Abstract
Migraine is a common brain disorder with high disability rates which involves a series of abnormal neuronal networks, interacting at different levels of the central and peripheral nervous system. An increase in the interest around migraine pathophysiology has allowed researchers to unravel certain neurophysiological mechanisms and neurotransmitter involvement culminating in the recent development of novel therapies, which might substantially change the clinical approach to migraine patients. The present review will highlight the current aspects of migraine pathophysiology, covering an understanding of the complex workings of the migraine state and the brain regions responsible for them. We will further discuss the therapeutic agents which have appeared in the most recent years for migraine care, from calcitonin gene-related peptide (CGRP) receptor antagonists, gepants; through serotonin 5-HT1F receptor agonists, ditans, and CGRP or CGRP receptor monoclonal antibodies to invasive and non-invasive neuromodulation techniques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is the most common neurological cause of disability in the world [1]. Notwithstanding, clinicians and researchers have seen little progress in the therapeutic options available to treat this condition in the last two decades. Recent advances in our understanding of migraine pathophysiology have allowed the development of pharmacological and non-pharmacological treatments that offer the advantage of targeting mechanisms known to be active in the disorder leading to better management of patients.
The current review follows this bench to bedside approach [2], with an outline of relevant mechanisms in migraine biology, followed by an up-to-date summary of the most important therapies used in migraine at the present stage.
Migraine pathophysiology
Over the last two decades our knowledge of the biology of migraine has improved considerably, with a series of basic science and imaging studies that demonstrate how vascular changes, first thought to explain migrainous pain, are neither necessary, nor sufficient in migraine [3, 4]. From a vascular theory the field has moved on to Neuronal theories involving the central or peripheral nervous system, or both. Much research has focused on specific brain structures thought to be at the basis of pain, arguably the primary migraine symptom. With these advances, it has become clear that the concept of a unique migraine generator may not be useful, in view of the variety of overlapping phases that constitute the migraine attack.
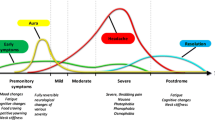
It is now widely accepted that migraine should be viewed as a complex brain network disorder with a strong genetic basis that involves multiple cortical, subcortical and brainstem regions to account for the pain and the wide constellation of symptoms characterizing the attack [4,5,6]. Here we will describe some important advances in our understanding of the different brain areas known to be directly involved in the premonitory, aura, pain and postdromal phases of migraine.
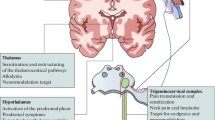
The trigeminal vascular system and brainstem nuclei
The trigeminovascular system is one of the key actors in the expression of migraine headache. It consists of peripheral axons from the trigeminal ganglion that reach the meninges and intracranial arteries and converge centrally in the trigeminocervical complex releasing, among other transmitters, calcitonin gene-related peptide (CGRP) [7, 8]. The trigeminocervical complex (TCC) consists of the trigeminal nucleus caudalis along with the dorsal horn of C1–C2 segments of the spinal cord [9, 10]. Its activation is thought to lead to the cascade of events resulting in the migraine pain due to its direct connection with key brain centres such as diencephalic and brainstem nuclei [11, 12].
In the late 1980s it was proposed that migraine pain may be due to a sterile neurogenically induced inflammation of the dura mater [13, 14]. However, the failure of specific plasma protein extravasation blockers as acute or preventive migraine treatments in randomized controlled trials suggested other explanations were needed [15, 16]. Human observational [17] and brain imaging studies [18,19,20] have suggested a role of brainstem regions, such as the periaqueductal grey matter (PAG) and the dorsolateral pons (DLP), in migraine attacks: the ‘migraine generator’. In addition, a series of laboratory experiments have proposed that the brainstem might act as a driver of changes in cortical activity during migraine [21, 22]. Although the validity of the brainstem generator theory has been widely debated in the last few years [23], the role of relevant brainstem nuclei—including the rostral ventral medulla, the locus coeruleus, the superior salivatory and cuneiform nucleus—in modulating trigeminovascular pain transmission and autonomic responses in migraine is well established [4, 18, 19]. Furthermore, there is evidence showing antimigraine drugs such as triptans [24, 25], ergot derivatives [26, 27] and the novel CGRP receptor antagonists [28, 29] can specifically modulate activity in the TCC, which might explain their effect in aborting migraine.
The hypothalamus
The central role of the hypothalamus in cluster headache and other trigeminal autonomic cephalalgias is well established [30,31,32]. Several studies have recently highlighted its possible involvement in migraine as well. Evidence shows that this brain structure has direct and indirect anatomical connections to the thalamus [33], trigeminovascular neurons [34, 35] and sympathetic and parasympathetic brainstem nuclei [36], supporting its role in nociceptive and autonomic modulation in migraine patients. Previous positron emission tomography studies have shown hypothalamic activation during spontaneous migraine headache [37] and during the premonitory phase [38]. Recently, Schulte and May performed an elegant study in which a migraine patient underwent functional neuroimaging for 30 consecutive days. During the 24 h preceding the attack as well as throughout the ictal phase an altered functional connectivity between the hypothalamus and the areas of the brainstem generator was found, leading the authors to hypothesize that this network change might be the real driver of attacks [39]. The key involvement of the hypothalamus in migraine explains symptoms that begin in the early ictal stages and last throughout the attack, such as craving, mood changes, yawning and fatigue [4, 40].
