Abstract
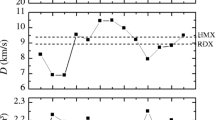
Density functional theory calculations were used to study energetic and stability properties for a series of pentaprismane (C10H10) derivatives with different substituent groups (NO2, NO, CN, N3, NH2, NHNO2, and ONO2). The results indicated that the N3 and CN groups greatly increase while the ONO2 group decreases the heats of formation. Moreover, the NO2, NHNO2, and ONO2 derivatives possess better detonation properties (detonation velocities = 8.92–9.72 km s−1 and detonation pressures = 38.37–45.24 GPa) than those of other derivatives due to high densities (1.97–2.08 g cm−3) and large heats of detonation (1189.22–1807.45 kJ mol−1). An analysis of the bond dissociation energies (BDE) revealed that all investigated compounds meet the qualification of energetic material (BDE > 84 kJ mol−1) even though the initiation decomposition steps are diverse (breaking of C–C bonds for NO2, N3, NH2, and CN derivatives, N–NO2 bond for NHNO2 derivative, O–NO2 bond for ONO2 derivative, and cage–NO bond for NO derivative). Considering both detonation performance and thermal stability, NO2 and NHNO2 derivatives are proposed to be potential candidates of high energy density materials.






Similar content being viewed by others
References
Thottempudi V, Gao H, Shreeve JM (2011) Trinitromethyl-substituted 5-nitro-or 3-azo-1,2,4-triazoles: synthesis, characterization, and energetic properties. J Am Chem Soc 133(16):6464–6471
Zhang J, Mitchell LA, Parrish DA, Shreeve JM (2015) Enforced layer-by-layer stacking of energetic salts towards high-performance insensitive energetic materials. J Am Chem Soc 137(33):10532–10535
Liu Y, Zhang L, Wang G, Wang L, Gong X (2012) High-pressure studies on azido-tetrazole chain–ring conversion in crystalline 2-azido-4,6-dichloro-1,3,5-triazine. Theor Chem Acc 131(8):1–12
Bian C, Zhang M, Li C, Zhou Z (2015) 3-Nitro-1-(2H-tetrazol-5-yl)-1H-1,2,4-triazol-5-amine (HANTT) and its energetic salts: highly thermally stable energetic materials with low sensitivity. J Mater Chem A 3(1):163–169
Gao H, Shreeve JM (2015) The many faces of FOX-7: a precursor to high-performance energetic materials. Angew Chem Inter Ed 54(21):6335–6338
Zhang X, Liu Y, Wang F, Gong X (2014) A theoretical study on the structure, intramolecular interactions, and detonation performance of hydrazinium dinitramide. Chem Asian J 9(1):229–236
Sikder A, Sikder N (2004) A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications. J Hazard Mater 112(1):1–15
Guo Y-Y, Chi W-J, Li Z-S, Li Q-S (2015) Molecular design of N–NO2 substituted cycloalkanes derivatives Cm(N–NO2)m for energetic materials with high detonation performance and low impact sensitivity. RSC Adv 5(48):38048–38055
Steinhauser G, Klapötke TM (2008) “Green” pyrotechnics: a chemists’ challenge. Angew Chem Inter Ed 47(18):3330–3347
Yin P, Parrish DA, Shreeve JM (2014) N-diazo-bridged nitroazoles: catenated nitrogen-atom chains compatible with nitro functionalities. Chem Eur J 20(22):6707–6712
Zhang Y, Huang Y, Parrish DA, Jean’ne MS (2011) 4-Amino-3, 5-dinitropyrazolate salts—highly insensitive energetic materials. J Mater Chem 21(19):6891–6897
Nielsen AT, Chafin AP, Christian SL, Moore DW, Nadler MP, Nissan RA, Vanderah DJ, Gilardi RD, George CF, Flippen-Anderson JL (1998) Synthesis of polyazapolycyclic caged polynitramines. Tetrahedron 54(39):11793–11812
Zhang M-X, Eaton PE, Gilardi R (2000) Hepta-and octanitrocubanes. Angew Chem Inter Ed 39(2):401–404
