitude of thalamocortical low-frequency oscillations in patients with migraine. J Neurosci 36(30):8026–8036
Tu Y, Fu Z, Zeng F, Maleki N, Lan L, Li Z et al (2019) Abnormal thalamocortical network dynamics in migraine. Neurology 92(23):e2706–e2716
Lim M, Jassar H, Kim DJ, Nascimento TD, DaSilva AF (2021) Differential alteration of fMRI signal variability in the ascending trigeminal somatosensory and pain modulatory pathways in migraine. J Headache Pain 22(1):4
Shields KG, Goadsby PJ (2006) Serotonin receptors modulate trigeminovascular responses in ventroposteromedial nucleus of thalamus: a migraine target? Neurobiol Dis 23(3):491–501
Shields KG, Goadsby PJ (2005) Propranolol modulates trigeminovascular responses in thalamic ventroposteromedial nucleus: a role in migraine? Brain 128(Pt 1):86–97
Tepe N, Filiz A, Dilekoz E, Akcali D, Sara Y, Charles A et al (2015) The thalamic reticular nucleus is activated by cortical spreading depression in freely moving rats: prevention by acute valproate administration. Eur J Neurosci 41(1):120–128
Andreou AP, Shields KG, Goadsby PJ (2010) GABA and valproate modulate trigeminovascular nociceptive transmission in the thalamus. Neurobiol Dis 37(2):314–323
Summ O, Charbit AR, Andreou AP, Goadsby PJ (2010) Modulation of nocioceptive transmission with calcitonin gene-related peptide receptor antagonists in the thalamus. Brain J Neurol 133(9):2540–2548
Puledda F, Shields K (2018) Non-pharmacological approaches for migraine. Neurother J Am Soc Exp Neurother 15(2):336–345
Andreou AP, Holland PR, Akerman S, Summ O, Fredrick J, Goadsby PJ (2016) Transcranial magnetic stimulation and potential cortical and trigeminothalamic mechanisms in migraine. Brain J Neurol 139(Pt 7):2002–2014
Olesen J, Larsen B, Lauritzen M (1981) Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann Neurol 9(4):344–352
Barral E, Martins Silva E, García-Azorín D, Viana M, Puledda F (2023) Differential diagnosis of visual phenomena associated with migraine: spotlight on aura and visual snow syndrome. Diagnostics 13(2):252
Leão AAP (1944) Spreading depression of activity in the cerebral cortex. J Neurophysiol 7:359–390
Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischl B et al (2001) Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci USA 98(8):4687–4692
Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA (2002) Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med 8(2):136–142
Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R (2011) Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol 69(5):855–865
Melo-Carrillo A, Noseda R, Nir RR, Schain AJ, Stratton J, Strassman AM et al (2017) Selective inhibition of trigeminovascular neurons by fremanezumab: a humanized monoclonal anti-CGRP antibody. J Neurosci 37(30):7149–7163
Charles A (2018) The migraine aura. Continuum (Minneapolis, Minn). 24(4, Headache):1009–1022
Goadsby PJ (2002) Parallel concept of migraine pathogenesis. Ann Neurol 51:140
Messina R, Filippi M, Goadsby PJ (2018) Recent advances in headache neuroimaging. Curr Opin Neurol 31(4):379–385
Coppola G, Parisi V, Di Renzo A, Pierelli F (2020) Cortical pain processing in migraine. J Neural Transm (Vienna) 127(4):551–566
Magon S, May A, Stankewitz A, Goadsby PJ, Schankin C, Ashina M et al (2019) Cortical abnormalities in episodic migraine: a multi-center 3T MRI study. Cephalalgia 39(5):665–673
Park S, Lee DA, Lee HJ, Shin KJ, Park KM (2022) Brain networks in migraine with and without aura: an exploratory arterial spin labeling MRI study. Acta Neurol Scand 145(2):208–214
Arngrim N, Hougaard A, Schytz HW, Vestergaard MB, Britze J, Amin FM et al (2019) Effect of hypoxia on BOLD fMRI response and total cerebral blood flow in migraine with aura patients. J Cereb Blood Flow Metab 39(4):680–689
