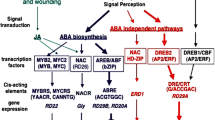
Abstract
The current agriculture is facing a continuous challenge of reaching up to 70% increase in crop productivity by 2050. An alarming expansion of human population, global climate changes, increasing soil salinity, and freshwater scarcity put the sustainable food production in a serious question. The inanimate life of plants makes them surrounded by a myriad of diverse biotic and abiotic stress conditions, which are mostly unavoidable. During the course of evolution, plants have evolved a robust and complicated mechanism of growth and defense trade off when responding to stress conditions. Secondary metabolite compounds like terpenoids, flavonols, flavones, and stilbenes are considered as the stress-inducible phytochemicals play crucial role in the development of plant immunity. It is now well established that transcription factors enable plants to counteract unfavorable conditions via the modulation of secondary metabolite genes, and they are now considered as potential genomic candidates for their wide applications in crop breeding strategies. Defensive molecular switches involve transcription factors which act as mediators of stress signals and regulate stress-responsive gene expression. To counteract the stress factors, different transcription factor families including AP2/ERF, WRKY, bHLH, bZIP, MYB, and NAC regulate the secondary metabolite biosynthesis genes. Classical breeding approaches for the generation of stress-resilient crops seem to be time-consuming and often the outcome is less effective. Recent advancement in the applications related to promoter engineering for the metabolite biosynthesis found to be novel approach for improved crop yield. The transcriptional and posttranscriptional modulation of TFs can facilitate molecular breeding and genetic moderation of plants for the increased production of secondary metabolites. Moreover, the synthetic promoters and transcription factors have been immensely powerful and effective as components for the regulation of targeted plant transgene expression. In this present chapter, we have highlighted on the function of TFs and the recent advancement of promoter engineering in the generation of stress-tolerant plants in the context of enhanced metabolite production.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Aida, M., Ishida, T., Fukaki, H., Fujisawa, H., & Tasaka, M. (1997). Genes involved in organ separation in Arabidopsis: An analysis of the cup-shaped cotyledon mutant. Plant Cell, 9, 841–857.
Alfieri, M., Vaccaro, M. C., Cappetta, E., Ambrosone, A., Tommasi, N. D., & Leone, A. (2018). Coactivation of MEP-biosynthetic genes and accumulation of abietane diterpenes in Salvia sclarea by heterologous expression of WRKY and MYC2 transcription factors. Scientific Reports, 8, 11009.
Ali, S., & Kim, W. C. (2019). A fruitful decade using synthetic promoters in the improvement of transgenic plants. Frontiers in Plant Science, 10, 1433. https://doi.org/10.3389/fpls.2019.01433
Allen, M. D., Yamasaki, K., Ohme-Takagi, M., Tateno, M., & Suzuki, M. A. (1998). Novel mode of DNA recognition by a β-sheet revealed by the solution structure of the GCC-box binding domain in complex with DNA. The EMBO Journal, 17, 5484–5496.
An, J. P., Li, H. H., Song, L. Q., Su, L., Liu, X., You, C. X., Wang, X. F., & Hao, Y. J. (2016). The molecular cloning and functional characterization of MdMYC2, a bHLH transcription factor in apple. Plant Physiology and Biochemistry, 108, 24–31.
An, J. P., Qu, F. J., Yao, J. F., Wang, X. N., You, C. X., Wang, X. F., & Hao, Y. J. (2017). The bZIP transcription factor MdHY5 regulates anthocyanin accumulation and nitrate assimilation in apple. Horticulture Research, 4, 17023.
Avato, P., Bucci, R., Tava, A., Vitali, C., Rosato, A., Bialy, Z., & Jurzysta, M. (2006). Antimicrobial activity of saponins from Medicago sp.: Structure-activity relationship. Phytotherapy Research, 20, 454–457.
Babitha, K. C., Ramu, S. V., Pruthvi, V., Mahesh, P., Nataraja, K. N., & Udayakumar, M. (2013). Co-expression of AtbHLH17 and AtWRKY28 confers resistance to abiotic stress in Arabidopsis. Transgenic Research, 22, 327–341.
Bai, Y., Pattanaik, S., Patra, B., Werkman, J. R., Xie, C. H., & Yuan, L. (2011). Flavonoid-related basic helix–loop–helix regulators, NtAn1a and NtAn1b, of tobacco have originated from two ancestors and are functionally active. Planta, 234, 363–375.
Bakshi, M., & Oelmüller, R. (2014). WRKY transcription factors: Jack of many trades in plants. Plant Signaling & Behavior, 9, e27700.
Banerjee, A., & Roychoudhury, A. (2015). Abscisic-acid-dependent basic leucine zipper (bZIP) transcription factors in plant abiotic stress. Protoplasma, 254, 3–16.
Bednarek, P., & Osbourn, A. (2009). Plant-microbe interactions: Chemical diversity in plant defense. Science, 324, 746–748.
Besseau, S., Hoffmann, L., Geoffroy, P., Lapierre, C., Pollet, B., & Legrand, M. (2007). Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell, 19, 148–162.
Boch J, Bonas U (2010) Xanthomonas AvrBs3 family-type III effectors: Discovery and function. Annual Review of Phytopathology 48:419–436.
Bogdanove, A. J., Schornack, S., & Lahaye, T. (2010). TAL effectors: Finding plant genes for disease and defense. Current Opinion in Plant Biology, 13, 394–401.
Cao, Y., Zhai, J., Wang, Q., Yuan, H., & Huang, X. (2017). Function of Hevea brasiliensis NAC1 in dehydration-induced laticifer differentiation and latex biosynthesis. Planta, 245, 31–44.
Capell, T., & Christou, P. (2004). Progress in plant metabolic engineering. Current Opinion in Biotechnology, 15, 148–154.
Cárdenas, P. D., Sonawane, P. D., Pollier, J., Bossche, R. V., Dewangan, V., Weithorn, E., Tal, L., Meir, S., Rogachev, I., & Malitsky, S. (2016). GAME9 regulates the biosynthesis of steroidal alkaloids and upstream isoprenoids in the plant mevalonate pathway. Nature Communications, 7, 10654.
Celenza, J. L., Quiel, J. A., Smolen, G. A., Merrikh, H., Silvestro, A. R., Normanly, J., & Bender, J. (2005). The Arabidopsis ATR1 Myb transcription factor controls indolic glucosinolate homeostasis. Plant Physiology, 137, 253–262.
Chen, H., Chen, W., Zhou, J., He, H., Chen, L., Chen, H., & Deng, X. W. (2012a). Basic leucine zipper transcription factor OsbZIP16 positively regulates drought resistance in rice. Plant Science, 193–194, 8–17. https://doi.org/10.1016/j.plantsci.2012.05.003
Chen, L., Song, Y., Li, S., Zhang, L., Zou, C., & Yu, D. (2012b). The role of WRKY transcription factors in plant abiotic stresses. Biochimica et Biophysica Acta, 1819, 120–128.
Dalman, K., Wind, J. J., Nemesio-Gorriz, M., Hammerbacher, A., Lundén, K., Ezcurra, I., & Elfstrand, M. (2017). Overexpression of PaNAC03, a stress induced NAC gene family transcription factor in Norway spruce leads to reduced flavonol biosynthesis and aberrant embryo development. BMC Plant Biology, 17, 6.
