Abstract
Background
Migraine is one of the most severe primary headache disorders. The nature of the headache and the associated symptoms during the attack suggest underlying functional alterations in the brain. In this study, we examined amplitude, the resting state fMRI fluctuation in migraineurs with and without aura (MWA, MWoA respectively) and healthy controls.
Methods
Resting state functional MRI images and T1 high-resolution images were acquired from all participants. For data analysis we compared the groups (MWA-Control, MWA-MWoA, MWoA-Control). The resting state networks were identified by MELODIC. The mean time courses of the networks were identified for each participant for all networks. The time-courses were decomposed into five frequency bands by discrete wavelet decomposition. The amplitude of the frequency-specific activity was compared between groups. Furthermore, the preprocessed resting state images were decomposed by wavelet analysis into five specific frequency bands voxel-wise. The voxel-wise amplitudes were compared between groups by non-parametric permutation test.
Results
In the MWA-Control comparison the discrete wavelet decomposition found alterations in the lateral visual network. Higher activity was measured in the MWA group in the highest frequency band (0.16–0.08 Hz).
In case of the MWA-MWoA comparison all networks showed higher activity in the 0.08–0.04 Hz frequency range in MWA, and the lateral visual network in in higher frequencies.
In MWoA-Control comparison only the default mode network revealed decreased activity in MWoA group in the 0.08–0.04 Hz band.
The voxel-wise frequency specific analysis of the amplitudes found higher amplitudes in MWA as compared to MWoA in the in fronto-parietal regions, anterior cingulate cortex and cerebellum.
Discussion
The amplitude of the resting state fMRI activity fluctuation is higher in MWA than in MWoA. These results are in concordance with former studies, which found cortical hyperexcitability in MWA.
Similar content being viewed by others
Background
Migraine is a common disabling disease, affecting about 10% of the population [1]. While not life threatening, it has a significant effect on the quality of life. Two major forms of the disease present with or without transient, focal neurological symptoms, called aura [2]. While the pathomechanism of migraine is not entirely understood, the two subgroups of the disease thought to have different background [3, 4]. It was suggested that cortical spreading depression, a slow depolarization wave traveling anteriorly in the brain is responsible for the aura symptoms and occur exclusively in migraine with aura (MWA) [5, 6]. Cortical hyperexcitability was mentioned as the potential trigger of migraine [7–12] and this hyperexcitability is also present in MWA more robustly [13].
Information about the resting brain activity can be noninvasively gathered by BOLD fMRI. Traditionally fMRI studies compare signal in various phases of a task, but acquiring BOLD signal in rest, allows the studying of resting brain activity fluctuations. Interestingly, remote areas show synchronous activity, which renders resting state activity into functional networks [14, 15].
Several studies investigated the activity of the resting state functional networks in migraine and found various alterations of networks that are implicated in pain processing [16–19]. Furthermore, a few studies investigated patients with MWA and migraine without aura (MWoA) and reported various aspects of altered connectivity in the subgroups of the disease [20–25]. Remarkably some of the studies found increased functional connectivity, in comparison to decreased functional connectivity in migraine.
Most of the resting state fMRI studies investigated the connectivity between various regions and therefore build on the coherent activity in spatially distributed networks. The variation in the frequency and the amplitude of the resting state BOLD signal is usually neglected. The BOLD resting state fluctuation is a low frequency fluctuation [14, 15]. To filter out the non-neural noise from the raw BOLD time courses most of the studies apply filters [16, 26, 27]. However, neural signal could be detected also in the higher frequencies [28]. Furthermore, a few recent studies started to analyse the amplitude of the low frequency fluctuation of the resting state signals [29, 30] offering a unique insight into the resting brain activity in various diseases but not in migraine.
Based on the above described premises, the increased cortical excitability in migraine might be related to the frequency specific alteration of amplitude of the resting BOLD activity. MWA and MWoA being different in respect to cortical excitability might also appear in the differential frequency spectrum of the resting brain activity in the two subgroups of the disease.
In the current study we investigated the resting state BOLD fluctuations in migraine with special focus on the amplitude and the frequency of the activity.
Methods
Participants
Fifty-three patients with migraine were recruited into this study from the Headache Outpatients Clinic, at the Department of Neurology, University of Szeged. All of the patients were diagnosed with episodic migraine and were scanned during the interictal phase, having had the the scanning at least one week to the last attack. The diagnosis set up by the International Classification of Headache Disorders [2]. Eighteen patients suffered from MWA (17 visual aura, 1 sensory aura), the other 35 patients never experienced aura. Patients had no other neurological or psychiatric disorders.