Farago P, Tuka B, Toth E, Szabo N, Kiraly A, Csete G et al (2017) Interictal brain activity differs in migraine with and without aura: resting state fMRI study. J Headache Pain 18(1):8
Vereb D, Szabo N, Tuka B, Tajti J, Kiraly A, Farago P et al (2020) Temporal instability of salience network activity in migraine with aura. Pain 161(4):856–864
Russo A, Tessitore A, Silvestro M, Di Nardo F, Trojsi F, Del Santo T et al (2019) Advanced visual network and cerebellar hyperresponsiveness to trigeminal nociception in migraine with aura. J Headache Pain 20(1):46
Puledda F, Ffytche DH, O’Daly O, Goadsby PJ (2019) Imaging the visual network in the migraine spectrum. Front Neurol 10:1325
Wang ZW, Yin ZH, Wang X, Zhang YT, Xu T, Du JR et al (2022) Brain structural and functional changes during menstrual migraine: relationships with pain. Front Mol Neurosci 15:967103
Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D (2012) Concurrent functional and structural cortical alterations in migraine. Cephalalgia Int J Headache 32(8):607–620
Sheng L, Zhao P, Ma H, Yuan C, Zhong J, Dai Z et al (2020) A lack of consistent brain grey matter alterations in migraine. Brain 143(6):e45
Chen ZH, Cui YL, Sun JT, Li YT, Zhang C, Zhang YM et al (2022) The brain structure and function abnormalities of migraineurs: a systematic review and neuroimaging meta-analysis. Front Neurol 13:1022793
Hamedani AG, Rose KM, Peterlin BL, Mosley TH, Coker LH, Jack CR et al (2013) Migraine and white matter hyperintensities: the ARIC MRI study. Neurology 81(15):1308–1313
Kruit MC, van Buchem MA, Launer LJ, Terwindt GM, Ferrari MD (2010) Migraine is associated with an increased risk of deep white matter lesions, subclinical posterior circulation infarcts and brain iron accumulation: the population-based MRI CAMERA study. Cephalalgia 30(2):129–136
Monteith T, Gardener H, Rundek T, Dong C, Yoshita M, Elkind MS et al (2014) Migraine, white matter hyperintensities, and subclinical brain infarction in a diverse community: the northern Manhattan study. Stroke 45(6):1830–1832
Dobrynina LA, Suslina AD, Gubanova MV, Belopasova AV, Sergeeva AN, Evers S et al (2021) White matter hyperintensity in different migraine subtypes. Sci Rep 11(1):10881
Ahmed SR, Mohamed AAM, Salem HH, Helmy S, Moustafa RR, Borham SMF (2022) Association of white matter hyperintensities with migraine phenotypes and response to treatment. Acta Neurol Belg. https://doi.org/10.1007/s13760-022-02015-x
Coppola G, Ambrosini A, Di Clemente L, Magis D, Fumal A, Gerard P et al (2007) Interictal abnormalities of gamma band activity in visual evoked responses in migraine: an indication of thalamocortical dysrhythmia? Cephalalgia Int J Headache 27(12):1360–1367
Ambrosini A, Rossi P, De Pasqua V, Pierelli F, Schoenen J (2003) Lack of habituation causes high intensity dependence of auditory evoked cortical potentials in migraine. Brain J Neurol 126(Pt 9):2009–2015
Afra J, Cecchini AP, De Pasqua V, Albert A, Schoenen J (1998) Visual evoked potentials during long periods of pattern-reversal stimulation in migraine. Brain J Neurol 121(Pt 2):233–241
Coppola G, Di Lorenzo C, Schoenen J, Pierelli F (2013) Habituation and sensitization in primary headaches. J Headache Pain 14:65
Freilinger T, Anttila V, de Vries B, Malik R, Kallela M, Terwindt GM et al (2012) Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat Genet 44(7):777–782
Younis S, Hougaard A, Vestergaard MB, Larsson HBW, Ashina M (2017) Migraine and magnetic resonance spectroscopy: a systematic review. Curr Opin Neurol 30(3):246–262
Ferrari MD, Goadsby PJ, Burstein R, Kurth T, Ayata C, Charles A et al (2022) Migraine. Nat Rev Dis Primers 8(1):2
Moreno-Ajona D, Villar-Martínez MD, Goadsby PJ (2021) Targets for migraine treatment: beyond calcitonin gene-related peptide. Curr Opin Neurol 34(3):363–372
Garelja ML, Hay DL (2022) A narrative review of the calcitonin peptide family and associated receptors as migraine targets: calcitonin gene-related peptide and beyond. Headache 62(9):1093–1104