Danielsson, M., Lundén, K., Elfstrand, M., Hu, J., Zhao, T., Arnerup, J., Ihrmark, K., Swedjemark, G., Borg-Karlson, A. K., & Stenlid, J. (2011). Chemical and transcriptional responses of Norway spruce genotypes with different susceptibility to Heterobasidion spp. infection. BMC Plant Biology, 11:154.
Datta, K., Baisakh, N., Ganguly, M., Krishnan, S., Yamaguchi Shinozaki, K., & Datta, S. K. (2012). Overexpression of Arabidopsis and rice stress genes inducible transcription factor confers drought and salinity tolerance to rice. Plant Biotechnology Journal, 10, 579–586. https://doi.org/10.1111/j.1467-7652.2012.00688.x
de Lange, O., Schreiber, T., Schandry, N., Radeck, J., Braun, K. H., Koszinowski, J., Heuer, H., Straub, A., & Lahaye, T. (2013). Breaking the DNA binding code of Ralstonia solanacearum TAL effectors provides new possibilities to generate plant resistance genes against bacterial wilt disease. The New Phytologist, 199, 773–786.
Deng, B., Huang, Z., Ge, F., Liu, D., Lu, R., & Chen, C. (2017). An AP2/ERF family transcription factor PnERF1 raised the biosynthesis of saponins in Panax notoginseng. Journal of Plant Growth Regulation, 36, 691–701.
Devic, M., Guilleminot, J., Debeaujon, I., Bechtold, N., Bensaude, E., Koornneef, M., Pelletier, G., & Delseny, M. (1999). The BANYULS gene encodes a DFR-like protein and is a marker of early seed coat development. The Plant Journal, 19, 387–398.
Dong, Y., Wang, C., Han, X., Tang, S., Liu, S., Xia, X., & Yin, W. (2014). A novel bHLH transcription factor PebHLH35 from Populus euphratica confers drought tolerance through regulating stomatal development, photosynthesis and growth in Arabidopsis. Biochemical and Biophysical Research Communications, 450, 453–458.
Duan, M., Zhang, R., Zhu, F., Zhang, Z., Gou, L., Wen, J., Dong, J., & Wang, T. (2017). A lipid-anchored NAC transcription factor is translocated into the nucleus and activates glyoxalase I expression during drought stress. Plant Cell, 29, 1748–1772.
Dubos, C., Gourrierec, J. L., Baudry, A., Huep, G., Lanet, E., Debeaujon, I., Routaboul, J. M., Alboresi, A., Weisshaar, B., & Lepiniec, L. (2008). MYBL2 is a new regulator of flavonoid biosynthesis in Arabidopsis thaliana. The Plant Journal, 55, 940–953.
Espley, R. V., Hellens, R. P., Putterill, J., Stevenson, D. E., Kutty-Amma, S., & Allan, A. C. (2007). Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. The Plant Journal, 49, 414–427.
Eulgem, T., Rushton, P. J., Robatzek, S., & Somssich, I. E. (2000). The WRKY superfamily of plant transcription factors. Trends in Plant Science, 5, 199–206.
Feller, A., Machemer, K., Braun, E. L., & Grotewold, E. (2011). Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. The Plant Journal, 66, 94–116.
Frerigmann, H. (2016). Glucosinolate regulation in a complex relationship–MYC and MYB–no one can act without each other. Advances in Botanical Research Elsevier, 80, 57–97.
Frerigmann, H., Berger, B., & Gigolashvili, T. (2014). bHLH05 is an interaction partner of MYB51 and a novel regulator of glucosinolate biosynthesis in Arabidopsis. Plant Physiology, 166, 349–369.
Fu, J., Liu, Q., Wang, C., Liang, J., Liu, L., & Wang, Q. (2017). ZmWRKY79 positively regulates maize phytoalexin biosynthetic gene expression and is involved in stress response. Journal of Experimental Botany, 69, 497–510.
Fujita, M., Fujita, Y., Maruyama, K., Seki, M., Hiratsu, K., Ohme-Takagi, M., Tran, L. S., Yamaguchi-Shinozaki, K., & Shinozaki, K. (2004). A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. The Plant Journal, 39, 863–876.
Gaj, T., Gersbach, C. A., & Barbas, C. F., III. (2013). ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends in Biotechnology, 31, 397–405.
Gao, H., Wright, D. A., Li, T., Wang, Y., Horken, K., Weeks, D. P., Yang, B., & Spalding, M. H. (2014). TALE activation of endogenous genes in Chlamydomonas reinhardtii. Algal Research, 5, 52–60.
Gigolashvili, T., Engqvist, M., Yatusevich, R., Müller, C., & Flügge, U. I. (2008). HAG2/MYB76 and HAG3/MYB29 exert a specific and coordinated control on the regulation of aliphatic glucosinolate biosynthesis in Arabidopsis thaliana. New Phytologist, 177, 627–642. https://doi.org/10.1111/j.1469-8137.2007.02295.x
Gonzalez, A., Zhao, M., Leavitt, J. M., & Lloyd, A. M. (2008). Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. The Plant Journal, 53, 814–827.
Guan, X., Stege, J., Kim, M., Dahmani, Z., Fan, N., Heifetz, P., Barbas, C. F., III, & Briggs, S. P. (2002). Heritable endogenous gene regulation in plants with designed polydactyl zinc finger transcription factors. Proceedings of the National Academy of Sciences, 99, 13296–13301.
Guan, Y., Meng, X., Khanna, R., LaMontagne, E., Liu, Y., & Zhang, S. (2014). Phosphorylation of a WRKY transcription factor by MAPKs is required for pollen development and function in Arabidopsis. PLoS Genetics, 10, e1004384.
Guo, W., Jin, L., Miao, Y., He, X., Hu, Q., Guo, K., Zhu, L., & Zhang, X. (2016). An ethylene response-related factor, GbERF1-like, from Gossypium barbadense improves resistance to Verticillium dahliae via activating lignin synthesis. Plant Molecular Biology, 91, 305–318.
Gust, A. A., Brunner, F., & Nürnberger, T. (2010). Biotechnological concepts for improving plant innate immunity. Current Opinion in Biotechnology, 21(2), 204–210. https://doi.org/10.1016/j.copbio.2010.02.004
Hanada, K., Zou, C., Lehti-Shiu, M. D., Shinozaki, K., & Shiu, S. H. (2008). Importance of lineage-specific expansion of plant tandem duplicates in the adaptive response to environmental stimuli. Plant Physiology, 148, 993–1003.
Hartmann, L., Pedrotti, L., Weiste, C., Fekete, A., Schierstaedt, J., Gottler, J., Kempa, S., Krischke, M., Dietrich, K., & Mueller, M. J. (2015). Crosstalk between two bZIP signaling pathways orchestrates salt-induced metabolic reprogramming in Arabidopsis roots. Plant Cell, 27, 2244–2260.
Higo, K., Ugawa, Y., Iwamoto, M., & Korenaga, T. (1999). Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Research, 27(1), 297–300. https://doi.org/10.1093/nar/27.1.297
Hilton, I. B., D’Ippolito, A. M., Vockley, C. M., Thakore, P. I., Crawford, G. E., Reddy, T. E., & Gersbach, C. A. (2015). Epigenome editing by a CRISPRCas9-based acetyl transferase activates genes from promoters and enhancers. Nature Biotechnology, 33, 510–519.
Hirai, H., Tani, T., & Kikyo, N. (2010). Structure and functions of powerful trans activators: VP16, MyoO and FoxA. The International Journal of Developmental Biology, 54, 1589–1596.