Thirty-two healthy volunteers were recruited. None of the controls had any records of any neurological or psychiatric disorders. For demographic data of patients and controls, see Table 1.
The study was approved by the ethics committee of the University of Szeged and all study participants gave written informed consent in accordance with the Declaration of Helsinki (authority number: 56/2011).
Image acquisition
The MR imaging was performed on a 1.5 T GE Signa Excite HDxt MRI Scanner (Milwaukee, WI, USA). The head motion was restricted with foam padding around the head and the noise of the scanner was attenuated with earplugs. For every participants high-resolution T1 weighted images (3D IR-FSPGR: TR/TE/TI: 10.3/4.2/450 ms, flip angle: 15°, ASSET: 2, FOV: 25*25 cm, matrix: 256*256, slice thickness: 1 mm,) and a resting state fMRI protocol with echo-planar imaging technique (TE: 40 ms, TR: 3000 ms, matrix: 64*64 cm, FOV: 30*30 cm, slice thickness: 6 mm, flip angle: 90°, NEX: 1, ASSET: 2,0 Ph, Phases per Loc: 128, volumes: 200) were acquired. Subjects were asked to be awake during the acquisition eyes closed.
Data processing
All image processing were performed by FMRIB’s Software Library (http://www.fmrib.ox.uk/fsl, Oxford, UK) toolkits.
Preprocessing
The pre-processing was carried out with FEAT (FMRI Expert Analysis Tool). The first two images were removed from all resting state datasets. The non-brain parts were removed using Brain Extraction (BET) [31]. Motion correction (MCFLIRT) [32] were applied in all images and spatially smoothed with Gaussian kernel of 6 mm FWHM. The high-pass filter cut-off was applied to all functional images (with sigma 100 s).
All pre-processed resting state images were registered to standard space (MNI152 T1 image; 2 mm slice thickness) and to their high-resolution T1 images with linear (FLIRT) and then with non-linear registration (FNIRT) [32]. All images were resampled to 4 mm isovoxels.
Independent component analysis
Resting state networks were identified by group independent component analysis as implemented in the MELODIC toolbox, part of the FSL software library (FMRIB Software Library, htttp://www.fmrib.ox.uk/fsl) [15]. The individual preprocessed and standard space registered functional images were concatenated after voxel-wise de-meaning and variance normalization. The 4D data were decomposed into a set of matrices that characterize the underlying processes in the spatial and temporal domains in such a way that spatial matrices are maximally non-Gaussian. The number of components was estimated by applying the Laplace approximation to the Bayesian evidence of a probabilistic principal component model [33]. Spatial maps were processed by using an alternative hypothesis test based on the fitting a Gaussian/Gamma mixture model to the distribution of voxel intensities within spatial maps and a posterior probability threshold of p > 0.5. The resulting subject-wise time courses are to be understood as the temporal characteristics of the network activity across the entire spatial map for each individual subject.
Amplitude of resting state activity
The frequency specific modulation of the amplitude of resting activity was analysed by discrete wavelet decomposition (Wavelet Toolbox of the Matlab software package; MathWorks Inc). The networks’ time courses were divided into five consecutive frequency bands (band1: 0.16–0.08 Hz, band2: 0.08–0.04 Hz, band3: 0.04–0.02 Hz; band4: 0.02–0.01 Hz; band5: 0.01–0 Hz) using discrete wavelet decomposition. Discrete wavelet decomposition is an implementation of the wavelet transform using a set of predefined wavelet scales and translations and decomposes the signal into mutually orthogonal set of wavelets. Wavelets are brief waves, they are finitely extended and their oscillations decay to zero rapidly, satisfying the admissibility condition:
∫Ψ(t)dt = 0.
By dilating and translating a “mother” wavelet (Ψ) and a “father” wavelet (Φ) (∫Φ(t)dt = 1) a wavelet family can be obtained:
where j is index of the scale S j = 2 j and k indexes the K = n/2 j location in time. The Daubechies wavelet was used as mother wavelet. The analysis by using a halfband filtering, decomposes the data over a hierarchy of scales (S j ). At each scale the data is split into two orthogonal components: details (d jk ) containing the high frequency information and approximations (a jk ) containing the low frequency information [34]. By five levels of decomposition the following frequency bands were derived 0–0.16 Hz: 0.16–0.08 Hz, 0.08–0.04 Hz, 0.04–0.02 Hz, 0.02–0.01 Hz and 0.01–0 Hz.