Houts CR, McGinley JS, Nishida TK, Buse DC, Wirth RJ, Dodick DW et al (2021) Systematic review of outcomes and endpoints in acute migraine clinical trials. Headache 61(2):263–275
McGinley JS, Houts CR, Nishida TK, Buse DC, Lipton RB, Goadsby PJ et al (2021) Systematic review of outcomes and endpoints in preventive migraine clinical trials. Headache 61(2):253–262
Lo SH, Gallop K, Smith T, Powell L, Johnston K, Hubig LT et al (2022) Real-world experience of interictal burden and treatment in migraine: a qualitative interview study. J Headache Pain 23(1):65
Goadsby PJ (2000) The pharmacology of headache. Prog Neurobiol 62(5):509–525
Goadsby PJ, Edvinsson L (1994) Joint 1994 Wolff Award Presentation. Peripheral and central trigeminovascular activation in cat is blocked by the serotonin (5HT)-1D receptor agonist 311C90. Headache 34(7):394–399
Ferrari MD, Goadsby PJ, Roon KI, Lipton RB (2002) Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. Cephalalgia Int J Headache 22(8):633–658
Brandes JL, Kudrow D, Stark SR, O’Carroll CP, Adelman JU, O’Donnell FJ et al (2007) Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA 297(13):1443–1454
Silberstein SD, Mannix LK, Goldstein J, Couch JR, Byrd SC, Ames MH et al (2008) Multimechanistic (sumatriptan-naproxen) early intervention for the acute treatment of migraine. Neurology 71(2):114–121
van Casteren DS, Kurth T, Danser AHJ, Terwindt GM, MaassenVanDenBrink A (2021) Sex differences in response to triptans: a systematic review and meta-analysis. Neurology 96(4):162–170
Sacco S, Lampl C, Amin FM, Braschinsky M, Deligianni C, Uludüz D et al (2022) European Headache Federation (EHF) consensus on the definition of effective treatment of a migraine attack and of triptan failure. J Headache Pain 23(1):133
Lombard L, Farrar M, Ye W, Kim Y, Cotton S, Buchanan AS et al (2020) A global real-world assessment of the impact on health-related quality of life and work productivity of migraine in patients with insufficient versus good response to triptan medication. J Headache Pain 21(1):41
Wu JW, Lai PY, Chen YL, Wang YF, Lirng JF, Chen ST et al (2022) The use of neuroimaging for predicting sumatriptan treatment response in patients with migraine. Front Neurol 13:798695
Roberto G, Raschi E, Piccinni C, Conti V, Vignatelli L, D’Alessandro R et al (2015) Adverse cardiovascular events associated with triptans and ergotamines for treatment of migraine: systematic review of observational studies. Cephalalgia 35(2):118–131
Dodick D, Lipton RB, Martin V, Papademetriou V, Rosamond W, MaassenVanDenBrink A et al (2004) Consensus statement: cardiovascular safety profile of triptans (5-HT agonists) in the acute treatment of migraine. Headache 44(5):414–425
Nelson DL, Phebus LA, Johnson KW, Wainscott DB, Cohen ML, Calligaro DO et al (2010) Preclinical pharmacological profile of the selective 5-HT1F receptor agonist lasmiditan. Cephalalgia 30(10):1159–1169
Vila-Pueyo M, Page K, Murdock PR, Loraine HJ, Woodrooffe AJ, Johnson KW et al (2022) The selective 5-HT(1F) receptor agonist lasmiditan inhibits trigeminal nociceptive processing: implications for migraine and cluster headache. Br J Pharmacol 179(3):358–370
Ferrari MD, Färkkilä M, Reuter U, Pilgrim A, Davis C, Krauss M et al (2010) Acute treatment of migraine with the selective 5-HT1F receptor agonist lasmiditan—a randomised proof-of-concept trial. Cephalalgia Int J Headache 30(10):1170–1178
Färkkilä M, Diener H-C, Géraud G, Láinez M, Schoenen J, Harner N et al (2012) Efficacy and tolerability of lasmiditan, an oral 5-HT1F receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol 11(5):405–413
Kuca B, Silberstein SD, Wietecha L, Berg PH, Dozier G, Lipton RB (2018) Lasmiditan is an effective acute treatment for migraine: a phase 3 randomized study. Neurology 91(24):e2222–e2232