Huang, Y. F., Vialet, S., Guiraud, J. L., Torregrosa, L., Bertrand, Y., Cheynier, V., This, P., & Terrier, N. (2014). A negative MYB regulator of proanthocyanidin accumulation, identified through expression quantitative locus mapping in the grape berry. New Phytologist, 201, 795–809.
Huot, B., Yao, J., Montgomery, B. L., & He, S. Y. (2014). Growth–defense tradeoffs in plants: A balancing act to optimize fitness. Molecular Plant, 7, 1267–1287.
Ishida T, Hattori S, Sano R, Inoue K, Shirano Y, Hayashi H, Shibata D, Sato S, Kato T, Tabata S (2007) Arabidopsis TRANSPARENT TESTA GLABRA2 is directly regulated by R2R3 MYB transcription factors and is involved in regulation of GLABRA2 transcription in epidermal differentiation. Plant Cell 19:2531–2543.
Ishihama, N., Yamada, R., Yoshioka, M., Katou, S., & Yoshioka, H. (2011). Phosphorylation of the Nicotiana benthamiana WRKY8 transcription factor by MAPK functions in the defense response. Plant Cell, 23, 1153–1170.
Ito, S., Song, Y. H., Josephson-Day, A. R., Miller, R. J., Breton, G., & Olmstead, R. G. (2010). Flowering bHLH transcriptional activators control expression of the photoperiodic flowering regulator CONSTANS in Arabidopsis. Proceedings of the National Academy of Sciences, 109, 3582–3587.
Ito, Y., Katsura, K., Maruyama, K., Taji, T., Kobayashi, M., & Seki, M. (2006). Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant & Cell Physiology, 47, 141–153. https://doi.org/10.1093/pcp/pci230
Iwaki, T., Guo, L., Ryals, J. A., Yasuda, S., Shimazaki, T., & Kikuchi, A. (2013). Metabolic profiling of transgenic potato tubers expressing Arabidopsis dehydration response element-binding protein 1A (DREB1A). Journal of Agricultural and Food Chemistry, 61, 893–900. https://doi.org/10.1021/jf304071n
Iwase, A., Matsui, K., & Ohme-Takagi, M. (2009). Manipulation of plant metabolic pathways by transcription factors. Plant Biotechnology, 26, 29–38. https://doi.org/10.5511/plantbiotechnology.26.29
Jakoby, M. (2002). bZIP transcription factors in Arabidopsis. Trends in Plant Science, 7, 106–111.
Jiang, J., Xi, H., Dai, Z., Lecourieux, F., Yuan, L., Liu, X., Patra, B., Wei, Y., Li, S., & Wang, L. (2018). VvWRKY8 represses stilbene synthase genes through direct interaction with VvMYB14 to control resveratrol biosynthesis in grapevine. Journal of Experimental Botany, 70, 715–729.
Johnson, C. S., Kolevski, B., & Smyth, D. R. (2002). TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell, 14, 1359–1375.
Joshi, R., Wani, S. H., & Singh, B. (2016). Transcription factors and plants response to drought stress: Current understanding and future directions. Frontiers in Plant Science, 7, 1029. https://doi.org/10.3389/fpls.2016.01029
Kadonaga, J. T. (2002). The DPE, a core promoter element for transcription by RNA polymerase II. Experimental & Molecular Medicine, 34, 259–264.
Kage, U., Yogendra, K. N., & Kushalappa, A. C. (2017). TaWRKY70 transcription factor in wheat QTL-2DL regulates downstream metabolite biosynthetic genes to resist Fusarium graminearum infection spread within spike. Scientific Reports, 7, 42596.
Karre, S., Kumar, A., Yogendra, K., Kage, U., Kushalappa, A., & Charron, J. B. (2019). HvWRKY23 regulates flavonoid glycoside and hydroxycinnamic acid amide biosynthetic genes in barley to combat Fusarium head blight. Plant Molecular Biology, 1–15.
Kashima, K., Yuki, Y., Mejima, M., Kurokawa, S., Suzuki, Y., & Minakawa, S. (2016). Good manufacturing practices production of a purification-free oral cholera vaccine expressed in transgenic rice plants. Plant Cell Reports, 35(3), 667–679. https://doi.org/10.1007/s00299-015-1911-9
Khan, S. A., Li, M. Z., Wang, S. M., & Yin, H. J. (2018). Revisiting the role of plant transcription factors in the battle against abiotic stress. International Journal of Molecular Sciences, 19(6), 1634. https://doi.org/10.3390/ijms19061634
Kishi-Kaboshi, M., Seo, S., Takahashi, A., & Hirochika, H. (2018). The MAMP-responsive MYB transcription factors MYB30, MYB55 and MYB110 activate the HCAA synthesis pathway and enhance immunity in Rice. Plant & Cell Physiology, 59, 903–915.
Kitsios, G., & Doonan, J. H. (2011). Cyclin dependent protein kinases and stress responses in plants. Plant Signaling & Behavior, 6, 204–209.
Knight, H., & Knight, M. R. (2001). Abiotic stress signalling pathways: Specificity and cross-talk. Trends in Plant Science, 6, 262–267.
Koschmann, J., Machens, F., Becker, M., Niemeyer, J., Schulze, J., & Bülow, L. (2012). Integration of bioinformatics and synthetic promoters leads to the discovery of novel elicitor-responsive cis-regulatory sequences in Arabidopsis. Plant Physiology, 160(1), 178–191. https://doi.org/10.1104/pp.112.198259
Kumar, S., AlAbed, D., Whitteck, J. T., Chen, W., Bennett, S., Asberry, A., Wang, X., DeSloover, D., Rangasamy, M., & Wright, T. R. (2015). A combinatorial bidirectional and bicistronic approach for coordinated multi-gene expression in corn. Plant Molecular Biology, 87, 341–353.
Kutchan, T. M. (2001). Ecological arsenal and developmental dispatcher. The paradigm of secondary metabolism. Plant Physiology, 125(1), 58–60. https://doi.org/10.1104/pp.125.1.58
Le Hir, R., Castelain, M., Chakraborti, D., Moritz, T., Dinant, S., & Bellini, C. (2017). AtbHLH68 transcription factor contributes to the regulation of ABA homeostasis and drought stress tolerance in Arabidopsis thaliana. Physiologia Plantarum, 160, 312–327.
Lee, S. B., Kim, H., Kim, R. J., & Suh, M. C. (2014). Overexpression of Arabidopsis MYB96 confers drought resistance in Camelina sativa via cuticular wax accumulation. Plant Cell Reports, 33, 1535–1546.
Lescot, M., Déhais, P., Thijs, G., Marchal, K., Moreau, Y., & Van de Peer, Y. (2002). PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Research, 30(1), 325–327. https://doi.org/10.1093/nar/30.1.325
Li, J., Zhang, K., Meng, Y., Hu, J., Ding, M., Bian, J., Yan, M., Han, J., & Zhou, M. (2018). Jasmonic acid/ethylene signaling coordinates hydroxycinnamic acid amides biosynthesis through ORA 59 transcription factor. The Plant Journal, 95, 444–457.
Li, S., Wang, H., Li, F., Chen, Z., Li, X., Zhu, L., Wang, G., Yu, J., Huang, D., & Lang, Z. (2015). The maize transcription factor EREB 58 mediates the jasmonate-induced production of sesquiterpene volatiles. The Plant Journal, 84, 296–308.
Li, S., Zhang, P., Zhang, M., Fu, C., & Yu, L. (2013a). Functional analysis of a WRKY transcription factor involved in transcriptional activation of the DBAT gene in Taxus chinensis. Plant Biology, 15, 19–26.