The thalamus
The thalamus is a nociceptive relay station where inputs from the dura mater as well as cutaneous regions are conveyed through second-order trigeminovascular neurons. It is a central area for the processing and integration of pain stimuli and its connection to a wide variety of cortical areas such as the somatosensory, motor, visual, auditory, olfactory and limbic regions can explain part of the complexity of migraine features [41]. Thalamo-cortical transmission is constantly modulated by different pathways involved in cognition, emotion and autonomic responses [42]. Several studies have reported structural [43,44,45] and functional [19, 46,47,48,49] thalamic alterations in migraineurs during the ictal and interictal phase, which can be detected since the paediatric age and might influence the onset of the migraine attack. Furthermore, the thalamus has shown to be a pivotal area for the development of sensory hypersensitivity to visual stimuli [50] and mechanical allodynia [51].
Several acute [24] and preventive [52,53,54,55] migraine therapies are thought to act centrally through the modulation of thalamic neurons. Recently, Andreou et al. [56] showed that the efficacy of single pulse transcranial magnetic stimulation (sTMS) in the treatment of migraine with and without aura [57] might be related not only to its capability to block cortical spreading depression (CSD) but also to its modulation of thalamo-cortical activity.
The cortex
Even if the role of the cortical wave of spreading depression first identified by Leão [58, 59] in the generation of aura is well established [60, 61], its activity as a potential trigger for migraine headache is less clear. Those in favour of this theory argue that experimental studies in rats have shown that CSD can trigger neurogenic meningeal inflammation and subsequently activate the trigeminovascular system [62, 63]; however, this has not been confirmed in humans. Many changes in the structure and function of key cortical areas have been reported over the last years in migraine patients both with and without aura. Specifically, cortical changes in the ictal and interictal period have been shown in regions normally associated with pain processing such as the insular, somatosensory, prefrontal, and cingulate cortex [64, 65].
A large body of evidence has pointed to an increased sensitivity to different sensory stimuli in migraineurs during the attack and in the interictal phase [66]. In addition, several neurophysiological studies have reported a reduction of the common physiological response known as habituation, in which repeated stimulations cause a decrement in the amplitudes of sensory responses [67, 68]. The lack of habituation in migraine, measured for different sensory modalities, usually occurs during the pain-free period and reverts during the ictal phase or with attacks becoming more frequent [66]. Although the neural mechanisms underlying sensitization and habituation deficits remain poorly understood, the presence of a widespread cortical dyshabituation has been hypothesized as one of the main contributors to this deficit [69].
Recent large genome-wide association studies have identified 13 susceptibility gene variants in migraine patients which are mainly involved in glutamatergic neurotransmission and synaptic plasticity, and whose impairment may, therefore, be considered a key mechanism underlying an abnormal cortical excitability [70, 71].
Finally, positive results from the use of novel therapeutic approaches capable of modulating neuronal activity in the cortex also confirm the possibility of an abnormal cortical responsivity in migraine [56], as will be highlighted further.
Novel therapies in migraine
Migraine therapy has historically been divided between acute and preventive treatments, a structure that for simplicity is followed in this review. It is, however, becoming evident that this dichotomous principle might in fact be dated [2], especially by observing the mechanism of action of novel migraine therapies such as the CGRP antagonists, which have been studied as both acute and preventive migraine agents.
Acute therapies
Treatment for the acute migraine attack ranges from nonspecific medications—such as non-steroidal anti-inflammatory drugs and combination analgesics—to migraine-specific drugs, including ergotamine preparations and triptans. Triptans, which act by targeting 5-HT1B and 5-HT1D serotonin receptors, were the first drugs specifically developed as acute migraine therapies [72]. Although they can be very effective in many individuals, they often have significant limitations to their use caused by adverse effects. Furthermore, lack of efficacy and recurrence of migraine symptoms are seen in over 50% of cases in most studies [73, 74]. As a consequence, in the last years there has been a search for promising novel therapeutic agents to better treat migraine patients.
CGRP is a neuropeptide widely expressed in both peripheral and central neurons. Aside from its action as a potent cerebral arteriolar dilatator, substantial evidence has pointed to its role in modulating central and peripheral pain circuits. Studies showing the mediating action of CGRP on second- and third-order neurons seem to underline its regulatory role in central pain mechanisms. Furthermore, elevation of this molecule in migraineurs is thought to be linked to a decrease in descending inhibitory mechanisms which in turn might lead to migraine susceptibility through sensitization of multiple central neuronal circuits [8]. These findings have progressively led to the development of new drugs that target the CGRP pathway. Six different CGRP receptor antagonists, the gepants, have been developed for use in acute migraine [72]. Remarkably, each study reported positive outcomes on the primary endpoint of pain freedom when comparing the new drugs to placebo. However, two studies were stopped due to liver toxicity [75, 76] and three because of lack of interest from the companies [77,78,79]. One study testing the molecule ubrogepant is currently in phase III [80]. Notably, these medicines have a better tolerability in terms of central nervous system and vascular side effects compared to triptans and they seem to present a lower risk of causing medication overuse [2, 73].