Goadsby PJ, Wietecha LA, Dennehy EB, Kuca B, Case MG, Aurora SK et al (2019) Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain J Neurol 142(7):1894–1904
Ashina M, Reuter U, Smith T, Krikke-Workel J, Klise SR, Bragg S et al (2021) Randomized, controlled trial of lasmiditan over four migraine attacks: findings from the CENTURION study. Cephalalgia Int J Headache 41(3):294–304
Shapiro RE, Hochstetler HM, Dennehy EB, Khanna R, Doty EG, Berg PH et al (2019) Lasmiditan for acute treatment of migraine in patients with cardiovascular risk factors: post-hoc analysis of pooled results from 2 randomized, double-blind, placebo-controlled, phase 3 trials. J Headache Pain 20(1):90
Puledda F, Younis S, Huessler E-M, Haghdoost F, Lisicki M, Goadsby PJ et al (2023) Efficacy, safety and indirect comparisons of lasmiditan, rimegepant, and ubrogepant for the acute treatment of migraine: a systematic review and network meta-analysis of the literature. Cephalalgia Int J Headache 43(3):0333
Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X et al (2008) Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet 372(9656):2115–2123
Hewitt DJ, Aurora SK, Dodick DW, Goadsby PJ, Ge YJ, Bachman R et al (2011) Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia Int J Headache 31(6):712–722
Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U et al (2004) Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med 350(11):1104–1110
Diener HC, Barbanti P, Dahlof C, Reuter U, Habeck J, Podhorna J (2011) BI 44370 TA, an oral CGRP antagonist for the treatment of acute migraine attacks: results from a phase II study. Cephalalgia 31(5):573–584
Food and Drug Administration (2020). Drug Approval Package: NURTEC ODT. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/212728Orig1s000TOC.cfm. Accessed 13 Mar 2022
Food and Drug Administration (2019) FDA approves new treatment for adults with migraine. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-adults-migraine. Accessed 13 Mar 2022
Dodick DW, Lipton RB, Ailani J, Lu K, Finnegan M, Trugman JM et al (2019) Ubrogepant for the treatment of migraine. N Engl J Med 381(23):2230–2241
Lipton RB, Dodick DW, Ailani J, Lu K, Finnegan M, Szegedi A et al (2019) Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II randomized clinical trial. JAMA 322(19):1887–1898
Lipton R, Coric V, Stock E, Stock D, Morris B, McCormack T, et al (2018). Rimegepant 75 mg, an oral calcitonin gene-related peptide antagonist, for the acute treatment of migraine: two phase 3, double-blind, randomized, placebo-controlled trials. Cephalalgia Int J Headache
Lipton RB, Croop R, Stock EG, Stock DA, Morris BA, Frost M et al (2019) Rimegepant, an oral calcitonin gene-related peptide receptor antagonist, for migraine. N Engl J Med 381(2):142–149
Croop R, Goadsby PJ, Stock DA, Conway CM, Forshaw M, Stock EG et al (2019) Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet 394(10200):737–745
Croop R, Madonia J, Stock DA, Thiry A, Forshaw M, Murphy A et al (2022) Zavegepant nasal spray for the acute treatment of migraine: a Phase 2/3 double-blind, randomized, placebo-controlled, dose-ranging trial. Headache 62(9):1153–1163
Moreno-Ajona D, Villar-Martinez MD, Goadsby PJ (2022) New generation gepants: migraine acute and preventive medications. J Clin Med 11(6):1656
Hutchinson S, Dodick DW, Treppendahl C, Bennett NL, Yu SY, Guo H et al (2021) Ubrogepant for the acute treatment of migraine: pooled efficacy, safety, and tolerability from the ACHIEVE I and ACHIEVE II phase 3 randomized trials. Neurol Ther 10(1):235–249
Al-Hassany L, Goadsby PJ, Danser AHJ, MaassenVanDenBrink A (2022) Calcitonin gene-related peptide-targeting drugs for migraine: how pharmacology might inform treatment decisions. Lancet Neurol 21(3):284–294