Li, T., Huang, S., Zhou, J., & Yang, B. (2013b). Designer TAL effectors induce disease susceptibility and resistance to Xanthomonas oryzae pv. oryzae in rice. Molecular Plant, 6, 781–789.
Li, X., Xue, C., Li, J., Qiao, X., Li, L., Yu, L., Huang, Y., & Wu, J. (2016). Genome-wide identification, evolution and functional divergence of MYB transcription factors in Chinese white pear (Pyrus bretschneideri). Plant & Cell Physiology, 57, 824–847.
Li, Y. Y., Mao, K., & Zhao, C. (2012). MdCOP1 ubiquitin E3 ligases interact with MdMYB1 to regulate light-induced anthocyanin biosynthesis and red fruit coloration in apple. Plant Physiology, 160(2), 1011–1022. https://doi.org/10.1104/pp.112.199703
Liu, C., Long, J., Zhu, K., Liu, L., Yang, W., Zhang, H., Li, L., Xu, Q., & Deng, X. (2016). Characterization of a citrus R2R3-MYB transcription factor that regulates the flavonol and hydroxycinnamic acid biosynthesis. Scientific Reports, 6, 25352.
Liu, C., Mao, B., Ou, S., Wang, W., Liu, L., Wu, Y., Chu, C., & Wang, X. (2014). OsbZIP71, a bZIP transcription factor, confers salinity and drought tolerance in rice. Plant Molecular Biology, 84(1–2), 19–36. https://doi.org/10.1007/s11103-013-0115-3
Liu, C. C., Chi, C., Jin, L. J., Zhu, J., Yu, J. Q., & Zhou, Y. H. (2018). The bZip transcription factor HY5 mediates CRY1a-induced anthocyanin biosynthesis in tomato. Plant, Cell & Environment, 41, 1762–1775.
Liu, J., Osbourn, A., & Ma, P. (2015a). MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Molecular Plant, 8, 689–708.
Liu, W., & Stewart, C. N., Jr. (2016). Plant synthetic promoters and transcription factors. Current Opinion in Biotechnology, 37, 36–44. https://doi.org/10.1016/j.copbio.2015.10.001
Liu, W., Yuan, J. S., & Stewart, C. N., Jr. (2013). Advanced genetic tools for plant biotechnology. Nature Reviews. Genetics, 14(11), 781–793. https://doi.org/10.1038/nrg3583
Liu Y, Ji X, Nie X, Qu M, Zheng L, Tan Z, Zhao H, Huo L, Liu S, Zhang B (2015b) Arabidopsis AtbHLH112 regulates the expression of genes involved in abiotic stress tolerance by binding to their E-box and GCG-box motifs. The New Phytologist 207:692–709.
Lowder, L. G., Zhang, D., Baltes, N. J., Paul, J. W., III, Tang, X., Zheng, X., Voytas, D. F., Hsieh, T. F., Zhang, Y., & Qi, Y. (2015). A CRISPR/Cas9 Toolbox for multiplexed plant genome editing and transcriptional regulation. Plant Physiology, 169(2), 971–985. https://doi.org/10.1104/pp.15.00636
Lu, X., Zhang, L., Zhang, F., Jiang, W., Shen, Q., Zhang, L., Lv, Z., Wang, G., & Tang, K. (2013). AaORA, a trichome-specific AP2/ERF transcription factor of Artemisia annua, is a positive regulator in the artemisinin biosynthetic pathway and in disease resistance to Botrytis cinerea. The New Phytologist, 198, 1191–1202.
Luca, V. D., & Pierre, B. S. (2000). The cell and developmental biology of alkaloid biosynthesis. Trends in Plant Science, 5, 168–173.
Luo, M., Dennis, E. S., Berger, F., Peacock, W. J., & Chaudhury, A. (2005). MINISEED3 (MINI3), a WRKY family gene, and HAIKU2 (IKU2), a leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America, 102, 17531–17536.
Luo, Q. J., Mittal, A., Jia, F., & Rock, C. D. (2012). An autoregulatory feedback loop involving PAP1 and TAS4 in response to sugars in Arabidopsis. Plant Molecular Biology, 80, 117–129.
Ma, D., Pu, G., Lei, C., Ma, L., Wang, H., Guo, Y., Chen, J., Du, Z., Wang, H., & Li, G. (2009). Isolation and characterization of AaWRKY1, an Artemisia annua transcription factor that regulates the amorpha-4,11-diene synthase gene, a key gene of artemisinin biosynthesis. Plant & Cell Physiology, 50, 2146–2161.
Ma, R., Xiao, Y., Lv, Z., Tan, H., Chen, R., Li, Q., Chen, J., Wang, Y., Yin, J., Zhang, L., & Chen, W. (2017). AP2/ERF transcription factor, Ii049, positively regulates lignan biosynthesis in Isatis indigotica through activating salicylic acid signaling and lignan/lignin pathway genes. Frontiers in Plant Science, 8, 1361.
Madanala, R., Gupta, V., Pandey, A. K., Srivastava, S., Pandey, V., & Singh, P. K. (2015). Tobacco chloroplasts as bioreactors for the production of recombinant superoxide dismutase in plants, an industrially useful enzyme. Plant Molecular Biology, 33(4), 1107–1115. https://doi.org/10.1007/s11105-014-0805-2
Mahmood, K., Xu, Z., El-Kereamy, A., Casaretto, J. A., & Rothstein, S. J. (2016). The Arabidopsis transcription factor ANAC032 represses anthocyanin biosynthesis in response to high sucrose and oxidative and abiotic stresses. Frontiers in Plant Science, 7, 1548.
Maston, G. A., Evans, S. K., & Green, M. R. (2006). Transcriptional regulatory elements in the human genome. Annual Review of Genomics and Human Genetics, 7, 29–59.
Matys, V., Fricke, E., Geffers, R., Gößling, E., Haubrock, M., & Hehl, R. (2003). TRANSFAC®: Transcriptional regulation, from patterns to profiles. Nucleic Acids Research, 31(1), 374–378. https://doi.org/10.1093/nar/gkg108
Mehrotra, R., & Mehrotra, S. (2010). Promoter activation by ACGT in response to salicylic and abscisic acids is differentially regulated by the spacing between two copies of the motif. Journal of Plant Physiology, 167, 1214–1218.
Menke, F. L. H., Champion, A., Kijne, J. W., & Memelink, J. (1999). A novel jasmonate- and elicitor-responsive element in the periwinkle secondary metabolite biosynthetic gene Str interacts with a jasmonate- and elicitor-inducible AP2-domain transcription factor, ORCA2. EMBO, 18, 4455–4463. https://doi.org/10.1093/emboj/18.16.4455
Meraj, T. A., Fu, J., & Raza, M. A. (2020). Transcriptional factors regulate plant stress responses through mediating secondary metabolism. Genes, 11(4), 346. https://doi.org/10.3390/genes11040346
Mertens, J., Pollier, J., Bossche, R. V., Lopez-Vidriero, I., Franco-Zorrilla, J. M., & Goossens, A. (2016). The bHLH transcription factors TSAR1 and TSAR2 regulate triterpene saponin biosynthesis in Medicago truncatula. Plant Physiology, 170, 194–210.
Mishra, S., Triptahi, V., Singh, S., Phukan, U. J., Gupta, M. M., Shanker, K., & Shukla, R. K. (2013). Wound induced transcriptional regulation of benzylisoquinoline pathway and characterization of wound inducible PsWRKY transcription factor from Papaver somniferum. PLoS One, 8, e52784.
