Abstract
Key message
The first Good Manufacturing Practices production of a purification-free rice-based oral cholera vaccine (MucoRice-CTB) from transgenic plants in a closed cultivation system yielded a product meeting regulatory requirements.
Abstract
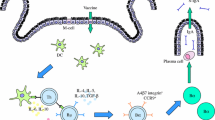
Despite our knowledge of their advantages, plant-based vaccines remain unavailable for human use in both developing and industrialized countries. A leading, practical obstacle to their widespread use is producing plant-based vaccines that meet governmental regulatory requirements. Here, we report the first production according to current Good Manufacturing Practices of a rice-based vaccine, the cholera vaccine MucoRice-CTB, at an academic institution. To this end, we established specifications and methods for the master seed bank (MSB) of MucoRice-CTB, which was previously generated as a selection-marker-free line, evaluated its propagation, and given that the stored seeds must be renewed periodically. The production of MucoRice-CTB incorporated a closed hydroponic system for cultivating the transgenic plants, to minimize variations in expression and quality during vaccine manufacture. This type of molecular farming factory can be operated year-round, generating three harvests annually, and is cost- and production-effective. Rice was polished to a ratio of 95 % and then powdered to produce the MucoRice-CTB drug substance, and the identity, potency, and safety of the MucoRice-CTB product met pre-established release requirements. The formulation of MucoRice-CTB made by fine-powdering of drug substance and packaged in an aluminum pouch is being evaluated in a physician-initiated phase I study.





Similar content being viewed by others
Abbreviations
- Ab:
-
Antibody
- BSA:
-
Bovine serum albumin
- Ch:
-
Chromosome
- CT:
-
Cholera toxin
- CTB:
-
Cholera toxin B-subunit
- ELISA:
-
Enzyme-Linked ImmunoSorbent Assay
- IgA:
-
Immunoglobulin A
- IgG:
-
Immunoglobulin G
- JP:
-
The Japanese Pharmacopeia
- MSB:
-
Master seed bank
- MS/MS:
-
Tandem mass spectrometry
- PBS:
-
Phosphate-buffered saline
- PCR:
-
Polymerase chain reaction
- RNAi:
-
RNA interference
- SDS–PAGE:
-
SDS–polyacrylamide gel electrophoresis
- WT:
-
Wild type rice
References
Azegami T, Itoh H, Kiyono H, Yuki Y (2015) Novel transgenic rice-based vaccines. Arch Immunol Ther Ex 63:87–99
Chichester JA, Jones RM, Green BJ, Stow M, Miao F, Moonsammy G, Streatfield SJ, Yusibov V (2012) Safety and immunogenicity of a plant-produced recombinant hemagglutinin-based influenza vaccine (HAI-05) derived from A/Indonesia/05/2005 (H5N1) influenza virus: a phase 1 randomized, double-blind, placebo-controlled, dose-escalation study in healthy adults. Viruses 4:3227–3244
Chory J (1993) Out of darkness: mutants reveal pathways controlling light-regulated development in plants. Trends Genet 9:167–172
Cummings JF, Guerreroa ML, Moona JE, Watermana P, Nielsena RK, Jeffersonb S, F. Grossb FL, Hancockb K, Katzb JM, Yusibovc V (2014) Safety and immunogenicity of a plant-produced recombinantmonomer hemagglutinin-based influenza vaccine derived frominfluenza A (H1N1) pdm09 virus: A Phase 1 dose-escalation study inhealthy adults. Vaccine 32: 2251–2259
Curtiss RI, Cardineau CA (1997) Oral immunization by transgenic plants. US Patent 5,686,079
Daniell H, Singh ND, Mason H, Streatfield SJ (2009) Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci 14:669–679
Dertzbaugh M, Elson CO (1993) Reduction in oral immunogenicity of cholera toxin B subunit by N-terminal peptide addition. Infect Immun 42:914–923
Haq TA, Mason HS, Clements JD, Arntzen CJ (1995) Oral immunization with a recombinant bacterial antigen produced in transgenic plants. Science 268:714–716
Idso S, Kimball B, Anderson M, Mauney J (1987) Effects of atmospheric CO2 enrichment on plant growth: the interactive role of air temperature. Agr Ecosyst Environ 20:1–10
Iwasaki T, Saito Y, Harada E, Kasai M, Shoji K, Miyao M, Yamamoto N (1997) Cloning of cDNA encoding the rice 22 kDa protein of photosystem II (PSII-S) and analysis of light-induced expression of the gene. Gene 185:223–229
Jackson MT (1997) Conservation of rice genetic resources: the role of the International Rice Genebank at IRRI. Plant Mol Biol 35:61–67
Kajiura H, Wasai M, Kasahara S, Takaiwa F, Fujiyama K (2012) N-glycosylation and N-glycan moieties of CTB expressed in rice seeds. Mol Biotechnol 54:784–794
