Abstract
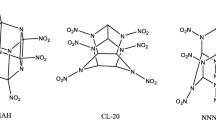
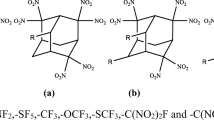
A series of new derivatives of cage 2,4,6,8,10,12,13,14,15-nanonitro-2,4,6,8,10,12,13,14, 15-nanoazaheptacyclo[5.5.1.13,11,15,9] pentadecane (NNNAHP) were designed by incorporating combination of heterocyclic and non-heterocyclic substituents and studied by using density functional theory. The results indicate that the –tetrazine and –N(NO2)2 are very beneficial structural fragments to increase their heat of formation. The introduction of different heterocyclic and non-heterocyclic groups can produce different effects on different properties: large densities (1.88–2.06 g cm−3), high detonation velocities (8.17–9.83 km s−1), excellent detonation pressures (30.55–46.02GPa), and outstanding heat of detonations (1169.80–1637.19 cal g−1). The analysis of bond dissociation energy values show that the N(cage)-NO2 is the weakest bond, and it may turn into a trigger bond during detonation. Almost all the derivatives are thermally more stable than the parent compound. All the substituted derivatives are insensitive as compared with the parent compound. According to excellent detonation properties, high thermal stability, and good insensitivity, 10 compounds may be chosen as potential high-energy density compounds.







Similar content being viewed by others
References
Spear RJ, Wilson WS (1984) Recent approaches to the synthesis of high explosive and energetic materials: a review. J Energ Mater 2(1–2):61–149
Fried LE, Manaa MR, Pagoria PF, Simpson RL (2001) Design and synthesis of energetic materials. Annu Rev Mater Res 31(1):291–321
Pagoria PF, Lee GS, Mitchell AR, Schmidt RD (2002) A review of energetic materials synthesis. Thermochim Acta 384(1–2):187–204
Thottempudi V, Shreeve JM (2011) Synthesis and promising properties of a new family of high-density energetic salts of 5-nitro-3-trinitromethyl-1 H-1, 2, 4-triazole and 5, 5′-Bis (trinitromethyl)-3, 3′-azo-1 H-1, 2, 4-triazole. J Am Chem Soc 133(49):19982–19992
Sabatini JJ, Oyler KD (2016) Recent advances in the synthesis of high explosive materials. Crystals 6(1):5
Klapötke TM, Krumm B, Ilg R, Troegel D, Tacke R (2007) The sila-explosives Si (CH2N3) 4 and Si (CH2ONO2) 4: silicon analogues of the common explosives pentaerythrityl tetraazide, C (CH2N3) 4, and pentaerythritol tetranitrate, C (CH2ONO2) 4. J Am Chem Soc 129(21):6908–6915
Pan Y, Zhu W (2018) Designing and looking for novel cage compounds based on bicyclo-HMX as high energy density compounds. RSC Adv 8(1):44–52
Xu X-J, Xiao H-M, Ju X-H, Gong X-D, Zhu W-H (2006) Computational studies on polynitrohexaazaadmantanes as potential high energy density materials. J Phys Chem A 110(17):5929–5933
Richard RM, Ball DW (2008) Enthalpies of formation of nitrobuckminsterfullerenes: extrapolation to C60 (NO2) 60. J Mol Struct Theochem 858(1–3):85–87
Ghule V, Jadhav P, Patil R, Radhakrishnan S, Soman T (2009) Quantum-chemical studies on hexaazaisowurtzitanes. J Phys Chem A 114(1):498–503
Jiao Y, Liu Z, Zhu W (2018) Searching for a new family of modified CL-20 cage derivatives with high energy and low sensitivity. Struct Chem 29(3):837–845