Misra, P., Pandey, A., Tiwari, M., Chandrashekar, K., Sidhu, O. P., Asif, M. H., Chakrabarty, D., Singh, P. K., Trivedi, P. K., & Nath, P. (2010). Modulation of transcriptome and metabolome of tobacco by Arabidopsis transcription factor, AtMYB12, leads to insect resistance. Plant Physiology, 152, 2258–2268.
Mittler, R., & Blumwald, E. (2010). Genetic engineering for modern agriculture: Challenges and perspectives. Annual Review of Plant Biology, 61, 443–462. https://doi.org/10.1146/annurev-arplant-042809-12116
Miyamoto, K., Matsumoto, T., Okada, A., Komiyama, K., Chujo, T., Yoshikawa, H., Nojiri, H., Yamane, H., & Okada, K. (2014). Identification of target genes of the bZIP transcription factor OsTGAP1, whose overexpression causes elicitor-induced hyperaccumulation of diterpenoid phytoalexins in rice cells. PLoS One, 9, e105823.
Miyamoto, K., Nishizawa, Y., Minami, E., Nojiri, H., Yamane, H., & Okada, K. (2015). Overexpression of the bZIP transcription factor OsbZIP79 suppresses the production of diterpenoid phytoalexin in rice cells. Journal of Plant Physiology, 173, 19–27.
Montero, C., Cristescu, S., Jiménez, J., Orea, J., te Lintel, H. S., Harren, F., & Urena, A. G. (2003). Trans-resveratrol and grape disease resistance. A dynamical study by high-resolution laser-based techniques. Plant Physiology, 131, 129–138.
Moore, R. C., & Purugganan, M. D. (2005). The evolutionary dynamics of plant duplicate genes. Current Opinion in Plant Biology, 8, 122–128.
Morbitzer, R., Romer, P., Boch, J., & Lahaye, T. (2010). Regulation of selected genome loci using de novo-engineered transcription activator-like effector (TALE)-type transcription factors. Proceedings of the National Academy of Sciences of the United States of America, 107, 21617–21622.
Morishita T, Kojima Y, Maruta T, Nishizawa-Yokoi A, Yabuta Y, Shigeoka S (2009) Arabidopsis NAC transcription factor, ANAC078, regulates flavonoid biosynthesis under high-light. Plant & Cell Physiology 50:2210–2222.
Nakayasu, M., Umemoto, N., Ohyama, K., Fujimoto, Y., Lee, H. J., Watanabe, B., Muranaka, T., Saito, K., Sugimoto, Y., & Mizutani, M. (2017). A dioxygenase catalyzes steroid 16-hydroxylation in steroidal glycoalkaloid biosynthesis. Plant Physiology, 175, 120–133.
Nijhawan, A., Jain, M., Tyagi, A. K., & Khurana, J. P. (2008). Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice. Plant Physiology, 146(2), 333–350. https://doi.org/10.1104/pp.107.112821
Ohno, S., Hosokawa, M., Hoshino, A., Kitamura, Y., Morita, Y., Park, K. I., Nakashima, A., Deguchi, A., Tatsuzawa, F., & Doi, M. (2011). A bHLH transcription factor, DvIVS, is involved in regulation of anthocyanin synthesis in dahlia (Dahlia variabilis). Journal of Experimental Botany, 62, 5105–5116.
Okada, A., Okada, K., Miyamoto, K., Koga, J., Shibuya, N., Nojiri, H., & Yamane, H. (2009). OsTGAP1, a bZIP transcription factor, coordinately regulates the inductive production of diterpenoid phytoalexins in rice. The Journal of Biological Chemistry, 284, 26510–26518.
Oksman-Caldentey, K. M., & Saito, K. (2005). Integrating genomics and metabolomics for engineering plant metabolic pathways. Current Opinion in Biotechnology, 16, 174–179.
Onkokesung, N., Reichelt, M., van Doorn, A., Schuurink, R. C., van Loon, J. J., & Dicke, M. (2014). Modulation of flavonoid metabolites in Arabidopsis thaliana through overexpression of the MYB75 transcription factor: Role of kaempferol-3, 7-dirhamnoside in resistance to the specialist insect herbivore Pieris brassicae. Journal of Experimental Botany, 65, 2203–2217.
Ordiz, M. I., Barbas, C. F., III, & Beachy, R. N. (2002). Regulation of transgene expression in plants with polydactyl zinc finger transcription factors. Proceedings of the National Academy of Sciences, 99, 13290–13295.
Pan, Q., Wang, C., Xiong, Z., Wang, H., Fu, X., Shen, Q., Peng, B., Ma, Y., Sun, X., & Tang, K. (2019). CrERF5, an AP2/ERF transcription factor, positively regulates the biosynthesis of bisindole alkaloids and their precursors in Catharanthus roseus. Frontiers in Plant Science, 10, 931.
Pandey, A., Misra, P., Khan, M. P., Swarnkar, G., Tewari, M. C., Bhambhani, S., Trivedi, R., Chattopadhyay, N., & Trivedi, P. K. (2014). Co-expression of Arabidopsis transcription factor, AtMYB12, and soybean isoflavone synthase, GmIFS1, genes in tobacco leads to enhanced biosynthesis of isoflavones and flavonols resulting in osteoprotective activity. Plant Biotechnology Journal, 12, 69–80.
Pandey, A., Misra, P., & Trivedi, P. K. (2015). Constitutive expression of Arabidopsis MYB transcription factor, AtMYB11, in tobacco modulates flavonoid biosynthesis in favor of flavonol accumulation. Plant Cell Reports, 34, 1515–1528.
Patra, B., Schluttenhofer, C., Wu, Y., Pattanaik, S., & Yuan, L. (2013). Transcriptional regulation of secondary metabolite biosynthesis in plants. Biochimica et Biophysica Acta, 1829(11), 1236–1247. https://doi.org/10.1016/j.bbagrm.2013.09.006
Peleg, Z., & Blumwald, E. (2011). Hormone balance and abiotic stress tolerance in crop plants. Current Opinion in Plant Biology, 14, 290–295.
Piatek, A., Ali, Z., Baazim, H., Li, L., Abulfaraj, A., Al-Shareef, S., Aouida, M., & Mahfouz, M. M. (2015). RNA-guided transcriptional regulation in planta via synthetic dCas9-based transcription factors. Plant Biotechnology Journal, 13, 578–589.
Puranik, S., Sahu, P. P., Srivastava, P. S., & Prasad, M. (2012). NAC proteins: Regulation and role in stress tolerance. Trends in Plant Science, 17, 369–381.
Qiu, D., Xiao, J., Xie, W., Liu, H., Li, X., Xiong, L., & Wang, S. (2008). Rice gene network inferred from expression profiling of plants overexpressing OsWRKY13, a positive regulator of disease resistance. Molecular Plant, 1, 538–551.
Qiu, Z., Wang, X., Gao, J., Guo, Y., Huang, Z., & Du, Y. (2016). The tomato Hoffman’s anthocyaninless gene encodes a bHLH transcription factor involved in anthocyanin biosynthesis that is developmentally regulated and induced by low temperatures. PLoS One, 11(3), e0151067.
Quattrocchio, F., Wing, J., van der Woude, K., Souer, E., de Vetten, N., Mol, J., & Koes, R. (1999). Molecular analysis of the anthocyanin2 gene of petunia and its role in the evolution of flower color. Plant Cell, 11, 1433–1444.