To measure the amplitude of the resting activity in the various frequency bands an envelope was fitted to the absolute values of each frequency bands: The minimums of the following function was identified:
and a linear interpolation of these points were used to create an envelope. The envelopes were averaged over time to describe the mean activity in the given frequency band.
The analysis outlined above was applied to (i) the mean activity of the ICA identified resting state networks and (ii) to the voxel-wise preprocessed fMRI data.
-
i.
To compare the amplitude of the mean network timecourses across groups in the individual frequency bands General Linear Model (GLM) based test was used. Gender and age were included in our analysis as covariate.
-
ii.
For voxel-wise comparison of the amplitude of the resting activity across groups a nonparametric permutation test was performed (5000 permutations) for each frequency bands. The design encoded for group membership, age and gender were used as nuisance variables. For statistical analysis threshold-free cluster enhancement were used (TFCE) and corrected for multiple comparisons (across space) within the permutation framework. Age and gender also included in this analysis as nuisance variable.
Additionally, we performed the voxel wise comparison of the resting state networks’ for the non-filtered data also.
Results
Demographical data
There were no significant difference between the groups’ age or gender distribution. There were no significant differences between the two migraineus group in disease duration.
Amplitude of the activity of the resting state functional networks
A MELODIC analysis found 33 components in the healthy controls. The artefact components were removed from our analysis based on previous studies [14, 15] and five networks were included for analysis: default mode network, right attention network, left attention network, medial visual network, lateral visual network.
MWA vs. healthy controls
There were no significant differences between the amplitude of the resting activity in MWA vs. healthy controls. While not significant, in the highest frequencies (0.16–0.08 Hz) the amplitude of the resting activity was slightly higher in the left attention network (p = 0.07) and in the right attention network (p = 0.059) in MWA as compared to healthy controls.
MWA vs. MWoA
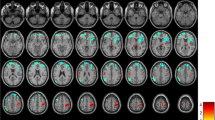
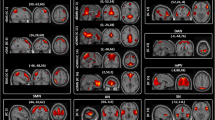
The amplitude of the activity was higher (p < 0.05) in all examined networks in the 0.08–0.04 Hz frequency range and in the lateral visual network also in the 0.16–0.08 Hz frequency band (Fig. 1 ) in the MWA group. There were no other significant results in any other frequency bands or in case of the non-filtered data.
All of the investigated network ((a) medial visual, (b) lateral visual, (c) default mode, (d) right attention, (e) right attention) showed higher amplitude in the 0.08–0.04 Hz frequency range in MWA compared to MWoA. The images on the left depicting the resting state networks are thresholded at p < 0.5 and overlaid on the standard MNI_152 brain. The boxplots depicting the amplitude of the activity of the networks in the 0.08-0.04 Hz frequency range. The central mark is the median, the edges of the box are the 25th and 75th percentiles, the whiskers extend to the most extreme datapoints
MWoA vs. Healthy controls
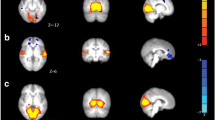
The amplitude of resting activity in the 0.08–0.04 Hz range was lower in patients without aura in the default mode network (p < 0.05) (Fig. 2 ). There were no other significant results in any other frequency bands or in case of the non-filtered data.
The default mode network showed higher amplitude in the 0.04–0.02 Hz in healthy group compared to MWoA. The images on the left depicting the resting state networks are thresholded at p < 0.5 and overlaid on the standard MNI_152 brain. The boxplots depicting the amplitude of the activity of the networks. The central mark is the median, the edges of the box are the 25th and 75th percentiles, the whiskers extend to the most extreme datapoints
Voxel-wise comparison of the amplitude of resting activity
The voxel-wise comparison of the amplitude of resting activity showed higher amplitudes (p < 0.05, corrected for multiple comparisons) in the left parietal lobe in all frequency ranges in MWA compared to MWoA. In addition, in the 0.08–0.04 Hz frequency band the amplitudes were higher in the bilateral cerebellum, in the left occipital pole and occipito-temporal junction, and a smaller cluster was found in the right inferior parietal lobule. In the 0.04–0.02 Hz frequency band the amplitudes were higher in MWA compared to MWoA in the left inferior parietal lobule, bilateral cerebellum and in the anterior cingulate gyrus. In the 0.02–0.01 Hz range next to the inferior parietal lobule, occipital pole and cingulate gyral differences amplitudes were found higher in the bilateral frontal lobe around the superior frontal sulcus and precentral gyrus (Table 2 and Fig. 3).