Zhang J-y, Du H-c, Wang F, Gong X-d, Ying S-j (2012) Crystal structure, detonation performance, and thermal stability of a new polynitro cage compound: 2, 4, 6, 8, 10, 12, 13, 14, 15-nonanitro-2, 4, 6, 8, 10, 12, 13, 14, 15-nonaazaheptacyclo [5.5. 1.1 3, 11. 1 5, 9] pentadecane. J Mol Model 18(6):2369–2376
Lai W-P, Lian P, Yu T, Bu J-H, Liu Y-Z, Zhu W-L, Lv J, Zhong-Xue G (2014) Theoretical study on the structure and stability of [1, 2, 5] oxadiazolo [3, 4-e][1, 2, 3, 4]-tetrazine-4, 6-di-N-dioxide (FTDO). J Mol Model 20(7):2343
Wu Q, Zhu W, Xiao H (2014) Designing and screening novel explosives with high energy and low sensitivity by appropriately introducing N-oxides, amino groups, and nitro groups into s-heptazine. RSC Adv 4(95):53000–53009
Yang J, Yan H, Wang G, Zhang X, Wang T, Gong X (2014) Computational investigations into the substituent effects of–N 3,–NF 2,–NO 2, and–NH 2 on the structure, sensitivity and detonation properties of N, N′-azobis (1, 2, 4-triazole). J Mol Model 20(4):2148
Zhang J, Shreeve JM (2014) 3, 3′-dinitroamino-4, 4′-azoxyfurazan and its derivatives: an assembly of diverse N–O building blocks for high-performance energetic materials. J Am Chem Soc 136(11):4437–4445
Deswal S, Ghule VD, Kumar TR, Radhakrishnan S (2015) Quantum-chemical design of tetrazolo [1, 5-b][1, 2, 4, 5] tetrazine based nitrogen-rich energetic materials. Computat Theoret Chem 1054:55–62
He C, Shreeve JM (2015) Energetic materials with promising properties: synthesis and characterization of 4, 4′-Bis (5-nitro-1, 2, 3-2H-triazole) derivatives. Angew Chem Int Ed 54(21):6260–6264
He P, Zhang J-G, Wang K, Yin X, Zhang T-L (2015) Combination multinitrogen with good oxygen balance: molecule and synthesis design of polynitro-substituted tetrazolotriazine-based energetic compounds. J Org Chem 80(11):5643–5651
Huang H, Shi Y, Liu Y, Yang J (2016) High-oxygen-balance furazan anions: a good choice for high-performance energetic salts. Chemistry 11(11):1688–1696
Klapötke TM, Leroux M, Schmid PC, Stierstorfer J (2016) Energetic materials based on 5, 5′-diamino-4, 4′-dinitramino-3, 3′-bi-1, 2, 4-triazole. Chemistry 11(6):844–851
Khan RU, Zhu S, Zhu W (2019) DFT studies on nitrogen-rich pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine-based high-energy density compounds. J Mol Model 25(9):283. https://doi.org/10.1007/s00894-019-4167-4
Ullah Khan R, Zhu W (2019) Theoretical studies on energetic nitrogen-rich heterocyclic substituted derivatives of pyrazino [2, 3-e] [1, 2, 3, 4] tetrazine-1, 3-di-N-oxide. ChemistrySelect 4(46):13646–13655. https://doi.org/10.1002/slct.201903605
Agrawal JP (1998) Recent trends in high-energy materials. Prog Energy Combust Sci 24(1):1–30
Xue H, Gao Y, Twamley B, Shreeve JM (2005) New energetic salts based on nitrogen-containing heterocycles. Chem Mater 17(1):191–198
Wu Q, Zhu W, Xiao H (2014) A new design strategy for high-energy low-sensitivity explosives: combining oxygen balance equal to zero, a combination of nitro and amino groups, and N-oxide in one molecule of 1-amino-5-nitrotetrazole-3 N-oxide. J Mater Chem A 2(32):13006–13015
Khan RU, Zhu W (2020) Designing and looking for novel low-sensitivity and high-energy cage derivatives based on the skeleton of nonanitro nonaaza pentadecane framework. Struct Chem. https://doi.org/10.1007/s11224-020-01506-y