Another encouraging new acute treatment for migraine is represented by the drug class of 5-HT1F receptor agonist called ditans. Several studies have shown that 5-HT1F receptors are not expressed in the vasculature [81] and that ditans inhibit activation of cells in the trigeminal nucleus caudalis evoked by trigeminal stimulation [82, 83]. Lasmiditan has been studied in two randomized, placebo-controlled double-blind trials which showed significant improvement, measured in terms of headache freedom at 2 h [84, 85], with its use. The main advantage of this new drug is the lack of any cardiovascular and cerebrovascular effects [86], although mild side effects such as dizziness, fatigue, vertigo and somnolence have been reported in the randomized controlled trials (RCT).
Glutamatergic targets, including both metabotropic and ionotropic glutamate receptors, are also expected to have a prominent role in future migraine therapy. Recent experimental and clinical studies have shown an effect of NMDA, AMPA, iGluR5 and mGluR5 receptor antagonists in migraine, although their efficacy was lower than that of sumatriptan and related visual side effects were observed [87,88,89]. The NMDA receptor, however, could prove to be an important target for the management of migraine with aura, as shown by small RCT testing the effects of ketamine in reducing the severity of auras [90].
Preventive therapies
Preventive therapies are recommended in patients with chronic migraine and in more than a third of episodic migraine patients, especially in the case of frequent attacks or in subjects who do not tolerate and respond to acute treatments [91]. Many drugs of different pharmacological categories—such as β blockers, anticonvulsants, tricyclic antidepressants and calcium channel modulators—have been approved for migraine prevention or have class A evidence supporting their use. Patients’ compliance and adherence to these medications, however, is often poor due to their modest efficacy and adverse effects [86]. Therefore, more effective and better tolerated drugs are currently being studied for preventive use in migraine, mainly represented by monoclonal antibodies (mAB) to either CGRP peptide (galcanezumab, eptinezumab or TEV-48125) or its canonical receptor (erenumab). Data from a total of five RCTs performed on episodic migraine patients [92,93,94,95,96] revealed that these compounds present a therapeutic gain—measured through 50% responder rates for migraine/probable migraine days—ranging from 17 to 31. In the two placebo-controlled RCTs for chronic migraine [97, 98] the therapeutic gain was of 16 and 24 [2]. Even though monoclonal antibodies are very likely to represent the future strategy for effective migraine prevention, there are several caveats to their use that need to be considered. First, given the relatively short duration of the ongoing studies, evidence is needed to exclude long-term issued linked to the use of mAB. Furthermore, there is little knowledge regarding the development of autoantibodies against these compounds following prolonged treatment. Lastly, the elevated cost of these molecules must be counterbalanced by a high patient benefit to justify their extensive use.
Other targets for migraine therapy focusing on the supposed pathophysiological role of neuroinflammation in inducing migraine attacks—such as substance P, neurokinin 1 receptors [99] and orexin receptors [100]—have consistently failed in clinical trials in recent years. This evidence once more suggests that targeted migraine therapies must focus on specific neuronal mechanisms [2, 86].
Neuromodulation
Neuromodulation is a promising approach that has emerged in recent years with both acute and preventive migraine treatment strategies. These exciting techniques range from invasive approaches such as occipital nerve stimulation (ONS) and sphenopalatine ganglion (SPG) stimulation, which have been used for several years and are largely positioned in intractable chronic patients, to more modern non-invasive devices that target the nervous system transcutaneously. The latter are mainly represented by TMS, non-invasive vagus nerve stimulation (nVNS), supraorbital nerve stimulation and transcranial direct current stimulation (tDCS).
ONS has been investigated as a prevention in chronic migraine patients in three randomized controlled trials: each was negative [101,102,103]. A later open-label follow-up study has shown a modest 12-month efficacy rate of ONS for headache pain and disability, although the complication rates associated with this procedure were still high [104].
Several experimental studies have demonstrated that the SPG has connections with the trigeminovascular system [105], explaining the presence of cranial autonomic symptoms in primary headaches and suggesting a potential role for the SPG in pain modulation [106]. Preliminary studies reported an improvement in pain intensity after lidocaine-induced SPG block [107, 108] or electrical SPG stimulation [109] during acute migraine attacks. In addition, a trend of reduction in migraine days per month and an amelioration in several quality of life measures were reported after repetitive SPG blockades with 0.5% bupivacaine [110]. Two RCTs are currently evaluating the acute use of a surgically implanted SPG neurostimulator in high disability migraine (NCT01540799, NCT01294046) and results are awaited. The positive results of a double-blind, randomized, sham-controlled trial performed on 67 episodic migraine patients (the PREMICE study) [111] followed by an audit on more than 2000 patients [112] have led to the approval of the non-invasive transcutaneous supraorbital nerve stimulator (Cefaly®) as a preventive treatment for migraine. A current RCT (NCT02590939) is testing the Cefaly® device as an acute treatment; however, further studies with a focus on blinding issues are needed to confirm its efficacy as a preventive treatment in migraine.
Early studies on patients with comorbid epilepsy or depression and headache supported a possible effect of vagus nerve stimulation in migraine. Different open-label studies for the treatment of acute migraine attacks using a novel portable device for nVNS (GammaCore®) demonstrated that its effect was comparable to that of most commonly used triptans with mild and well-tolerated side effects [113,114,115]. Regarding its preventive use, a double-blind, sham-controlled study in chronic migraine patients revealed a modest reduction in headache days in the active group compared to the sham group after two months (−1.4 vs. −0.2 days; p = 0.56) [116]. However, the open-label extension data suggests that longer term use of nVNS might be effective. Another recent open-label study on menstrual-related migraine reported a significant reduction in the number of migraine days and analgesic use following a 12-week treatment period in 56 patients [117].