The frequency specific voxel wise comparison of the amplitude of the resting activity showed higher amplitudes in MWA as compared to MWoA in all freqeuncy ranges. The voxel wise changes in each frequency range. The images are thresholded at p < 0.05 corrected for multiple comparisons and overlaid on the standard MNI_152 brain. We marked the sidedness with 'R' as the right side. The X-Y-Z letters indicates the axes of the pictures above them
There were no differences between the MWoA and healthy group or between the MWA and control group.
Discussion
This study identifies novel frequency specific alterations of resting fluctuation of fMRI measured brain activity. Our analysis showed that amplitude of resting state BOLD fluctuation is higher in MWA in the cingulate cortex, superior parietal lobule, cerebellum and bilateral frontal regions compared to MWoA. Furthermore, amplitude of the activity of the resting state networks in the 0.08–0.04 Hz frequency range was higher in MWA than in MWoA in all examined resting state networks. In MWoA the amplitude of the activity fluctuation of the default mode network was lower than in healthy controls.
Several lines of evidence supports the hyperexcitability of the cortex in migraine [7–12]. The amplitude of visual evoked potentials (VEP) were showed higher in migraineurs [11, 34–36]. However, recent reports showed that the VEP measured hyperexcitability predominantly true for migraine with aura [37, 38].
The threshold of transcranial magnetic stimulation evoked phosphenes are also lower in migraineurs and the prevalence of phosphenes are higher [9]. Interestingly, recent metaanalysis pointed out that, similar to the VEP results, this kind of TMS measured hyperexcitability is not evident for MWoA and only true for patients experiencing aura [39].
Similarly, neuroimaging PET and fMRI studies found higher activation for visual stimuli in migraineurs [40–42], but recent reports showed that patients with aura show higher BOLD response to photic stimuli in contrasts to those migraineurs not experiencing aura symptoms [43, 44].
A possible explanation of such interictal hyperexcitability in migraine might be the higher interictal glutamate/glutamine ratio [45] or the lower gamma-amino butyric acid level [46] in the occipital cortex as shown by MR spectroscopy studies. A recent investigation also showed larger reduction of N-acetyl-aspartate levels in a functional MR spectroscopy study in MWA but not in MWoA [47] indicating a less effective mitochondrial functioning in MWA.
These above mentioned studies, indicating cortical hyperexcitability in MWA are in line with our current results. The amplitude of the resting BOLD fluctuation was higher in MWA patients in regions highly related to pain processing and in certain frequencies, the amplitude of the activity of the resting state networks was also higher. While there is no direct evidence that various electrophysiological measures of cortical excitability are represented as a variation in the amplitude or the frequency of the resting BOLD fluctuations, one might speculate that they are strongly related and both depict important features of cortical function.
Several researches investigated the brain resting fMRI activity in migraine with various approaches [17, 19–21, 23, 43, 48, 49]. These approaches are not giving direct information on the cortical hyperexcitability, but a measure of functional interaction. A few studies investigated the amplitude of the resting state BOLD fluctuation in chronic pain conditions [50, 51] and found higher amplitude of resting activity in chronic back pain, irritable bowel syndrome, knee osteoarthritis and complex regional pain syndrome. Only single study investigated the amplitude of the low frequency fluctuations in 24 migraineurs, without grouping the patients based on the aura symptoms [52]. Concentrating on only the low frequency component of the BOLD signal (0.01–0.08 Hz) they found lower amplitude in migraineus in the cerebellum, bilateral frontal and occipital regions and increased amplitudes in the brainstem and insula. Also our results sustain the well known importance to investigate patients with and without aura symptoms in separate groups. Only the patients experiencing aura symptoms were the ones who had higher amplitude BOLD fluctuation in our study. This was true for the amplitude of the resting activity and also for the amplitude of the activity of the resting state networks.
The importance of the various frequencies of BOLD fluctuations is not yet known but recently a few studies started to explore this feature [53], especially in pain conditions [30, 54]. It was proposed that functional connectivity of various brain regions are represented in different dominant frequency bands [55]. Another option might be that migraine was proposed to be a neurovascular disease, and the altered neurovascular coupling may affect the frequency of the resting BOLD fluctuations [56] by acting as a filter. Furthermore, the group difference in the resting state network activity might well be the result of improved signal to noise ratio by filtering out the low and high frequency artifact. Since most of the slow frequency fluctuation in our analysis with a relatively long TR were shown to be neural origin [57], this hypothesis seems rather unlikely.