Ramsay, N. A., & Glover, B. J. (2005). MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends in Plant Science, 10, 63–70.
Rashid, M., Guangyuan, H., Guangxiao, Y., Hussain, J., & Xu, Y. (2012). AP2/ERF transcription factor in rice: Genome-wide canvas and syntenic relationships between monocots and eudicots. Evolutionary Bioinformatics Online, 8, 321–355. https://doi.org/10.4137/EBO.S9369
Robatzek, S., & Somssich, I. E. (2002). Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes & Development, 16, 1139–1149.
Roepke, J., Salim, V., Wu, M., Thamm, A. M., Murata, J., Ploss, K., Boland, W., & De Luca, V. (2010). Vinca drug components accumulate exclusively in leaf exudates of Madagascar periwinkle. Proceedings of the National Academy of Sciences of the United States of America, 107, 15287–15292.
Rushton, P. J., Somssich, I. E., Ringler, P., & Shen, Q. J. (2010). WRKY transcription factors. Trends in Plant Science, 15, 247–258.
Saga, H., Ogawa, T., Kai, K., Suzuki, H., Ogata, Y., Sakurai, N., Shibata, D., & Ohta, D. (2012). Identification and characterization of ANAC042, a transcription factor family gene involved in the regulation of camalexin biosynthesis in Arabidopsis. Molecular Plant-Microbe Interactions, 25, 684–696.
Sahoo, D. K., Sarkar, S., Sumita, R., Maiti, I. B., & Dey, N. (2014). Comparative analysis of synthetic DNA promoters for high-level gene expression in plants. Planta, 240, 855–875.
Samira, R., Li, B., Kliebenstein, D., Li, C., Davis, E., Gillikin, J. W., & Long, T. A. (2018). The bHLH transcription factor ILR3 modulates multiple stress responses in Arabidopsis. Plant Molecular Biology, 97, 297–309.
Sawant, S. V., Kiran, K., Mehrotra, R., Chaturvedi, C. P., Ansari, S. A., Singh, P., Lodhi, N., & Tuli, R. (2005). A variety of synergistic and antagonistic interactions mediated by cis-acting DNA motifs regulate gene expression in plant cells and modulate stability of the transcription complex formed on a basal promoter. Journal of Experimental Botany, 56, 2345–2353.
Schluttenhofer, C., Pattanaik, S., Patra, B., & Yuan, L. (2014). Analyses of Catharanthus roseus and Arabidopsis thaliana WRKY transcription factors reveal involvement in jasmonate signaling. BMC Genomics, 15, 50.
Schwinn, K., Venail, J., Shang, Y., Mackay, S., Alm, V., Butelli, E., Oyama, R., Bailey, P., Davies, K., & Martin, C. (2006). A small family of MYB-regulatory genes controls floral pigmentation intensity and patterning in the genus Antirrhinum. The Plant Cell, 18, 831–851.
Sears, M. T., Zhang, H., Rushton, P. J., Wu, M., Han, S., Spano, A. J., & Timko, M. P. (2014). NtERF32: A non-NIC2 locus AP2/ERF transcription factor required in jasmonate-inducible nicotine biosynthesis in tobacco. Plant Molecular Biology, 84, 49–66.
Sewelam, N., Kazan, K., Thomas-Hall, S. R., Kidd, B. N., Manners, J. M., & Schenk, P. M. (2013). Ethylene response factor 6 is a regulator of reactive oxygen species signaling in Arabidopsis. PLoS One, 8, e70289.
Shao, H., Wang, H., & Tang, X. (2015). NAC transcription factors in plant multiple abiotic stress responses: Progress and prospects. Frontiers in Plant Science, 6, 902.
Shen, Y., Sun, T., Pan, Q., Anupol, N., Chen, H., Shi, J., Liu, F., Deqiang, D., Wang, C., & Zhao, J. (2019). RrMYB5-and RrMYB10-regulated flavonoid biosynthesis plays a pivotal role in feedback loop responding to wounding and oxidation in Rosa rugosa. Plant Biotechnology Journal, 17, 2078–2095.
Shikata, M., & Ohme-Takagi, M. (2008). The utility of transcription factors for manipulation of floral traits. Plant Biotechnology, 25, 31–36.
Shoji, T., & Hashimoto, T. (2011). Tobacco MYC2 regulates jasmonate-inducible nicotine biosynthesis genes directly and by way of the NIC2-locus ERF genes. Plant & Cell Physiology, 52, 1117–1130.
Shoji, T., Kajikawa, M., & Hashimoto, T. (2010). Clustered transcription factor genes regulate nicotine biosynthesis in tobacco. Plant Cell, 22, 3390–3409.
Singh, A. K., Kumar, S. R., Dwivedi, V., Rai, A., Pal, S., Shasany, A. K., & Nagegowda, D. A. (2017). A WRKY transcription factor from Withania somnifera regulates triterpenoid withanolide accumulation and biotic stress tolerance through modulation of phytosterol and defense pathways. New Phytologist, 215, 1115–1131.
Singh, B., & Sharma, R. A. (2015). Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech, 5(2), 129–151. https://doi.org/10.1007/s13205-014-0220-2
Song, S., Qi, T., Fan, M., Zhang, X., Gao, H., Huang, H., Wu, D., Guo, H., & Xie, D. (2013). The bHLH subgroup IIId factors negatively regulate jasmonate-mediated plant defense and development. PLoS Genetics, 9, e1003653.
Song, S. Y., Chen, Y., Chen, J., Dai, X. Y., & Zhang, W. H. (2011). Physiological mechanisms underlying OsNAC5-dependent tolerance of rice plants to abiotic stress. Planta, 234, 331–345.
Souer, E., Houwelingen, A. V., Kloos, D., Mol, J., & Koes, R. (1996). The no apical meristem gene of petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell, 85, 159–170.
Srivastava, R., Rai, K. M., Srivastava, M., Kumar, V., Pandey, B., Singh, S. P., Bag, S. K., Singh, B. D., Tuli, R., & Sawant, S. V. (2014). Distinct role of core promoter architecture in regulation of light-mediated responses in plant genes. Molecular Plant, 7(4), 626–641. https://doi.org/10.1093/mp/sst146
Stege, J. T., Guan, X., Ho, T., Beachy, R. N., & Barbas, C. F., III. (2002). Controlling gene expression in plants using synthetic zinc finger transcription factors. The Plant Journal, 32, 1077–1086.
Sun, Y., & Yu, D. (2015). Activated expression of AtWRKY53 negatively regulates drought tolerance by mediating stomatal movement. Plant Cell Reports, 34, 1295–1306.
Suttipanta, N., Pattanaik, S., Kulshrestha, M., Patra, B., Singh, S. K., & Yuan, L. (2011). The transcription factor CrWRKY1 positively regulates the terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Plant Physiology, 157, 2081–2093.
Takahashi, A., & Ohnishi, T. (2004). The significance of the study about the biological effects of solar ultraviolet radiation using the exposed facility on the international space station. Biological Sciences in Space, 18, 255–260.
Takasaki, H., Maruyama, K., Kidokoro, S., Ito, Y., Fujita, Y., Shinozaki, K., Yamaguchi-Shinozaki, K., & Nakashima, K. (2010). The abiotic stress-responsive NAC-type transcription factor OsNAC5 regulates stress-inducible genes and stress tolerance in rice. Molecular Genetics and Genomics, 284, 173–183.