Sang CN, Ramadan NM, Wallihan RG, Chappell AS, Freitag FG, Smith TR et al (2004) LY293558, a novel AMPA/GluR5 antagonist, is efficacious and well-tolerated in acute migraine. Cephalalgia 24(7):596–602
Waung MW, Akerman S, Wakefield M, Keywood C, Goadsby PJ (2016) Metabotropic glutamate receptor 5: a target for migraine therapy. Ann Clin Transl Neurol 3(8):560–571
Gomez-Mancilla B, Brand R, Jurgens TP, Gobel H, Sommer C, Straube A et al (2014) Randomized, multicenter trial to assess the efficacy, safety and tolerability of a single dose of a novel AMPA receptor antagonist BGG492 for the treatment of acute migraine attacks. Cephalalgia 34(2):103–113
Goadsby PJ (2013) Therapeutic prospects for migraine: can paradise be regained? Ann Neurol 74(3):423–434
Afridi SK, Giffin NJ, Kaube H, Goadsby PJ (2013) A randomized controlled trial of intranasal ketamine in migraine with prolonged aura. Neurology 80(7):642–647
Eigenbrodt AK, Ashina H, Khan S, Diener H-C, Mitsikostas DD, Sinclair AJ et al (2021) Diagnosis and management of migraine in ten steps. Nat Rev Neurol 17(8):501–514
Diener HC, Charles A, Goadsby PJ, Holle D (2015) New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol 14(10):1010–1022
American HS (2019) The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache 59(1):1–18
Goadsby PJ, Reuter U, Hallström Y, Broessner G, Bonner JH, Zhang F et al (2017) A controlled trial of erenumab for episodic migraine. N Engl J Med 377(22):2123–2132
Reuter U, Goadsby PJ, Lanteri-Minet M, Wen S, Hours-Zesiger P, Ferrari MD et al (2018) Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet 392(10161):2280–2287
Dodick DW, Ashina M, Brandes JL, Kudrow D, Lanteri-Minet M, Osipova V et al (2018) ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia Int J Headache 38(6):1026–1037
Silberstein SD, Dodick DW, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T et al (2017) Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med 377(22):2113–2122
Ferrari MD, Diener HC, Ning X, Galic M, Cohen JM, Yang R et al (2019) Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet (London, England) 394(10203):1030–1040
Dodick DW, Silberstein SD, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T et al (2018) Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA 319(19):1999–2008
Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK (2018) Galcanezumab in chronic migraine. The randomized, double-blind, placebo-controlled REGAIN study. Neurology 91(24):e2211–e2221
Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR (2018) Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol 75(9):1080–1088
Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY (2018) Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 Phase 3 randomized controlled clinical trial. Cephalalgia Int J Headache 38(8):1442–1454
Ashina M, Saper J, Cady R, Schaeffler BA, Biondi DM, Hirman J et al (2020) Eptinezumab in episodic migraine: a randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia Int J Headache 40(3):241–254
Lipton RB, Goadsby PJ, Smith J, Schaeffler BA, Biondi DM, Hirman J et al (2020) Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology 94(13):e1365–e1377
Mulleners WM, Kim B-K, Láinez MJA, Lanteri-Minet M, Pozo-Rosich P, Wang S et al (2020) Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol 19(10):814–825
Ashina M, Lanteri-Minet M, Pozo-Rosich P, Ettrup A, Christoffersen CL, Josiassen MK et al (2022) Safety and efficacy of eptinezumab for migraine prevention in patients with two-to-four previous preventive treatment failures (DELIVER): a multi-arm, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol 21(7):597–607
Haghdoost F, Puledda F, Huessler EM, Messina R, Pozo-Rosich P (2023) Evaluating the efficacy of CGRP mAbs and gepants for the preventive treatment of migraine: a systematic review and network meta-analysis of phase 3 randomised controlled trials. Cephalalgia Int J Headache 43(3):0331