Another important aspect of our results is the spatial localisation of the amplitude differences. In the various frequency ranges extensive fronto-parietal and cerebellar areas showed higher activity in MWA patients. Most of these regions were associated with various aspects of the pain processing. Primary and secondary somatosensory cortices, insula, cingulate cortex and prefrontal cortex and subcortical regions, such as the periaqueductal gray matter, hypothalamus, amygdala, hippocampus and cerebellum are commonly activating in response to painful stimuli and referred to as the pain matrix [58]. These key regions were reported to subserve multiple functions in pain processing, including sensory discrimination, motivation-affect, motor, attention and also arousal and response selection functions [59].
Cingulate cortex in particular was shown to be one of the key region in experiencing pain [60–62] and it is also part of the descending pain modulatory system [63]. Anterior cingulate cortex is connected to the periaqueductal grey matter, a key region in migraine pathomechanism [64]. In a recent work we also showed that chronic inflammatory pain related sensitization is mediated by the altered activation and connectivity of the cingulate cortex in rats [65].
It was also shown that cerebellum actively contributes to various aspects of pain processing [66]. Subclinical vestibulo-cerebellar dysfunctions were found in migraine with and without aura [67, 68]. The macro and microstructure of is cerebellum is altered in migraine [69, 70]. The interictal perfusion of the cerebellum was not found affected by the disease, independently of aura symptoms [71]. In migraine patients Wang and colleagues found altered amplitude of low frequency fluctuation [52], higher and lower amplitudes were found in various parts of the cerebellum. Importantly, in their study no information was given if the patients experienced aura.
The fronto-parietal regions showing increased amplitude of resting activity fluctuation in our study are presumably the same, which are considered to be associated with executive functions. The subclinical executive dysfunction is a known feature of the disease [49, 72, 73]. A recent investigation also described the altered expression of the fronto-parietal resting state network in MWoA patients [49] in highly similar regions what we have found in our analysis.
Conclusion
In summary, the the regions we found to have increased amplitude of activity fluctuation in MWA patients are those known to be affected by the disease and responsible for the functional alterations also.
References
Lipton RB, Bigal ME, Steiner TJ, Silberstein SD, Olesen J (2004) Classification of primary headaches. Neurology 63:427–435
Headache Classification Committee of the International Headache (2013) The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 33:629–808
Manzoni GC, Torelli P (2008) Migraine with and without aura: a single entity? Neurol sci 29(Suppl 1):S40–43
Ranson R, Igarashi H, MacGregor EA, Wilkinson M (1991) The similarities and differences of migraine with aura and migraine without aura: a preliminary study. Cephalalgia 11:189–192
Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, Kwong KK, Cutrer FM, Rosen BR, Tootell RB, Sorensen AG, Moskowitz MA (2001) Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A 98:4687–4692
Petrusic I, Zidverc-Trajkovic J (2014) Cortical spreading depression: origins and paths as inferred from the sequence of events during migraine aura. Funct Neurol 29:207–212
Antal A, Temme J, Nitsche MA, Varga ET, Lang N, Paulus W (2005) Altered motion perception in migraineurs: evidence for interictal cortical hyperexcitability. Cephalalgia 25:788–794
Pierelli F, Iacovelli E, Bracaglia M, Serrao M, Coppola G (2013) Abnormal sensorimotor plasticity in migraine without aura patients. Pain 154:1738–1742
Aurora SK, Ahmad BK, Welch KM, Bhardhwaj P, Ramadan NM (1998) Transcranial magnetic stimulation confirms hyperexcitability of occipital cortex in migraine. Neurology 50:1111–1114
Chadaide Z, Arlt S, Antal A, Nitsche MA, Lang N, Paulus W (2007) Transcranial direct current stimulation reveals inhibitory deficiency in migraine. Cephalalgia 27:833–839