Takos, A. M., Jaffe, F. W., Jacob, S. R., Bogs, J., Robinson, S. P., & Walker, A. R. (2006). Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiology, 142, 1216–1232.
Todaka, D., Shinozaki, K., & Yamaguchi-Shinozaki, K. (2015). Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants. Frontiers in Plant Science, 6, 84. https://doi.org/10.3389/fpls.2015.00084
Todd, A. T., Liu, E., Polvi, S. L., Pammett, R. T., & Page, J. E. (2010). A functional genomics screen identifies diverse transcription factors that regulate alkaloid biosynthesis in Nicotiana benthamiana. The Plant Journal, 62, 589–600.
Tripathi, P., Rabara, R., & Rushton, P. (2014). A systems biology perspective on the role of WRKY transcription factors in drought responses in plants. Planta, 239, 255–266.
Tungmunnithum, D., Thongboonyou, A., Pholboon, A., & Yangsabai, A. (2018). Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects an overview. Medicine, 5(3), 93. https://doi.org/10.3390/medicines5030093
Van der Fits, L., & Memelink, J. (2000). ORCA3, a Jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science, 289, 295–297.
Vannozzi, A., Wong, D. C. J., Höll, J., Hmmam, I., Matus, J. T., Bogs, J., Ziegler, T., Dry, I., Barcaccia, G., & Lucchin, M. (2018). Combinatorial regulation of stilbene synthase genes by WRKY and MYB transcription factors in grapevine (Vitis vinifera L.). Plant & Cell Physiology, 59, 1043–1059.
Venter, M. (2007). Synthetic promoters: Genetic control through cis engineering. Trends in Plant Science, 12, 118–124.
Wan, X., Marsafari, M., & Xu, P. (2019). Engineering metabolite-responsive transcriptional factors to sense small molecules in eukaryotes: Current state and perspectives. Microbial Cell Factories, 18, 61. https://doi.org/10.1186/s12934-019-1111-3
Wang, C., Deng, P., Chen, L., Wang, X., Ma, H., Hu, W., Yao, N., Feng, Y., Chai, R., & Yang, G. (2013). A wheat WRKY transcription factor TaWRKY10 confers tolerance to multiple abiotic stresses in transgenic tobacco. PLoS One, 8, e65120.
Wang, F., Kong, W., Wong, G., Fu, L., Peng, R., Li, Z., & Yao, Q. (2016a). AtMYB12 regulates flavonoids accumulation and abiotic stress tolerance in transgenic Arabidopsis thaliana. Molecular Genetics and Genomics, 291, 1545–1559.
Wang, F., Zhu, H., Chen, D., Li, Z., Peng, R., & Yao, Q. (2016b). A grape bHLH transcription factor gene, VvbHLH1, increases the accumulation of flavonoids and enhances salt and drought tolerance in transgenic Arabidopsis thaliana. Plant Cell, Tissue and Organ Culture, 125, 387–398.
Wang, F., Zhu, H., Kong, W., Peng, R., Liu, Q., & Yao, Q. (2016c). The Antirrhinum AmDEL gene enhances flavonoids accumulation and salt and drought tolerance in transgenic Arabidopsis. Planta, 244, 59–73.
Wang, H., Avci, U., Nakashima, J., Hahn, M. G., Chen, F., & Dixon, R. A. (2010a). Mutation of WRKY transcription factors initiates pith secondary wall formation and increases stem biomass in dicotyledonous plants. Proceedings of the National Academy of Sciences of the United States of America, 107, 22338–22343.
Wang, K. L., Bolitho, K., Grafton, K., Kortstee, A., Karunairetnam, S., McGhie, T. K., Espley, R. V., Hellens, R. P., & Allan, A. C. (2010b). An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC Plant Biology, 10, 50.
Wang, L., Ran, L., Hou, Y., Tian, Q., Li, C., Liu, R., Fan, D., & Luo, K. (2017). The transcription factor MYB115 contributes to the regulation of proanthocyanidin biosynthesis and enhances fungal resistance in poplar. New Phytologist, 215, 351–367.
Wang, L., & Wang, Y. (2019). Transcription factor VqERF114 regulates stilbene synthesis in Chinese wild Vitis quinquangularis by interacting with VqMYB35. Plant Cell Reports, 38, 1347–1360.
Wang, Q., Guan, Y., Wu, Y., Chen, H., Chen, F., & Chu, C. (2008). Overexpression of a rice OsDREB1F gene increases salt, drought, and low temperature tolerance in both Arabidopsis and rice. Plant Molecular Biology, 67, 589–602.
Wang, W. L., Wang, Y. X., Li, H., Liu, Z. W., Cui, X., & Zhuang, J. (2018). Two MYB transcription factors (CsMYB2 and CsMYB26) are involved in flavonoid biosynthesis in tea plant [Camellia sinensis (L.) O. Kuntze]. BMC Plant Biol, 18, 288.
Wang, Z., Su, G., Li, M., Ke, Q., Kim, S. Y., Li, H., Huang, J., Xu, B., Deng, X. P., & Kwak, S. S. (2016d). Overexpressing Arabidopsis ABF3 increases tolerance to multiple abiotic stresses and reduces leaf size in alfalfa. Plant Physiology and Biochemistry, 109, 199–208.
Wei, K., Chen, J., & Wang, Y. (2012). Genome-wide analysis of bZIP-encoding genes in maize. DNA Research, 19(6), 463–476. https://doi.org/10.1093/dnares/dss026
Wei, T., Deng, K., Liu, D., Gao, Y., Liu, Y., Yang, M., Zhang, L., Zheng, X., Wang, C., & Song, W. (2016). Ectopic expression of DREB transcription factor, AtDREB1A, confers tolerance to drought in transgenic Salvia miltiorrhiza. Plant & Cell Physiology, 57, 1593–1609.
Winkel-Shirley, B. (2002). Biosynthesis of flavonoids and effects of stress. Current Opinion in Plant Biology, 5(3), 218–223. https://doi.org/10.1016/s1369-5266(02)00256-x
Wu, C. Y., Suzuki, A., Washida, H., & Takaiwa, F. (1998). The GCN4 motif in a rice glutelin gene is essential for endosperm-specific gene expression and is activated by Opaque-2 in transgenic rice plants. The Plant Journal, 14, 673–683.
Wu, H., Fu, B., Sun, P., Xiao, C., & Liu, J. H. (2016). A NAC transcription factor represses putrescine biosynthesis and affects drought tolerance. Plant Physiology, 172, 1532–1547.
Xiao, H., Pearson, A., Coulombe, B., Truant, R., Zhang, S., Regier, J. L., Triezenberg, S. J., Reinberg, D., Flores, O., & Ingles, C. J. (1994). Binding of basal transcription factor TFIIH to the acidic activation domains of VP16 and p53. Molecular and Cellular Biology, 14, 7013–7024.
Xu, Z. S., Ni, Z. Y., Li, Z. Y., Li, L. C., Chen, M., Gao, D. Y., Yu, X. D., Liu, P., & Ma, Y. Z. (2009). Isolation and functional characterization of HvDREB1: A gene encoding a dehydration-responsive element binding protein in Hordeum vulgare. Journal of Plant Research, 122, 121–130.
Yamada, Y., Motomura, Y., & Sato, F. (2015). CjbHLH1 homologs regulate sanguinarine biosynthesis in Eschscholzia californica cells. Plant & Cell Physiology, 56, 1019–1030.