Liang L, Huang H, Wang K, Bian C, Song J, Ling L, Zhao F, Zhou Z (2012) Oxy-bridged bis (1H-tetrazol-5-yl) furazan and its energetic salts paired with nitrogen-rich cations: highly thermally stable energetic materials with low sensitivity. J Mater Chem 22(41):21954–21964
Pan Y, Zhu W, Xiao H (2018) Molecular design on a new family of azaoxaadamantane cage compounds as potential high-energy density compounds. Can J Chem 97(2):86–93
Chen Z, Xiao J, Xiao H, Chiu Y (1999) Studies on heats of formation for tetrazole derivatives with density functional theory B3LYP method. J Phys Chem A 103(40):8062–8066
Chen P, Chieh Y, Tzeng S (2003) Density functional calculations of the heats of formation for various aromatic nitro compounds. J Mol Struct Theochem 634(1–3):215–224
Wang F, Xu X, Xiao H, Zhang J (2003) Theoretical studies on heat of formation and stability for polynitroadamantanes. Acta Chimica Sin-Chinese Edition 61(12):1939–1943
Ju X-H, Li Y-M, Xiao H-M (2005) Theoretical studies on the heats of formation and the interactions among the difluoroamino groups in polydifluoroaminocubanes. J Phys Chem A 109(5):934–938
Wei T, Zhu W, Zhang X, Li Y-F, Xiao H (2009) Molecular design of 1, 2, 4, 5-tetrazine-based high-energy density materials. J Phys Chem A 113(33):9404–9412
Zhang X, Zhu W, Xiao H (2009) Comparative theoretical studies of energetic substituted carbon-and nitrogen-bridged difurazans. J Phys Chem A 114(1):603–612
Khan RU, Zhu W (2019) Computational study of energetic derivatives of 3,3′-bridged ditriazoles. Can J Chem 98(3):115–127. https://doi.org/10.1139/cjc-2019-0399
Politzer P, Murray JS, Edward Grice M, Desalvo M, Miller E (1997) Calculation of heats of sublimation and solid phase heats of formation. Mol Phys 91(5):923–928
Byrd EF, Rice BM (2006) Improved prediction of heats of formation of energetic materials using quantum mechanical calculations. J Phys Chem A 110(3):1005–1013
Kamlet MJ, Jacobs S (1968) Chemistry of detonations. I. A simple method for calculating detonation properties of C–H–N–O explosives. J Chem Phys 48(1):23–35
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbe A (2009) An electrostatic interaction correction for improved crystal density prediction. Mol Phys 107(19):2095–2101
Pospíšil M, Vávra P, Concha MC, Murray JS, Politzer P (2010) A possible crystal volume factor in the impact sensitivities of some energetic compounds. J Mol Model 16(5):895–901
Guo Y-Y, Chi W-J, Li Z-S, Li Q-S (2015) Molecular design of N–NO 2 substituted cycloalkanes derivatives C m (N–NO 2) m for energetic materials with high detonation performance and low impact sensitivity. RSC Adv 5(48):38048–38055
Tian M, Chi W-J, Li Q-S, Li Z-S (2016) Theoretical design of highly energetic poly-nitro cage compounds. RSC Adv 6(53):47607–47615
Jin X, Hu B, Lu W, Gao S, Liu Z, Lv C (2014) Theoretical study on a novel high-energy density material 4, 6, 10, 12-tetranitro-5, 11-bis (nitroimino)-2, 8-dioxa-4, 6, 10, 12-tetraaza-tricyclo [7, 3, 0, 0 3, 7] dodecane. RSC Adv 4(13):6471–6477
Jin X, Xiao M, Ding Y, Zhou J, Hu B (2018) Theoretical insights on a series of cyclic energetic derivatives. ChemistrySelect 3(40):11160–11166