On the basis of previous experimental studies [118] and recent evidence [56] supporting a positive effect of sTMS in inhibiting CSD and the activity of thalamo-cortical neurons, a handheld device (SpringTMS®) has been recently developed and approved for the treatment of acute migraine attacks. A preliminary multicentre, randomized, double-blind, parallel-group, sham-controlled study [57] on 164 migraine patients with aura demonstrated a superiority of sTMS over sham stimulation for pain freedom at 2 h (39 vs 22%, p = 0.018) and for sustained pain freedom at 24 (29 vs 16%, p = 0·04) and 48 (27 vs 13%, p = 0·03) hours. Moreover, a post-marketing phone-based survey [119] on 190 episodic and chronic migraine patients revealed that 62% had a reduction in their migraine headaches and 59% reported a decrease in the number of headache days after a 12-week treatment. There is, however, still a lack of large controlled RCTs to support the use of the sTMS for the prevention of migraine.
Another neuromodulation approach has focused on the application of repeated cathodal or anodal transcranial direct current stimulation over the visual cortex, although data on its therapeutic effect in migraineurs have been conflicting [120, 121].
It is clear from the available evidence that although very promising, neuromodulation techniques require further studies to confirm their efficacy in migraine.
Conclusions
The recent recognition of migraine as a debilitating neurological condition is an important advance in directing more resources to the development of new treatments and their deployment to patients. The last two decades have seen a number of important studies in the area of primary headaches leading to an extremely exciting era for researchers interested in this disorder. New treatments are rapidly becoming available for patients and a better understanding of its pathophysiological mechanisms is allowing a greater awareness of the complexity of a brain disease which has often been overlooked and under-managed.
References
Global Burden of Disease Study 2013 Collaborators (2015) Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (Lond, Engl) 386:743–800
Goadsby PJ (2016) Bench to bedside advances in the 21st century for primary headache disorders: migraine treatments for migraine patients. Brain 139:2571–2577
Amin FM, Asghar MS, Hougaard A, Hansen AE, Larsen VA, de Koning PJ, Larsson HB, Olesen J, Ashina M (2013) Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross-sectional study. Lancet Neurol 12:454–461
Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S (2017) Pathophysiology of migraine—a disorder of sensory processing. Physiol Rev 97(2):553–622
Charles A (2013) Migraine: a brain state. Curr Opin Neurol 26:235–239
Ferrari MD, Klever RR, Terwindt GM, Ayata C, van den Maagdenberg AM (2015) Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol 14:65–80
Messlinger K, Fischer MJ, Lennerz JK (2011) Neuropeptide effects in the trigeminal system: pathophysiology and clinical relevance in migraine. Keio J Med 60:82–89
Ho TW, Edvinsson L, Goadsby PJ (2010) CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol 6:573–582
Goadsby PJ, Hoskin KL (1997) The distribution of trigeminovascular afferents in the nonhuman primate brain Macaca nemestrina: a c-fos immunocytochemical study. J Anat 190(Pt 3):367–375
Hoskin KL, Zagami A, Goadsby PJ (1999) Stimulation of the middle meningeal artery leads to Fos expression in the trigeminocervical nucleus: a comparative study of monkey and cat. J Anat 194:579–588
Goadsby PJ, Charbit AR, Andreou AP, Akerman S, Holland PR (2009) Neurobiology of migraine. Neuroscience 161:327–341
Akerman S, Holland PR, Goadsby PJ (2011) Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci 12:570–584
Moskowitz MA, Cutrer FM (1993) SUMATRIPTAN: a receptor-targeted treatment for migraine. Annu Rev Med 44:145–154
Markowitz S, Saito K, Moskowitz MA (1987) Neurogenically mediated leakage of plasma proteins occurs from blood vessels in dura mater but not brain. J Neurosci 7:4129–4136
May A, Goadsby PJ (2001) Substance P receptor antagonists in the therapy of migraine. Expert Opinion Investig Drugs 10:1–6
Peroutka SJ (2005) Neurogenic inflammation and migraine: implications for therapeutics. Mol Interv 5:306–313
Raskin NH, Hosobuchi Y, Lamb S (1987) Headache may arise from perturbation of brain. Headache 27:416–420
Weiller C, May A, Limmroth V, Juptner M, Kaube H, Schayck RV, Coenen HH, Diener HC (1995) Brain stem activation in spontaneous human migraine attacks. Nat Med 1:658–660
Bahra A, Matharu MS, Buchel C, Frackowiak RS, Goadsby PJ (2001) Brainstem activation specific to migraine headache. Lancet 357:1016–1017
Stankewitz A, Aderjan D, Eippert F, May A (2011) Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J Neurosci 31:1937–1943
Goadsby PJ, Duckworth JW (1989) Low frequency stimulation of the locus coeruleus reduces regional cerebral blood flow in the spinalized cat. Brain Res 476:71–77