Simpson R, Urtiew P, Ornellas D, Moody G, Scribner K, Hoffman D (1997) CL-20 performance exceeds that of HMX and its sensitivity is moderate. Propell Explos Pyrot 22(5):249–255
Dong K, Wang Y, Gong X-B, Zhang J, Sun C-H, Pang S-P (2013) Formyl azido substituted nitro hexaazaisowurtzitane–synthesis, characterization and energetic properties. New J Chem 37(11):3685–3691
Chen H, Chen S, Li L, Jiao Q, Wei T, Jin S (2010) Synthesis, single crystal structure and characterization of pentanitromonoformylhexaazaisowurtzitane. J Hazard Mater 175(1):569–574
Hariharan P, Kaufman JJ, Lowrey AH, Miller RS (1985) Ab initioMODPOT/VRDDO/MERGE calculations on energetic compounds. IV. Nitrocubanes: Mononitro to octanitro quantum chemical calculations and electrostatic molecular potential contour maps. Int J Quantum Chem 28(1):39–59
Wang F, Du H, Zhang J, Gong X (2011) Computational studies on the crystal structure, thermodynamic properties, detonation performance, and pyrolysis mechanism of 2,4,6,8-tetranitro-1,3,5,7-tetraazacubane as a novel high energy density material. J Phys Chem A 115(42):11788–11795
Wu Q, Zhu W, Xiao H (2014) Computer-aided design of two novel and super-high energy cage explosives: dodecanitrohexaprismane and hexanitrohexaazaprismane. RSC Adv 4(8):3789–3797
Rice BM, Hare JJ (2002) A quantum mechanical investigation of the relation between impact sensitivity and the charge distribution in energetic molecules. J Phys Chem A 106(9):1770–1783
Chi W-J, Li L-L, Li B-T, Wu H-S (2012) Density functional calculations for a high energy density compound of formula C6H6−n(NO2)n. J Mol Model 18(8):3695–3704
Chi W-J, Ze S-L (2015) Theoretical prediction of detonation performance and stability for energetic polydinitroaminoprismanes. RSC Adv 5(10):7766–7772
Chi W, Wang X, Li B, Wu H (2012) Theoretical investigation on the heats of formation and detonation performance in polydinitroaminocubanes. J Mol Model 18(9):4217–4223
Chi W-J, Li L-L, Li B-T, Wu H-S (2013) Looking for high energy density compounds among polynitraminecubanes. J Mol Model 19(2):571–580
Politzer P, Seminario JM (1990) Relative bond strengths in tetrahedrane, prismane, and some of their aza analogs. Struct Chem 1(1):29–32
Qiu L, Gong X, Ju X, Xiao H (2008) Substituent effect on the molecular stability, group interaction, detonation performance, and thermolysis mechanism of nitroamino-substituted cyclopentanes and cyclohexanes. Sci China Ser B 51(12):1231–1245
Chung G, Schmidt MW, Gordon MS (2000) An ab initio study of potential energy surfaces for N8 isomers. J Phys Chem A 104(23):5647–5650
Frisch M, Trucks G, Schlegel HB, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G (2009) Gaussian 09. D01, Gaussian, Inc. Wallingford
Ju XH, Wang X, Bei FL (2005) Substituent effects on heats of formation, group interactions, and detonation properties of polyazidocubanes. J Comput Chem 26(12):1263–1269
Ghule V, Jadhav P, Patil R, Radhakrishnan S, Soman T (2009) Quantum-chemical studies on hexaazaisowurtzitanes. J Phys Chem A 114(1):498–503
Brill TB, James KJ (1993) Kinetics and mechanisms of thermal decomposition of nitroaromatic explosives. Chem Rev 93(8):2667–2692
Politzer P, Murray JS, Edward Grice M, Desalvo M, Miller E (1997) Calculation of heats of sublimation and solid phase heats of formation. Mol Phys 91(5):923–928
Bulat FA, Toro-Labbé A, Brinck T, Murray JS, Politzer P (2010) Quantitative analysis of molecular surfaces: areas, volumes, electrostatic potentials and average local ionization energies. J Mol Model 16(11):1679–1691
Kamlet MJ, Jacobs SJ (1968) Chemistry of detonations. I. A simple method for calculating detonation properties of C–H–N–O explosives. J Chem Phys 48(1):23–35