Jin X, Zhou J, Hu B, Ma C (2017) Theoretical insights on a series of difluoramino group–based energetic molecules. J Phys Org Chem 30(12):e3704
Jin X, Zhou J, Wang S, Hu B (2016) Computational study on structure and properties of new energetic material 3, 7-bis (dinitromethylene)-2, 4, 6, 8-tetranitro-2, 4, 6, 8-tetraaza-bicyclo [3.3. 0] octane. Química Nova 39(4):467–473
Scott AP, Radom L (1996) Harmonic vibrational frequencies: an evaluation of Hartree− Fock, Møller− Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J Phys Chem 100(41):16502–16513
Lide D (2004) The 84th Edition of the CRC handbook of chemistry and physics. CRC Press Boca Raton, FL
Neutz J, Grosshardt O, Schäufele S, Schuppler H, Schweikert W (2003) Synthesis, characterization and thermal behaviour of guanidinium-5-aminotetrazolate (GA)–a new nitrogen-rich compound. Propellants, Explosives, Pyrotechnics 28(4):181–188
Ciezak JA, Trevino S (2005) The inelastic neutron scattering spectra of α-3-amino-5-nitro-1, 2, 4-2H-triazole: experiment and DFT calculations. Chem Phys Lett 403(4–6):329–333
Wei T, Zhu W, Zhang J, Xiao H (2010) DFT study on energetic tetrazolo-[1, 5-b]-1, 2, 4, 5-tetrazine and 1, 2, 4-triazolo-[4, 3-b]-1, 2, 4, 5-tetrazine derivatives. J Hazard Mater 179(1–3):581–590
Talawar M, Sivabalan R, Mukundan T, Muthurajan H, Sikder A, Gandhe B, Rao AS (2009) Environmentally compatible next generation green energetic materials (GEMs). J Hazard Mater 161(2–3):589–607
Pan Y, Li J, Cheng B, Zhu W, Xiao H (2012) Computational studies on the heats of formation, energetic properties, and thermal stability of energetic nitrogen-rich furazano [3, 4-b] pyrazine-based derivatives. Computat Theoret Chem 992:110–119
Qiu L, Xiao H, Gong X, Ju X, Zhu W (2006) Theoretical studies on the structures, thermodynamic properties, detonation properties, and pyrolysis mechanisms of spiro nitramines. J Phys Chem A 110(10):3797–3807
Zhang J-y, Du H-c, Wang F, Gong X-d, Huang Y-s (2011) DFT studies on a high energy density cage compound 4-trinitroethyl-2, 6, 8, 10, 12-pentanitrohezaazaisowurtzitane. J Phys Chem A 115(24):6617–6621
Zhang J-y, Du H-c, Wang F, Gong X-d, Huang Y-s (2012) Theoretical investigations of a high density cage compound 10-(1-nitro-1, 2, 3, 4-tetraazol-5-yl) methyl-2, 4, 6, 8, 12-hexanitrohexaazaisowurtzitane. J Mol Model 18(1):165–170
Oliveira MA, Borges Jr I (2019) On the molecular origin of the sensitivity to impact of cyclic nitramines. Int J Quantum Chem 119(8):e25868
Anders G, Borges Jr I (2011) Topological analysis of the molecular charge density and impact sensitivy models of energetic molecules. J Phys Chem A 115(32):9055–9068
Politzer P, Murray JS (2014) Impact sensitivity and crystal lattice compressibility/free space. J Mol Model 20(5):2223
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 21773119) and Science Challenging Program (No. TZ2016001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Khan, R.U., Zhu, W. Computer-aided design and property prediction of novel insensitive high-energy heterocycle-substituted derivatives of cage NNNAHP. J Mol Model 26, 239 (2020). https://doi.org/10.1007/s00894-020-04513-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04513-2