Vinogradova LV (2015) Comparative potency of sensory-induced brainstem activation to trigger spreading depression and seizures in the cortex of awake rats: implications for the pathophysiology of migraine aura. Cephalalgia 35:979–986
Borsook D, Burstein R (2012) The enigma of the dorsolateral pons as a migraine generator. Cephalalgia 32:803–812
Kroger IL, May A (2015) Triptan-induced disruption of trigemino-cortical connectivity. Neurology 84:2124–2131
Goadsby PJ, Hoskin KL (1996) Inhibition of trigeminal neurons by intravenous administration of the serotonin (5HT)1B/D receptor agonist zolmitriptan (311C90): are brain stem sites therapeutic target in migraine? Pain 67:355–359
Goadsby PJ, Gundlach AL (1991) Localization of 3H-dihydroergotamine-binding sites in the cat central nervous system: relevance to migraine. Ann Neurol 29:91–94
Hoskin KL, Kaube H, Goadsby PJ (1996) Central activation of the trigeminovascular pathway in the cat is inhibited by dihydroergotamine. A c-Fos and electrophysiological study. Brain 119(Pt 1):249–256
Pozo-Rosich P, Storer RJ, Charbit AR, Goadsby PJ (2015) Periaqueductal gray calcitonin gene-related peptide modulates trigeminovascular neurons. Cephalalgia 35:1298–1307
Storer RJ, Akerman S, Goadsby PJ (2004) Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br J Pharmacol 142:1171–1181
May A, Bahra A, Buchel C, Frackowiak RS, Goadsby PJ (1998) Hypothalamic activation in cluster headache attacks. Lancet (Lond, Engl) 352:275–278
Goadsby PJ (2012) Trigeminal autonomic cephalalgias. Continuum (Minneapolis, Minn) 18:883–895
May A (2005) Cluster headache: pathogenesis, diagnosis, and management. Lancet (Lond, Engl) 366:843–855
Kagan R, Kainz V, Burstein R, Noseda R (2013) Hypothalamic and basal ganglia projections to the posterior thalamus: possible role in modulation of migraine headache and photophobia. Neuroscience 248:359–368
Abdallah K, Artola A, Monconduit L, Dallel R, Luccarini P (2013) Bilateral descending hypothalamic projections to the spinal trigeminal nucleus caudalis in rats. PLoS One 8:e73022
Robert C, Bourgeais L, Arreto CD, Condes-Lara M, Noseda R, Jay T, Villanueva L (2013) Paraventricular hypothalamic regulation of trigeminovascular mechanisms involved in headaches. J Neurosci 33:8827–8840
Moulton EA, Becerra L, Johnson A, Burstein R, Borsook D (2014) Altered hypothalamic functional connectivity with autonomic circuits and the locus coeruleus in migraine. PLoS One 9:e95508
Denuelle M, Fabre N, Payoux P, Chollet F, Geraud G (2007) Hypothalamic activation in spontaneous migraine attacks. Headache 47:1418–1426
Maniyar FH, Sprenger T, Monteith T, Schankin C, Goadsby PJ (2014) Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain 137:232–241
Schulte LH, May A (2016) The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 139:1987–1993
Oliveira MM, Akerman S, Tavares I, Goadsby PJ (2016) Neuropeptide Y inhibits the trigeminovascular pathway through NPY Y1 receptor: implications for migraine. Pain 157:1666–1673
Noseda R, Jakubowski M, Kainz V, Borsook D, Burstein R (2011) Cortical projections of functionally identified thalamic trigeminovascular neurons: implications for migraine headache and its associated symptoms. J Neurosci 31:14204–14217
Noseda R, Kainz V, Borsook D, Burstein R (2014) Neurochemical pathways that converge on thalamic trigeminovascular neurons: potential substrate for modulation of migraine by sleep, food intake, stress and anxiety. PLoS One 9:e103929
Messina R, Rocca MA, Colombo B, Pagani E, Falini A, Comi G, Filippi M (2015) White matter microstructure abnormalities in pediatric migraine patients. Cephalalgia 35:1278–1286
Coppola G, Tinelli E, Lepre C, Iacovelli E, Di Lorenzo C, Di Lorenzo G, Serrao M, Pauri F, Fiermonte G, Bianco F, Pierelli F (2014) Dynamic changes in thalamic microstructure of migraine without aura patients: a diffusion tensor magnetic resonance imaging study. Eur J Neurol 21:287
Magon S, May A, Stankewitz A, Goadsby PJ, Tso AR, Ashina M, Amin FM, Seifert CL, Chakravarty MM, Muller J, Sprenger T (2015) Morphological abnormalities of thalamic subnuclei in migraine: a multicenter MRI study at 3 tesla. J Neurosci 35:13800–13806
Porcaro C, Di Lorenzo G, Seri S, Pierelli F, Tecchio F, Coppola G (2016) Impaired brainstem and thalamic high-frequency oscillatory EEG activity in migraine between attacks. Cephalalgia
Hodkinson DJ, Wilcox SL, Veggeberg R, Noseda R, Burstein R, Borsook D, Becerra L (2016) Increased amplitude of thalamocortical low-frequency oscillations in patients with migraine. J Neurosci 36:8026–8036
Afridi SK, Matharu MS, Lee L, Kaube H, Friston KJ, Frackowiak RS, Goadsby PJ (2005) A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain 128:932–939
Afridi SK, Giffin NJ, Kaube H, Friston KJ, Ward NS, Frackowiak RS, Goadsby PJ (2005) A positron emission tomographic study in spontaneous migraine. Arch Neurol 62:1270–1275
Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, Burstein R (2010) A neural mechanism for exacerbation of headache by light. Nat Neurosci 13:239–245
Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, Becerra L, Borsook D (2010) Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol 68:81–91
Shields KG, Goadsby PJ (2005) Propranolol modulates trigeminovascular responses in thalamic ventroposteromedial nucleus: a role in migraine? Brain 128:86–97
Tepe N, Filiz A, Dilekoz E, Akcali D, Sara Y, Charles A, Bolay H (2015) The thalamic reticular nucleus is activated by cortical spreading depression in freely moving rats: prevention by acute valproate administration. Eur J Neurosci 41:120–128
Andreou AP, Shields KG, Goadsby PJ (2010) GABA and valproate modulate trigeminovascular nociceptive transmission in the thalamus. Neurobiol Dis 37:314–323
Summ O, Charbit AR, Andreou AP, Goadsby PJ (2010) Modulation of nocioceptive transmission with calcitonin gene-related peptide receptor antagonists in the thalamus. Brain 133:2540–2548
Andreou AP, Holland PR, Akerman S, Summ O, Fredrick J, Goadsby PJ (2016) Transcranial magnetic stimulation and potential cortical and trigeminothalamic mechanisms in migraine. Brain 139:2002–2014
Lipton RB, Dodick DW, Silberstein SD, Saper JR, Aurora SK, Pearlman SH, Fischell RE, Ruppel PL, Goadsby PJ (2010) Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol 9:373–380
Leão AAP (1944) Spreading depression of activity in the cerebral cortex. J Neurophysiol 7:359–390
Leao AAP (1944) Pial circulation and spreading activity in the cerebral cortex. J Neurophysiol 7:391–396
Bhaskar S, Saeidi K, Borhani P, Amiri H (2013) Recent progress in migraine pathophysiology: role of cortical spreading depression and magnetic resonance imaging. Eur J Neurosci 38:3540–3551
Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, Kwong KK, Cutrer FM, Rosen BR, Tootell RB, Sorensen AG, Moskowitz MA (2001) Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci USA 98:4687–4692
Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA (2002) Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med 8:136–142
Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R (2011) Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol 69:855–865
Sprenger T, Borsook D (2012) Migraine changes the brain: neuroimaging makes its mark. Curr Opin Neurol 25:252–262
Chong CD, Schwedt TJ, Dodick DW (2016) Migraine: what imaging reveals. Curr Neurol Neurosci Rep 16:64
Coppola G, Di Lorenzo C, Schoenen J, Pierelli F (2013) Habituation and sensitization in primary headaches. J Headache Pain 14:65
Ambrosini A, Rossi P, De Pasqua V, Pierelli F, Schoenen J (2003) Lack of habituation causes high intensity dependence of auditory evoked cortical potentials in migraine. Brain 126:2009–2015
Afra J, Cecchini AP, De Pasqua V, Albert A, Schoenen J (1998) Visual evoked potentials during long periods of pattern-reversal stimulation in migraine. Brain 121(Pt 2):233–241
Schoenen J (2006) Neurophysiological features of the migrainous brain. Neurol Sci 27(suppl 2):S77–S81
Gasparini CF, Smith RA, Griffiths LR (2016) Genetic insights into migraine and glutamate: a protagonist driving the headache. J Neurol Sci 367:258–268
Freilinger T, Anttila V, de Vries B, Malik R, Kallela M, Terwindt GM, Pozo-Rosich P, Winsvold B, Nyholt DR, van Oosterhout WPJ, Artto V, Todt U, Hamalainen E, Fernandez-Morales J, Louter MA, Kaunisto MA, Schoenen J, Raitakari O, Lehtimaki T, Vila-Pueyo M, Gobel H, Wichmann E, Sintas C, Uitterlinden AG, Hofman A, Rivadeneira F, Heinze A, Tronvik E, van Duijn CM, Kaprio J, Cormand B, Wessman M, Frants RR, Meitinger T, Muller-Myhsok B, Zwart J-A, Farkkila M, Macaya A, Ferrari MD, Kubisch C, Palotie A, Dichgans M, van den Maagdenberg AMJM (2012) Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat Genet 44:777–782
Humphrey PP, Feniuk W, Perren MJ, Beresford IJ, Skingle M, Whalley ET (1990) Serotonin and migraine. Ann N Y Acad Sci 600:587–598 (discussion 598–600)
Goadsby PJ, Sprenger T (2010) Current practice and future directions in the prevention and acute management of migraine. Lancet Neurol 9:285–298
Ferrari MD, Goadsby PJ, Roon KI, Lipton RB (2002) Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. Cephalalgia 22:633–658
Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, Leibensperger H, Froman S, Assaid C, Lines C, Koppen H, Winner PK (2008) Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet 372:2115–2123
Hewitt DJ, Aurora SK, Dodick DW, Goadsby PJ, Ge YJ, Bachman R, Taraborelli D, Fan X, Assaid C, Lines C, Ho TW (2011) Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia 31:712–722
Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM, Group BBCPoCS (2004) Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med 350:1104–1110