Yamaguchi, K., Takahashi, Y., Berberich, T., Imai, A., Takahashi, T., Michael, A. J., & Kusano, T. A. (2007). A protective role for the polyamine spermine against drought stress in Arabidopsis. Biochemical and Biophysical Research Communications, 352, 486–490.
Yamamura, C., Mizutani, E., Okada, K., Nakagawa, H., Fukushima, S., Tanaka, A., Maeda, S., Kamakura, T., Yamane, H., & Takatsuji, H. (2015). Diterpenoid phytoalexin factor, a bHLH transcription factor, plays a central role in the biosynthesis of diterpenoid phytoalexins in rice. The Plant Journal, 84, 1100–1113.
Yang, Z., Patra, B., Li, R., Pattanaik, S., & Yuan, L. (2013). Promoter analysis reveals cis-regulatory motifs associated with the expression of the WRKY transcription factor CrWRKY1 in Catharanthus roseus. Planta, 238, 1039–1049.
Yogendra, K. N., Dhokane, D., Kushalappa, A. C., Sarmiento, F., Rodriguez, E., & Mosquera, T. (2017). StWRKY8 transcription factor regulates benzylisoquinoline alkaloid pathway in potato conferring resistance to late blight. Plant Science, 256, 208–216.
Yogendra, K. N., Kumar, A., Sarkar, K., Li, Y., Pushpa, D., Mosa, K. A., Duggavath, R., & Kushalappa, A. C. (2015). Transcription factor StWRKY1 regulates phenylpropanoid metabolites conferring late blight resistance in potato. Journal of Experimental Botany, 66, 7377–7389.
Yokotani, N., Sato, Y., Tanabe, S., Chujo, T., Shimizu, T., Okada, K., Yamane, H., Shimono, M., Sugano, S., & Takatsuji, H. (2013). WRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance. Journal of Experimental Botany, 64, 5085–5097.
You, J., Zong, W., Hu, H., Li, X., Xiao, J., & Xiong, L. (2014). A STRESS-RESPONSIVE NAC1-regulated protein phosphatase gene rice protein phosphatase18 modulates drought and oxidative stress tolerance through abscisic acid-independent reactive oxygen species scavenging in rice. Plant Physiology, 166, 2100–2114. https://doi.org/10.1104/pp.114.251116
Young, D., Michelotti, E., Swindell, C., & Krauss, N. (1992). Antifungal properties of taxol and various analogues. Experientia, 48, 882–885.
Younger, A. K. D., Dalvie, N. C., Rottinghaus, A. G., & Leonard, J. N. (2017). Engineering modular biosensors to confer metabolite responsive regulation of transcription. ACS Synthetic Biology, 6(2), 311–325.
Yu, Y., Wang, L., Chen, J., Liu, Z., Park, C. M., & Xiang, F. (2018). WRKY71 acts antagonistically against salt-delayed flowering in Arabidopsis thaliana. Plant & Cell Physiology, 59, 414–422.
Yu, Z. X., Li, J. X., Yang, C. Q., Hu, W. L., Wang, L. J., & Chen, X. Y. (2012). The jasmonate responsive AP2/ERF transcription factors AaERF1 and AaERF2 positively regulate artemisinin biosynthesis in Artemisia annua L. Molecular Plant, 5, 353–365.
Yuan, Y., Qi, L., Yang, J., Wu, C., Liu, Y., & Huang, L. (2015). A Scutellaria baicalensis R2R3-MYB gene, SbMYB8, regulates flavonoid biosynthesis and improves drought stress tolerance in transgenic tobacco. Plant Cell, Tissue and Organ Culture, 120, 961–972.
Zhai, Y., Zhang, L., Xia, C., Fu, S., Zhao, G., Jia, J., & Kong, X. (2016). The wheat transcription factor, TabHLH39, improves tolerance to multiple abiotic stressors in transgenic plants. Biochemical and Biophysical Research Communications, 473, 1321–1327.
Zhang, C., Li, C., Liu, J., Lv, Y., Yu, C., Li, H., Zhao, T., & Liu, B. (2017). The OsABF1 transcription factor improves drought tolerance by activating the transcription of COR413-TM1 in rice. Journal of Experimental Botany, 68, 4695–4707.
Zhang, C., Liu, J., Zhao, T., Gomez, A., Li, C., Yu, C., Li, H., Lin, J., Yang, Y., & Liu, B. (2016a). A drought-inducible transcription factor delays reproductive timing in rice. Plant Physiology, 171, 334–343.
Zhang, F., Fu, X., Lv, Z., Lu, X., Shen, Q., Zhang, L., Zhu, M., Wang, G., Sun, X., & Liao, Z. (2015a). A basic leucine zipper transcription factor, AabZIP1, connects abscisic acid signaling with artemisinin biosynthesis in Artemisia annua. Molecular Plant, 8, 163–175.
Zhang, M., Li, S., Nie, L., Chen, Q., Xu, X., Yu, L., & Fu, C. (2015b). Two jasmonate-responsive factors, TcERF12 and TcERF15, respectively act as repressor and activator of tasy gene of taxol biosynthesis in Taxus chinensis. Plant Molecular Biology, 89, 463–473.
Zhang, Y., Xu, Z., Ji, A., Luo, H., & Song, J. (2018). Genomic survey of bZIP transcription factor genes related to tanshinone biosynthesis in Salvia miltiorrhiza. Acta Pharmaceutica Sinica B, 8, 295–305.
Zhang, Z., Hu, X., Zhang, Y., Miao, Z., Xie, C., Meng, X. Z., Deng, J., Wen, J., Mysore, K. S., & Frugier, F. (2016b). Opposing control by transcription factors MYB61 and MYB3 increases freezing tolerance by relieving c-repeat binding factor suppression. Plant Physiology, 172, 1306–1323.
Zhou, F., Sun, T. H., Zhao, L., Pan, X. W., & Lu, S. (2015). The bZIP transcription factor HY5 interacts with the promoter of the monoterpene synthase gene QH6 in modulating its rhythmic expression. Frontiers in Plant Science, 6, 304.
Zhou, H., Peng, Q., Zhao, J., Owiti, A., Ren, F., Liao, L., Wang, L., Deng, X., Jiang, Q., & Han, Y. (2016). Multiple R2R3-MYB transcription factors involved in the regulation of anthocyanin accumulation in peach flower. Frontiers in Plant Science, 7, 1557.
Acknowledgments
The authors gratefully acknowledge the Council of Scientific and Industrial Research, Govt. of India (Ref. No. 38(1587)/16/EMR-II, dated: 17/05/2016 to SR), UGC, Govt. of India (Start-Up research grant No. F.30-158/2015 (BSR), SERB, DST, Govt. of India (Ref. No. ECR/2016/000539 to SR) and DST & BT, Govt. of WB (Ref. No: ST/P/S & T/1G-5/2018 to SR) for providing financial supports for performing research related to the topic discussed in this review. SB and PR are thankful to CSIR, Govt. of India and UGC, Govt. of India for the research fellowship. We apologize to those authors whose work could not be cited due to space limitation.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Banerjee, S., Roy, P., Roy, S. (2022). Transcription Factor Mediated Plant Metabolite Production in Response to Environmental Stress Factors: Current Understanding and Future Aspects. In: Aftab, T., Hakeem, K.R. (eds) Metabolic Engineering in Plants. Springer, Singapore. https://doi.org/10.1007/978-981-16-7262-0_4
Download citation
DOI: https://doi.org/10.1007/978-981-16-7262-0_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-7261-3
Online ISBN: 978-981-16-7262-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)