Messina R, Huessler EM, Puledda F, Haghdoost F, Lebedeva ER, Diener HC (2023) Safety and tolerability of monoclonal antibodies targeting the CGRP pathway and gepants in migraine prevention: a systematic review and meta-analysis. Cephalalgia Int J Headache 43(3):0331
Vernieri F, Brunelli N, Messina R, Costa CM, Colombo B, Torelli P et al (2021) Discontinuing monoclonal antibodies targeting CGRP pathway after one-year treatment: an observational longitudinal cohort study. J Headache Pain 22(1):154
Nsaka M, Scheffler A, Wurthmann S, Schenk H, Kleinschnitz C, Glas M et al (2022) Real-world evidence following a mandatory treatment break after a 1-year prophylactic treatment with calcitonin gene-related peptide (pathway) monoclonal antibodies. Brain Behav 12(7):e2662
di Cola FS, Caratozzolo S, Venturelli E, Balducci U, Sidoti V, Pari E et al (2021) Erenumab discontinuation after 12-month treatment: a multicentric, observational real-life study. Neurol Clin Pract 11(6):e834–e900
Terhart M, Mecklenburg J, Neeb L, Overeem LH, Siebert A, Steinicke M et al (2021) Deterioration of headache impact and health-related quality of life in migraine patients after cessation of preventive treatment with CGRP(-receptor) antibodies. J Headache Pain 22(1):158
Sacco S, Amin FM, Ashina M, Bendtsen L, Deligianni CI, Gil-Gouveia R et al (2022) European Headache Federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention—2022 update. J Headache Pain 23(1):67
Ailani J, Lipton RB, Goadsby PJ, Guo H, Miceli R, Severt L et al (2021) Atogepant for the preventive treatment of migraine. N Engl J Med 385(8):695–706
Goadsby PJ, Dodick DW, Ailani J, Trugman JM, Finnegan M, Lu K et al (2020) Safety, tolerability, and efficacy of orally administered atogepant for the prevention of episodic migraine in adults: a double-blind, randomised phase 2b/3 trial. Lancet Neurol 19(9):727–737
Croop R, Lipton RB, Kudrow D, Stock DA, Kamen L, Conway CM et al (2021) Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet (London, England) 397(10268):51–60
Boinpally R, Jakate A, Butler M, Borbridge L, Periclou A (2021) Single-dose pharmacokinetics and safety of atogepant in adults with hepatic impairment: results from an open-label, phase 1 trial. Clin Pharmacol Drug Dev 10(7):726–733
Tong G, Savant I, Jariwala N, Burt D, Zheng N, Buzescu A et al (2013) Phase I single and multiple dose study to evaluate the safety, tolerability. J Headache Pain 14(Suppl 1):118
AbbVie. (2021) QULIPTA (atogepant) tablets, for oral use: US prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215206Orig1s000lbl.pdf. Accessed 13 Mar 2022
Min KC, Kraft WK, Bondiskey P, Colón-González F, Liu W, Xu J et al (2021) Atogepant is not associated with clinically meaningful alanine aminotransferase elevations in healthy adults. Clin Transl Sci 14(2):599–605
Boinpally R, McNamee B, Yao L, Butler M, McGeeney D, Borbridge L et al (2021) A single supratherapeutic dose of atogepant does not affect cardiac repolarization in healthy adults: results from a randomized, single-dose, phase 1 crossover trial. Clin Pharmacol Drug Dev 10(9):1099–1107
Pozo-Rosich P, Ailani J, Ashina M, Goadsby PJ, Lipton R, Reuter U et al (2022) Atogepant for the preventive treatment of chronic migraine: results from the PROGRESS phase 3 trial. Cephalalgia Int J Headache 42(1S):14–15
Lanteri-Minet M, Ducros A, Francois C, Olewinska E, Nikodem M, Dupont-Benjamin L (2022) Effectiveness of onabotulinumtoxinA (BOTOX(R)) for the preventive treatment of chronic migraine: a meta-analysis on 10 years of real-world data. Cephalalgia 42(14):1543–1564
Armanious M, Khalil N, Lu Y, Jimenez-Sanders R (2021) Erenumab and OnabotulinumtoxinA combination therapy for the prevention of intractable chronic migraine without aura: a retrospective analysis. J Pain Palliat Care Pharmacother 35(1):1–6