Gawel M, Connolly JF, Rose FC (1983) Migraine patients exhibit abnormalities in the visual evoked potential. Headache 23:49–52
Afra J, Proietti Cecchini A, Sandor PS, Schoenen J (2000) Comparison of visual and auditory evoked cortical potentials in migraine patients between attacks. Clin Neurophysiol 111:1124–1129
Brigo F, Storti M, Nardone R, Fiaschi A, Bongiovanni LG, Tezzon F, Manganotti P (2012) Transcranial magnetic stimulation of visual cortex in migraine patients: a systematic review with meta-analysis. J Headache Pain 13:339–349
Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M (2007) Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A 104:13170–13175
Beckmann CF, DeLuca M, Devlin JT, Smith SM (2005) Investigations into resting-state connectivity using independent component analysis. Philos T Roy Soc B 360:1001–1013
Xue T, Yuan K, Zhao L, Yu D, Zhao L, Dong T, Cheng P, von Deneen KM, Qin W, Tian J (2012) Intrinsic brain network abnormalities in migraines without aura revealed in resting-state fMRI. PloS One 7(12):e52927. doi:10.1371/journal.pone.0052927
Tessitore A, Russo A, Giordano A, Conte F, Corbo D, De Stefano M, Cirillo S, Cirillo M, Esposito F, Tedeschi G (2013) Disrupted default mode network connectivity in migraine without aura. J Headache Pain 14:89. doi:10.1186/1129-2377-14-89
Yuan K, Zhao L, Cheng P, Yu DH, Zhao LM, Dong T, Xing LH, Bi YZ, Yang XJ, von Deneen KM, Liang FR, Gong QY, Qin W, Tian J (2013) Altered structure and resting-state functional connectivity of the basal ganglia in migraine patients without aura. J Pain 14:836–844
Mainero C, Boshyan J, Hadjikhani N (2011) Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol 70:838–845
Tedeschi G, Russo A, Conte F, Corbo D, Caiazzo G, Giordano A, Conforti R, Esposito F, Tessitore A (2015) Increased interictal visual network connectivity in patients with migraine with aura. Cephalalgia: an international journal of headache. doi:10.1177/0333102415584360
Hadjikhani N, Ward N, Boshyan J, Napadow V, Maeda Y, Truini A, Caramia F, Tinelli E, Mainero C (2013) The missing link: enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia 33:1264–1268
Niddam DM, Lai KL, Fuh JL, Chuang CY, Chen WT, Wang SJ (2015) Reduced functional connectivity between salience and visual networks in migraine with aura. Cephalalgia: an international journal of headache. doi:10.1177/0333102415583144
Tessitore A, Russo A, Conte F, Giordano A, De Stefano M, Lavorgna L, Corbo D, Caiazzo G, Esposito F, Tedeschi G (2015) Abnormal connectivity within executive resting-state network in migraine with aura. Headache 55:794–805
Hougaard A, Amin FM, Magon S, Sprenger T, Rostrup E, Ashina M (2015) No abnormalities of intrinsic brain connectivity in the interictal phase of migraine with aura. Eur j neurol 22:702–e746
Hougaard A, Amin FM, Hoffmann MB, Larsson HB, Magon S, Sprenger T, Ashina M (2015) Structural gray matter abnormalities in migraine relate to headache lateralization, but not aura. Cephalalgia 35:3–9
Schwedt TJ, Schlaggar BL, Mar S, Nolan T, Coalson RS, Nardos B, Benzinger T, Larson-Prior LJ (2013) Atypical resting-state functional connectivity of affective pain regions in chronic migraine. Headache 53:737–751
Jin CW, Yuan K, Zhao LM, Zhao L, Yu DH, von Deneen KM, Zhang M, Qin W, Sun WX, Tian J (2013) Structural and functional abnormalities in migraine patients without aura. NMR Biomed 26:58–64
Boyacioglu R, Beckmann CF, Barth M (2013) An investigation of RSN frequency spectra using ultra-fast generalized inverse imaging. Front Hum Neurosci 7:156. doi:10.3389/fnhum.2013.00156.eCollection
Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF (2008) An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods 172:137–141
Kim JY, Kim SH, Seo J, Kim SH, Han SW, Nam EJ, Kim SK, Lee HJ, Lee SJ, Kim YT, Chang Y (2013) Increased power spectral density in resting-state pain-related brain networks in fibromyalgia. Pain 154:1792–1797
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841
Beckmann CF, Smith SM (2005) Tensorial extensions of independent component analysis for multisubject FMRI analysis. Neuroimage 25:294–311