Marcus R, Goadsby PJ, Dodick D, Stock D, Manos G, Fischer TZ (2014) BMS-927711 for the acute treatment of migraine: a double-blind, randomized, placebo controlled, dose-ranging trial. Cephalalgia 34:114–125
Diener HC, Barbanti P, Dahlof C, Reuter U, Habeck J, Podhorna J (2011) BI 44370 TA, an oral CGRP antagonist for the treatment of acute migraine attacks: results from a phase II study. Cephalalgia 31:573–584
Voss T, Lipton RB, Dodick DW, Dupre N, Ge JY, Bachman R, Assaid C, Aurora SK, Michelson D (2016) A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia 36:887–898
Nelson DL, Phebus LA, Johnson KW, Wainscott DB, Cohen ML, Calligaro DO, Xu YC (2010) Preclinical pharmacological profile of the selective 5-HT1F receptor agonist lasmiditan. Cephalalgia 30:1159–1169
Goadsby PJ, Classey JD (2003) Evidence for serotonin (5-HT)1B, 5-HT1D and 5-HT1F receptor inhibitory effects on trigeminal neurons with craniovascular input. Neuroscience 122:491–498
Vila-Pueyo M, Strother L, Page K, Loaraine H, Kovalchin J, Goadsby PJ, Holland PR (2016) Lasmiditan inhibits trigeminovascular nociceptive transmission. Cephalalgia 36:152
Ferrari MD, Farkkila M, Reuter U, Pilgrim A, Davis C, Krauss M, Diener HC, European COLI (2010) Acute treatment of migraine with the selective 5-HT1F receptor agonist lasmiditan—a randomised proof-of-concept trial. Cephalalgia 30:1170–1178
Farkkila M, Diener HC, Geraud G, Lainez M, Schoenen J, Harner N, Pilgrim A, Reuter U, Group CM-s (2012) Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol 11:405–413
Diener HC, Charles A, Goadsby PJ, Holle D (2015) New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol 14:1010–1022
Sang CN, Ramadan NM, Wallihan RG, Chappell AS, Freitag FG, Smith TR, Silberstein SD, Johnson KW, Phebus LA, Bleakman D, Ornstein PL, Arnold B, Tepper SJ, Vandenhende F (2004) LY293558, a novel AMPA/GluR5 antagonist, is efficacious and well-tolerated in acute migraine. Cephalalgia 24:596–602
Waung MW, Akerman S, Wakefield M, Keywood C, Goadsby PJ (2016) Metabotropic glutamate receptor 5: a target for migraine therapy. Ann Clin Transl Neurol 3:560–571
Gomez-Mancilla B, Brand R, Jurgens TP, Gobel H, Sommer C, Straube A, Evers S, Sommer M, Campos V, Kalkman HO, Hariry S, Pezous N, Johns D, Diener HC, Group BGGS (2014) Randomized, multicenter trial to assess the efficacy, safety and tolerability of a single dose of a novel AMPA receptor antagonist BGG492 for the treatment of acute migraine attacks. Cephalalgia 34:103–113
Afridi SK, Giffin NJ, Kaube H, Goadsby PJ (2013) A randomized controlled trial of intranasal ketamine in migraine with prolonged aura. Neurology 80:642–647
Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF (2007) Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 68:343–349
Dodick DW, Goadsby PJ, Silberstein SD, Lipton RB, Olesen J, Ashina M, Wilks K, Kudrow D, Kroll R, Kohrman B, Bargar R, Hirman J, Smith J, Investigators ALDs (2014) Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 13:1100–1107
Dodick DW, Goadsby PJ, Spierings EL, Scherer JC, Sweeney SP, Grayzel DS (2014) Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol 13:885–892
Oakes TZQ, Ferguson M, SklJarevski V, Martinez JM, Johnson KW et al (2016) Efficacy and safety of LY2951742 in a randomized double-blind, placebo-controlled, dose-ranging study in patients with migraine. Headache 46(Suppl 1):68
Bigal ME, Dodick DW, Rapoport AM, Silberstein SD, Ma Y, Yang R, Loupe PS, Burstein R, Newman LC, Lipton RB (2015) Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 14:1081–1090
Sun H, Dodick DW, Silberstein S, Goadsby PJ, Reuter U, Ashina M, Saper J, Cady R, Chon Y, Dietrich J, Lenz R (2016) Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 15:382–390
Bigal ME, Edvinsson L, Rapoport AM, Lipton RB, Spierings EL, Diener HC, Burstein R, Loupe PS, Ma Y, Yang R, Silberstein SD (2015) Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 14:1091–1100
Smith J, Dodick D, Goadsby PJ, Silberstein SD, Lipton RB, Chakava G, O’Brien T, Hill R, Krause RA, Bonner J, Koltun W, Hirman J (2016) Randomized, double-blind, placebo-controlled trial of ALD403, an anti-CGRP antibody in the prevention of chronic migraine. Headache 56:1391
Goldstein DJ, Offen WW, Klein EG, Phebus LA, Hipskind P, Johnson KW, Ryan RE Jr (2001) Lanepitant, an NK-1 antagonist, in migraine prevention. Cephalalgia 21:102–106
Chabi A, Zhang Y, Jackson S, Cady R, Lines C, Herring WJ, Connor KM, Michelson D (2015) Randomized controlled trial of the orexin receptor antagonist filorexant for migraine prophylaxis. Cephalalgia 35:379–388
Lipton RBGP, Cady RK, Aurora SK, Grosberg BM, Freitag FG et al (2009) PRISM study: occipital nerve stimulation for treatment refractory migraine. Cephalalgia 29(Suppl 1):30
Saper JR, Dodick DW, Silberstein SD, McCarville S, Sun M, Goadsby PJ, Investigators O (2011) Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia 31:271–285