Scuteri D, Tonin P, Nicotera P, Vulnera M, Altieri GC, Tarsitano A et al (2022) Pooled analysis of real-world evidence supports anti-CGRP mAbs and OnabotulinumtoxinA combined trial in chronic migraine. Toxins (Basel). 14(8):529
Cohen F, Armand C, Lipton RB, Vollbracht S (2021) Efficacy and tolerability of calcitonin gene-related peptide-targeted monoclonal antibody medications as add-on therapy to OnabotulinumtoxinA in patients with chronic migraine. Pain Med 22(8):1857–1863
Chowdhury D, Bansal L, Duggal A, Datta D, Mundra A, Krishnan A et al (2022) TOP-PRO study: a randomized double-blind controlled trial of topiramate versus propranolol for prevention of chronic migraine. Cephalalgia 42(4–5):396–408
Lai KL, Niddam DM, Fuh JL, Chen SP, Wang YF, Chen WT et al (2017) Flunarizine versus topiramate for chronic migraine prophylaxis: a randomized trial. Acta Neurol Scand 135(4):476–483
Messina R, Lastarria Perez CP, Filippi M, Goadsby PJ (2020) Candesartan in migraine prevention: results from a retrospective real-world study. J Neurol 267(11):3243–3247
Sanchez-Rodriguez C, Sierra A, Planchuelo-Gomez A, Martinez-Pias E, Guerrero AL, Garcia-Azorin D (2021) Real world effectiveness and tolerability of candesartan in the treatment of migraine: a retrospective cohort study. Sci Rep 11(1):3846
Liampas I, Siokas V, Brotis A, Vikelis M, Dardiotis E (2020) Endogenous melatonin levels and therapeutic use of exogenous melatonin in migraine: systematic review and meta-analysis. Headache 60(7):1273–1299
Tseng PT, Yang CP, Su KP, Chen TY, Wu YC, Tu YK et al (2020) The association between melatonin and episodic migraine: a pilot network meta-analysis of randomized controlled trials to compare the prophylactic effects with exogenous melatonin supplementation and pharmacotherapy. J Pineal Res 69(2):e12663
Zhou T, Tang Y, Zhu H (2022) Effectiveness and safety of memantine for headache: a meta-analysis of randomized controlled studies. Clin Neuropharmacol 45(3):40–44
Puledda F, Goadsby PJ (2017) An update on non-pharmacological neuromodulation for the acute and preventive treatment of migraine. Headache 57(4):685–691
Lipton RB, Dodick DW, Silberstein SD, Saper JR, Aurora SK, Pearlman SH et al (2010) Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol 9(4):373–380
Bhola R, Kinsella E, Giffin N, Lipscombe S, Ahmed F, Weatherall M et al (2015) Single-pulse transcranial magnetic stimulation (sTMS) for the acute treatment of migraine: evaluation of outcome data for the UK post market pilot program. J Headache Pain 16:535
Starling AJ, Tepper SJ, Marmura MJ, Shamim EA, Robbins MS, Hindiyeh N et al (2018) A multicenter, prospective, single arm, open label, observational study of sTMS for migraine prevention (ESPOUSE Study). Cephalalgia Int J Head 38(6):1038–1048
Lloyd JO, Hill B, Murphy M, Al-Kaisy A, Andreou AP, Lambru G (2022) Single-pulse transcranial magnetic stimulation for the preventive treatment of difficult-to-treat migraine: a 12-month prospective analysis. J Headache Pain 23(1):63
Chou DE, Shnayderman Yugrakh M, Winegarner D, Rowe V, Kuruvilla D, Schoenen J (2019) Acute migraine therapy with external trigeminal neurostimulation (ACME): a randomized controlled trial. Cephalalgia Int J Headache 39(1):3–14
Schoenen J, Vandersmissen B, Jeangette S, Herroelen L, Vandenheede M, Gerard P et al (2013) Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology 80(8):697–704
Vikelis M, Dermitzakis EV, Spingos KC, Vasiliadis GG, Vlachos GS, Kararizou E (2017) Clinical experience with transcutaneous supraorbital nerve stimulation in patients with refractory migraine or with migraine and intolerance to topiramate: a prospective exploratory clinical study. BMC Neurol 17(1):97