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbe A (2009) An electrostatic interaction correction for improved crystal density prediction. Mol Phys 107(19):2095–2101
Blanksby SJ, Ellison GB (2003) Bond dissociation energies of organic molecules. Acc Chem Res 36(4):255–263
Accelrys Software Materials studio (2007) Accelrys Software, Inc, San Diego
Mayo SL, Olafson BD, Goddard WA (1990) DREIDING: a generic force field for molecular simulations. J Phys Chem 94(26):8897–8909
He P, Zhang J-G, Wang K, Yin X, Jin X, Zhang T-L (2015) Extensive theoretical studies on two new members of the FOX-7 family: 5-(dinitromethylene)-1, 4-dinitramino-tetrazole and 1,1′-dinitro-4,4′-diamino-5,5′-bitetrazole as energetic compounds. Phys Chem Chem Phys 17(8):5840–5848
Liu Y, Gong X, Wang L, Wang G, Xiao H (2011) Substituent effects on the properties related to detonation performance and sensitivity for 2,2′,4,4′,6,6′-hexanitroazobenzene derivatives. J Phys Chem A 115(9):1754–1762
Akhavan J (ed) (2004) The chemistry of ExplosiVes, 2nd edn. Royal Society of Chemistry, Cambridge
Richard RM, Ball DW (2009) Density functional calculations on the thermodynamic properties of a series of nitrosocubanes having the formula C8H8−x(NO)x (x = 1–8). J Hazard Mater 164(2):1552–1555
Zhang Q, Zhang J, Qi X, Shreeve JM (2014) Molecular design and property prediction of high density polynitro [3.3. 3]-propellane-derivatized frameworks as potential high explosives. J Phys Chem A 118(45):10857–10865
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88(6):899–926
Elbeih A, Husarova A, Zeman S (2011) Path to ε-HNIW with reduced impact sensitivity. Cent Eur J Energy Mater 8(3):173–182
Politzer P, Murray JS (1996) Relationships between dissociation energies and electrostatic potentials of C–NO2 bonds: applications to impact sensitivities. J Mol Struct 376(1):419–424
Murray JS, Lane P, Politzer P, Bolduc PR (1990) A relationship between impact sensitivity and the electrostatic potentials at the midpoints of C–NO2 bonds in nitroaromatics. Chem Phys Lett 168(2):135–139
Peter J (1998) Effects of strongly electron-attracting components on molecular surface electrostatic potentials: application to predicting impact sensitivities of energetic molecules. Mol Phys 93(2):187–194
Pospíšil M, Vávra P, Concha MC, Murray JS, Politzer P (2011) Sensitivity and the available free space per molecule in the unit cell. J Mol Model 17(10):2569–2574
Politzer P, Murray JS (2015) Impact sensitivity and the maximum heat of detonation. J Mol Model 21(10):262–273
Politzer P, Murray JS (2015) Some molecular/crystalline factors that affect the sensitivities of energetic materials: molecular surface electrostatic potentials, lattice free space and maximum heat of detonation per unit volume. J Mol Model 21(2):25–36
Acknowledgments
This work is financially supported by the Major State Basic Research Development Programs of China (2011CBA00701), the National Natural Science Foundation of China (21473010, 20933001). This work is also supported by the opening project of State Key Laboratory of Explosion Science and Technology (Beijing Institute of Technology) (ZDKT12-03).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chi, WJ., Guo, YY., Li, QS. et al. Substituent effects on the properties related to detonation performance and stability for pentaprismane derivatives. Theor Chem Acc 135, 145 (2016). https://doi.org/10.1007/s00214-016-1885-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-1885-x