Oelkers R, Grosser K, Lang E, Geisslinger G, Kobal G, Brune K, Lotsch J (1999) Visual evoked potentials in migraine patients: alterations depend on pattern spatial frequency. Brain 122(Pt 6):1147–1155
Connolly JF, Gawel M, Rose FC (1982) Migraine patients exhibit abnormalities in the visual evoked potential. J Neurol Neurosurg Psychiatry 45:464–467
Diener HC, Scholz E, Dichgans J, Gerber WD, Jack A, Bille A, Niederberger U (1989) Central effects of drugs used in migraine prophylaxis evaluated by visual evoked potentials. Ann Neurol 25:125–130
Coppola G, Bracaglia M, Di Lenola D, Di Lorenzo C, Serrao M, Parisi V, Di Renzo A, Martelli F, Fadda A, Schoenen J, Pierelli F (2015) Visual evoked potentials in subgroups of migraine with aura patients. J Headache Pain 16:92
Sand T, Zhitniy N, White LR, Stovner LJ (2008) Visual evoked potential latency, amplitude and habituation in migraine: a longitudinal study. Clin Neurophysiol 119:1020–1027
Brigo F, Storti M, Tezzon F, Manganotti P, Nardone R (2013) Primary visual cortex excitability in migraine: a systematic review with meta-analysis. Neurol Sci 34:819–830
Vincent M, Pedra E, Mourao-Miranda J, Bramati IE, Henrique AR, Moll J (2003) Enhanced interictal responsiveness of the migraineous visual cortex to incongruent bar stimulation: a functional MRI visual activation study. Cephalalgia 23:860–868
Noseda R, Burstein R (2011) Advances in understanding the mechanisms of migraine-type photophobia. Curr Opin Neurol 24:197–202
Boulloche N, Denuelle M, Payoux P, Fabre N, Trotter Y, Geraud G (2010) Photophobia in migraine: an interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatry 81:978–984
Datta R, Aguirre GK, Hu S, Detre JA, Cucchiara B (2013) Interictal cortical hyperresponsiveness in migraine is directly related to the presence of aura. Cephalalgia 33:365–374
Cucchiara B, Datta R, Aguirre GK, Idoko KE, Detre J (2015) Measurement of visual sensitivity in migraine: validation of two scales and correlation with visual cortex activation. Cephalalgia 35:585–592
Gonzalez de la Aleja J, Ramos A, Mato-Abad V, Martinez-Salio A, Hernandez-Tamames JA, Molina JA, Hernandez-Gallego J, Alvarez-Linera J (2013) Higher glutamate to glutamine ratios in occipital regions in women with migraine during the interictal state. Headache 53:365–375
Bridge H, Stagg CJ, Near J, Lau CI, Zisner A, Cader MZ (2015) Altered neurochemical coupling in the occipital cortex in migraine with visual aura. Cephalalgia 35:1025–1030
Sarchielli P, Tarducci R, Presciutti O, Gobbi G, Pelliccioli GP, Stipa G, Alberti A, Capocchi G (2005) Functional 1H-MRS findings in migraine patients with and without aura assessed interictally. Neuroimage 24:1025–1031
Hougaard A, Amin FM, Hoffmann MB, Rostrup E, Larsson HB, Asghar MS, Larsen VA, Olesen J, Ashina M (2014) Interhemispheric differences of fMRI responses to visual stimuli in patients with side-fixed migraine aura. Hum Brain Mapp 35:2714–2723
Russo A, Tessitore A, Giordano A, Corbo D, Marcuccio L, De Stefano M, Salemi F, Conforti R, Esposito F, Tedeschi G (2012) Executive resting-state network connectivity in migraine without aura. Cephalalgia 32:1041–1048
Ma X, Li S, Tian J, Jiang G, Wen H, Wang T, Fang J, Zhan W, Xu Y (2015) Altered brain spontaneous activity and connectivity network in irritable bowel syndrome patients: a resting-state fMRI study. Clin Neurophysiol 126:1190–1197
Baliki MN, Mansour AR, Baria AT, Apkarian AV (2014) Functional reorganization of the default mode network across chronic pain conditions. PLoS One 9:e106133
Wang JJ, Chen X, Sah SK, Zeng C, Li YM, Li N, Liu MQ, Du SL (2016) Amplitude of low-frequency fluctuation (ALFF) and fractional ALFF in migraine patients: a resting-state functional MRI study. Clin Radiol 71:558–564
Gao L, Bai L, Zhang Y, Dai XJ, Netra R, Min Y, Zhou F, Niu C, Dun W, Gong H, Zhang M (2015) Frequency-dependent changes of local resting oscillations in sleep-deprived brain. PLoS One 10:e0120323
Otti A, Guendel H, Wohlschlager A, Zimmer C, Noll-Hussong M (2013) Frequency shifts in the anterior default mode network and the salience network in chronic pain disorder. BMC Psychiatry 13:84