Ordas CM, Cuadrado ML, Pareja JA, de-Las-Casas-Camara G, Gomez-Vicente L, Torres-Gaona G et al (2020) Transcutaneous supraorbital stimulation as a preventive treatment for chronic migraine: a prospective, open-label study. Pain Med 21(2):415–422
Tassorelli C, Grazzi L, de Tommaso M, Pierangeli G, Martelletti P, Rainero I et al (2018) Noninvasive vagus nerve stimulation as acute therapy for migraine: the randomized PRESTO study. Neurology 91(4):e364–e373
Diener HC, Goadsby PJ, Ashina M, Al-Karagholi MA, Sinclair A, Mitsikostas D et al (2019) Non-invasive vagus nerve stimulation (nVNS) for the preventive treatment of episodic migraine: the multicentre, double-blind, randomised, sham-controlled PREMIUM trial. Cephalalgia 39(12):1475–1487
Silberstein SD, Calhoun AH, Lipton RB, Grosberg BM, Cady RK, Dorlas S et al (2016) Chronic migraine headache prevention with noninvasive vagus nerve stimulation: the EVENT study. Neurology 87(5):529–538
Najib U, Smith T, Hindiyeh N, Saper J, Nye B, Ashina S et al (2022) Non-invasive vagus nerve stimulation for prevention of migraine: the multicenter, randomized, double-blind, sham-controlled PREMIUM II trial. Cephalalgia Int J Headache 42(7):560–569
Chen SP, Ay I, de Morais AL, Qin T, Zheng Y, Sadeghian H et al (2016) Vagus nerve stimulation inhibits cortical spreading depression. Pain 157(4):797–805
Akerman S, Simon B, Romero-Reyes M (2017) Vagus nerve stimulation suppresses acute noxious activation of trigeminocervical neurons in animal models of primary headache. Neurobiol Dis 102:96–104
Antal A, Kriener N, Lang N, Boros K, Paulus W (2011) Cathodal transcranial direct current stimulation of the visual cortex in the prophylactic treatment of migraine. Cephalalgia Int J Headache 31(7):820–828
Vigano A, D’Elia TS, Sava SL, Auve M, De Pasqua V, Colosimo A et al (2013) Transcranial direct current stimulation (tDCS) of the visual cortex: a proof-of-concept study based on interictal electrophysiological abnormalities in migraine. J Headache Pain 14:23
Viganò A, Toscano M, Puledda F, Di Piero V (2019) Treating chronic migraine with neuromodulation: the role of neurophysiological abnormalities and maladaptive plasticity. Front Pharmacol 10:32
Nir RR, Yarnitsky D (2015) Conditioned pain modulation. Curr Opin Support Palliat Care 9(2):131–137
Yarnitsky D, Dodick DW, Grosberg BM, Burstein R, Ironi A, Harris D et al (2019) Remote electrical neuromodulation (REN) relieves acute migraine: a randomized, double-blind, placebo-controlled, multicenter trial. Headache 59(8):1240–1252
Nierenburg H, Vieira JR, Lev N, Lin T, Harris D, Vizel M et al (2020) Remote electrical neuromodulation for the acute treatment of migraine in patients with chronic migraine: an open-label pilot study. Pain Ther 9(2):531–543
Renner T, Sollmann N, Trepte-Freisleder F, Albers L, Mathonia NM, Bonfert MV et al (2019) Repetitive peripheral magnetic stimulation (rPMS) in subjects with migraine-setup presentation and effects on skeletal musculature. Front Neurol 10:738
Borner C, Renner T, Trepte-Freisleder F, Urban G, Schandelmaier P, Lang M et al (2022) Response predictors of repetitive neuromuscular magnetic stimulation in the preventive treatment of episodic migraine. Front Neurol 13:919623
Tepper SJ, Grosberg B, Daniel O, Kuruvilla DE, Vainstein G, Deutsch L et al (2022) Migraine treatment with external concurrent occipital and trigeminal neurostimulation-A randomized controlled trial. Headache 62(8):989–1001
Daniel O, Tepper SJ, Deutsch L, Sharon R (2022) External concurrent occipital and trigeminal neurostimulation relieves migraine headache: a prospective, randomized, double-blind, Sham-controlled trial. Pain Ther 11(3):907–922
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Puledda, F., Silva, E.M., Suwanlaong, K. et al. Migraine: from pathophysiology to treatment. J Neurol 270, 3654–3666 (2023). https://doi.org/10.1007/s00415-023-11706-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11706-1