Lai H, Chen Q (2012) Bioprocessing of plant-derived virus-like particles of Norwalk virus capsid protein under current good manufacture practice regulations. Plant Cell Rep 31:573–584
Ma JK-C, Drossard J, Lewis D, Altmann F, Boyle J, Christou P, Cole T, Dale P, van Dolleweerd CJ, Isitt V, Katinger D, Lobedan M, Mertens H, Paul MJ, Rademacher T, Sack M, Hundleby PAC, Stiegler G, Stoge E, Twyman RM, Vcelar B, Fische R (2015) Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnol J 13(8):1106–1120
Matsui T, Eguchi H, Hanami Y, Handa S, Terajima T (1971) A growth cabinet for the study on biotronics. I, Design and performance. Environ Control Biol 9:37–46
Mejima M, Kashima K, Kuroda M, Takeyama N, Kurokawa S, Fukuyama Y, Kiyono H, Itoh K, Mitsui T, Yuki Y (2015) Determination of genomic location and structure of the transgenes in marker-free rice-based cholera vaccine by using whole genome resequencing approach. Plant Cell Tiss Org 120:35–48
Nochi T, Takagi H, Yuki Y, Yang L, Masumura T, Mejima M, Nakanishi U, Matsumura A, Uozumi A, Hiroi T, Morita S, Tanaka K, Takaiwa F, Kiyono H (2007) Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. P Natl Acad Sci USA 104:10986–10991
Obembe OO, Popoola JO, Leelavathi S, Reddy SV (2011) Advances in plant molecular farming. Biotechnol Adv 29:210–222
Oyama M, Kozuka-Hata H, Tasaki S, Semba K, Hattori S, Sugano S, Inoue J, Yamamoto T (2009) Temporal perturbation of tyrosine phosphoproteome dynamics reveals the system-wide regulatory networks. Mol Cell Proteomics 8:226–231
Stoger E, Fischer R, Molone M, Ma JK-C (2014) Plant molecular pharming for the treatment of chronic and infectious diseases. Annu Rev Plant Biol 65:743–768
Thomas MD (1955) Effect of ecological factors on photosynthesis. Annu Rev Plant Physio 6:135–156
Tiwari S, Verma PC, Singh PK, Tuli R (2009) Plants as bioreactors for the production of vaccine antigens. Biotechnol Adv 27:449–467
Tokuhara D, Yuki Y, Nochi T, Kodama T, Mejima M, Kurokawa S, Takahashi Y, Nanno M, Nakanishi U, Takaiwa F, Honda T, Kiyono H (2010) Secretory IgA-mediated protection against V. cholerae and heat-labile enterotoxin-producing enterotoxigenic Escherichia coli by rice-based vaccine. P Natl Acad Sci USA 107:8794–8799
Tsuge T, Inagaki N, Yoshizumi T, Shimada H, Kawamoto T, Matsuki R, Yamamoto N, Matsui M (2001) Phytochrome-mediated control of COP1 gene expression in rice plants. Mol Genet Genomics 265:43–50
Vessey J, York E, Henry L, Raper C Jr (1987) Uniformity of environmental conditions and plant growth in a hydroponic culture system for use in a growth room with aerial CO2 control. Biotronics 17:79–94
Went FW (1943) Plant growth under controlled conditions. I. The air-conditioned greenhouses at the California Institute of Technology. Am J Bot 30:157–163
Yuki Y, Mejima M, Kurokawa S, Hiroiwa T, Takahashi Y, Tokuhara D, Nochi T, Katakai Y, Kuroda M, Takeyama N, Kashima K, Abe M, Chen Y, Nakanishi U, Masumura T, Takeuchi Y, Kozuka-Hata H, Shibata H, Oyama M, Tanaka K, Kiyono H (2013) Induction of toxin-specific neutralizing immunity by molecularly uniform rice-based oral cholera toxin B subunit vaccine without plant-associated sugar modification. Plant Biotechnol J 11:799–808
Acknowledgments
We are grateful to Drs. Teruhide Yamaguchi, Nana Kawasaki, Eiji Goto, Yoshinori Yamamoto, Kunisuke Tanaka, and Takehiro Masumura and to Mr. Ushio Nakanishi for useful discussions and technical support. This work was supported by grants from the programs of the Ministry of Economy, Trade, and Industry (H.K.) and New Energy and Industrial Technology Development Organization (NEDO) (H.K.); the Programs of Special Coordination Funds for Promoting Science and Technology and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Y.Y., H.K.); the Ministry of Health, Labor and Welfare of Japan (Y.Y., H.K.); Translational Research Network Program, Seed C (H.K.); and the Adaptable and Seamless Technology Transfer Program through Target-driven R&D (A-step) (Y.Y.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict exists.
Additional information
Communicated by F. Sato.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 4 (MPG 35176 kb)
Rights and permissions
About this article
Cite this article
Kashima, K., Yuki, Y., Mejima, M. et al. Good manufacturing practices production of a purification-free oral cholera vaccine expressed in transgenic rice plants. Plant Cell Rep 35, 667–679 (2016). https://doi.org/10.1007/s00299-015-1911-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-015-1911-9