Silberstein SD, Dodick DW, Saper J, Huh B, Slavin KV, Sharan A, Reed K, Narouze S, Mogilner A, Goldstein J, Trentman T, Vaisman J, Ordia J, Weber P, Deer T, Levy R, Diaz RL, Washburn SN, Mekhail N (2012) Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia 32:1165–1179
Dodick DW, Silberstein SD, Reed KL, Deer TR, Slavin KV, Huh B, Sharan AD, Narouze S, Mogilner AY, Trentman TL, Ordia J, Vaisman J, Goldstein J, Mekhail N (2015) Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: long-term results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia 35:344–358
May A, Goadsby PJ (1999) The trigeminovascular system in humans: pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab 19:115–127
Puledda F, Goadsby PJ (2016) Current approaches to neuromodulation in primary headaches: focus on vagal nerve and sphenopalatine ganglion stimulation. Curr Pain Headache Rep 20:47
Maizels M, Geiger AM (1999) Intranasal lidocaine for migraine: a randomized trial and open-label follow-up. Headache 39:543–551
Cady R, Saper J, Dexter K, Manley HR (2015) A double-blind, placebo-controlled study of repetitive transnasal sphenopalatine ganglion blockade with tx360((R)) as acute treatment for chronic migraine. Headache 55:101–116
Tepper SJ, Rezai A, Narouze S, Steiner C, Mohajer P, Ansarinia M (2009) Acute treatment of intractable migraine with sphenopalatine ganglion electrical stimulation. Headache 49:983–989
Cady RK, Saper J, Dexter K, Cady RJ, Manley HR (2015) Long-term efficacy of a double-blind, placebo-controlled, randomized study for repetitive sphenopalatine blockade with bupivacaine vs. saline with the Tx360 device for treatment of chronic migraine. Headache 55:529–542
Schoenen J, Vandersmissen B, Jeangette S, Herroelen L, Vandenheede M, Gerard P, Magis D (2013) Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology 80:697–704
Magis D, Sava S, d’Elia TS, Baschi R, Schoenen J (2013) Safety and patients’ satisfaction of transcutaneous supraorbital neurostimulation (tSNS) with the Cefaly(R) device in headache treatment: a survey of 2,313 headache sufferers in the general population. J Headache Pain 14:95
Barbanti P, Grazzi L, Egeo G, Padovan AM, Liebler E, Bussone G (2015) Non-invasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: an open-label study. J Headache Pain 16:61
Goadsby PJ, Grosberg BM, Mauskop A, Cady R (2014) Effect of non-invasive vagus nerve stimulation on acute migraine: an open label pilot study. Cephalalgia 34:986–993
Moscato D, Moscato FR, Liebler EJ (2014) Efficacy of noninvasive vagus nerve stimulation (nVNS) in the treatment of acute migraine attacks. Headache 44:1418
Silberstein SD, Calhoun AH, Lipton RB, Grosberg BM, Cady RK, Dorlas S, Simmons KA, Mullin C, Liebler EJ, Goadsby PJ, Saper JR, Group ES (2016) Chronic migraine headache prevention with noninvasive vagus nerve stimulation: The EVENT study. Neurology 87:529–538
Grazzi L, Egeo G, Calhoun AH, McClure CK, Liebler E, Barbanti P (2016) Non-invasive Vagus Nerve Stimulation (nVNS) as mini-prophylaxis for menstrual/menstrually related migraine: an open-label study. J Headache Pain 17:91
Lipton RB, Pearlman SH (2010) Transcranial magnetic simulation in the treatment of migraine. Neurotherapeutics 7:204–212
Bhola R, Kinsella E, Giffin N, Lipscombe S, Ahmed F, Weatherall M, Goadsby PJ (2015) Single-pulse transcranial magnetic stimulation (sTMS) for the acute treatment of migraine: evaluation of outcome data for the UK post market pilot program. J Headache Pain 16:535
Antal A, Kriener N, Lang N, Boros K, Paulus W (2011) Cathodal transcranial direct current stimulation of the visual cortex in the prophylactic treatment of migraine. Cephalalgia 31:820–828
Vigano A, D’Elia TS, Sava SL, Auve M, De Pasqua V, Colosimo A, Di Piero V, Schoenen J, Magis D (2013) Transcranial direct current stimulation (tDCS) of the visual cortex: a proof-of-concept study based on interictal electrophysiological abnormalities in migraine. J Headache Pain 14:23
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
PJG reports grants and personal fees from Allergan, Amgen, and Eli-Lilly and Company; and personal fees from Akita Biomedical, Alder Biopharmaceuticals, Autonomic Technologies Inc, Avanir Pharma, Cipla Ltd, Colucid Pharmaceuticals, Ltd, Dr Reddy's Laboratories, eNeura, Electrocore LLC, Novartis, Pfizer Inc, Promius Pharma, Quest Diagnostics, Scion, Teva Pharmaceuticals, Trigemina Inc., Scion; and personal fees from MedicoLegal work, Journal Watch, Up-to-Date, Oxford University Press; and in addition, Dr. Goadsby has a patent Magnetic stimulation for headache pending assigned to eNeura.
Ethical standard
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Puledda, F., Messina, R. & Goadsby, P.J. An update on migraine: current understanding and future directions. J Neurol 264, 2031–2039 (2017). https://doi.org/10.1007/s00415-017-8434-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8434-y