Salvador R, Martinez A, Pomarol-Clotet E, Gomar J, Vila F, Sarro S, Capdevila A, Bullmore E (2008) A simple view of the brain through a frequency-specific functional connectivity measure. Neuroimage 39:279–289
Malinen S, Vartiainen N, Hlushchuk Y, Koskinen M, Ramkumar P, Forss N, Kalso E, Hari R (2010) Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proc Natl Acad Sci U S A 107:6493–6497
Boyacioglu R, Beckmann CF, Barth M (2013) An investigation of RSN frequency spectra using ultra-fast generalized inverse imaging. Front Hum Neurosci 7:156
Lee MC, Tracey I (2013) Imaging pain: a potent means for investigating pain mechanisms in patients. Br J Anaesth 111:64–72
Davis KD, Moayedi M (2013) Central mechanisms of pain revealed through functional and structural MRI. J Neuroimmune Pharmacol 8:518–534
Bliss TV, Collingridge GL, Kaang B-K, Zhuo M (2016) Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nature Reviews Neuroscience
Cohen S, Mao J (2014) Neuropathic Pain: Mechanisms & Clinical Implications + MP3. Br Med J 348:f7656
Treede R-D, Kenshalo DR, Gracely RH, Jones AK (1999) The cortical representation of pain. Pain 79:105–111
Denk F, McMahon SB, Tracey I (2014) Pain vulnerability: a neurobiological perspective. Nat Neurosci 17:192–200
Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R (2000) Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J Comp Neurol 422:556–578
Spisak T, Pozsgay Z, Aranyi C, David S, Kocsis P, Nyitrai G, Gajari D, Emri M, Czurko A, Kincses ZT, Central sensitization related changes of effective and functional connectivity in the rat inflammatory trigeminal pain model. Neuroscience (in press)
Moulton EA, Schmahmann JD, Becerra L, Borsook D (2010) The cerebellum and pain: passive integrator or active participator? Brain Res Rev 65:14–27
Sandor PS, Mascia A, Seidel L, de Pasqua V, Schoenen J (2001) Subclinical cerebellar impairment in the common types of migraine: a three-dimensional analysis of reaching movements. Ann Neurol 49:668–672
Harno H, Hirvonen T, Kaunisto MA, Aalto H, Levo H, Isotalo E, Kallela M, Kaprio J, Palotie A, Wessman M, Farkkila M (2003) Subclinical vestibulocerebellar dysfunction in migraine with and without aura. Neurology 61:1748–1752
Granziera C, Romascano D, Daducci A, Roche A, Vincent M, Krueger G, Hadjikhani N (2013) Migraineurs without aura show microstructural abnormalities in the cerebellum and frontal lobe. Cerebellum 12:812–818
Jin C, Yuan K, Zhao L, Zhao L, Yu D, von Deneen KM, Zhang M, Qin W, Sun W, Tian J (2013) Structural and functional abnormalities in migraine patients without aura. NMR Biomed 26:58–64
Arkink EB, Bleeker EJ, Schmitz N, Schoonman GG, Wu O, Ferrari MD, van Buchem MA, van Osch MJ, Kruit MC (2012) Cerebral perfusion changes in migraineurs: a voxelwise comparison of interictal dynamic susceptibility contrast MRI measurements. Cephalalgia 32:279–288
O’Bryant SE, Marcus DA, Rains JC, Penzien DB (2005) Neuropsychology of migraine: present status and future directions. Expert Rev Neurother 5:363–370
Camarda C, Monastero R, Pipia C, Recca D, Camarda R (2007) Interictal executive dysfunction in migraineurs without aura: relationship with duration and intensity of attacks. Cephalalgia 27:1094–1100
Acknowledgement
The authors declare that there are no conflicts of interest. The study was supported by the „Neuroscience Research Group of the Hungarian Academy of Sciences and University of Szeged”, the National Brain Research Program (Grant No. KTIA_13_NAP-A-II/20.) and an OTKA [PD 104715] grant. Dr. Szabó was supported by the Bolyai Scholarship Programme of the Hungarian Academy of Sciences.
Authors’ contributions
ÁP, JT, DSZ, BT and ZSTK participated in patients recruitment, diagnosis statement and clinical data collection. PF, ZSTK and NSZ participated in the manuscript writing. ET, PF, GCS and AK performed the analysis in the MRI and statistical data. LV and ZSTK supervised and directed the study. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Faragó, P., Tuka, B., Tóth, E. et al. Interictal brain activity differs in migraine with and without aura: resting state fMRI study. J Headache Pain 18, 8 (2017). https://doi.org/10.1186/s10194-016-0716-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-016-0716-8