Abstract
Fungi have been companions of mankind for millennia. Mushrooms inspired our eating culture, and yeasts and filamentous fungi were developed into highly efficient cell factories during the last 100 years to produce many products utilized in different industries worldwide. What more is to come in the next 100 years? We propose here that fungi can become important cell factories for life in space, especially regarding the filamentous fungus Aspergillus niger as the cutting-edge must-have for space travel in the twenty-first century and beyond. First, it is one of the most robust and efficient production systems used nowadays in industrial biotechnology. Second, it is a multipurpose cell factory that produces a diverse range of organic acids, proteins, enzymes and natural products. And third, it is a common fungal isolate of the International Space Station. A. niger could thus become an essential companion of astronauts for the autonomous production of food, enzymes and antibiotics during space travel. What needs to be done to achieve these visionary goals? In this chapter, we will discuss the opportunities of A. niger as a cell factory spanning from Earth to space. We summarize the current state of the art of A. niger biotechnology on Earth and discuss the general tools and technologies still in need of development to take a new step for mankind: space biotechnology.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Introduction
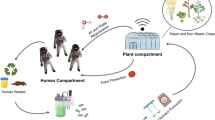
Human space exploration is envisioning long-term spaceflight missions that go far beyond low Earth orbit (LEO). However, maintaining the necessary resources on a long-duration manned mission has its many challenges. Currently, on the International Space Station (ISS), astronauts are provided with resources in resupply missions. These missions transport all kinds of resources such as food, spacecraft materials, medical supplies or scientific experiments. The frequent exchange between Earth and spacecraft will not be possible for long-duration far-reaching missions, such as a 500-day human mission to Mars or the colonization of the Moon. Moreover, the cost per kilo calculated to be around $12,600 when launching a spacecraft (Harper et al. 2016) makes it impractical to bring all the needed supplies at once. The success of space exploration requires the ability to be Earth-independent, particularly when it comes to resources. The ideal scenario would be to reduce the initial payload to a minimum and to produce the needed supplies in situ (e.g. aboard the spacecraft). Earth-independent space missions can ultimately be translated into the ability to maintain astronaut health and performance, as well as spacecraft safety. These mainly depend on:
-
1.
Protection from the isolated extreme environment of space
-
2.
Establishment of in situ resource utilization methods, e.g. production of compounds of interest such as food, materials or pharmaceuticals
-
3.
Development of sustainable, closed loop, life-support systems
In this sense, space crews should bring varied, lightweight stocks that are able to yield high biomass and/or produce compounds of interest according to the crews’ demand. Microorganisms are good examples of low-weight durable supplies, which can be cultured in different conditions according to the crew’s best interest. On Earth, the production of valuable resources relies largely on microbial biotechnology, spanning a wide variety of applications from pharmaceutics to the food industry (Barcelos et al. 2018). The exploration of deep space will likely depend on biotechnology to ensure sustainability and resource independence from Earth.
Filamentous fungi of the genus Aspergillus are key players in modern biotechnology, as they are being used in the production of compounds of interest (Cairns et al. 2018; Show et al. 2015; Meyer et al. 2015). These filamentous fungi are commonly associated with indoor-closed environments such as spacecraft where A. niger is one of the most common isolates. The ability of filamentous fungi to produce compounds of interest and their presence in space-related habitats make them attractive in long-term space missions and the colonization of other planetary bodies.
This chapter highlights the potential of fungal space biotechnology in providing new and sustainable solutions to some of the grand challenges faced by our society. Until now, reviews on biotechnology in space have focused on yeast, mammalian cell culture, tissue culture and engineering, protein crystallization or technologies applied to biology in space (such as PCR or sequencing technology) (Karouia et al. 2017; Betzel et al. 2017; Grimm 2017; Ronnie 2013). Here, the term biotechnology will follow the definition from the Organisation for Economic Co-operation and Development (OECD) as “…the application of science and technology to living organisms as well as parts, products and models thereof, to alter living or non-living materials for the production of knowledge, goods and services”. This chapter gives a historical perspective on biotechnological attempts in space and addresses highlights of modern fungal biotechnology on Earth, future challenges to be met in fungal-based space biotechnology and spaceflight sustainability by focusing on lessons learned from mankind’s aviation activities.
2 A Historical Perspective
Soon after the space age of the 1950s and 1960s, scientists questioned the effect of space and the spaceflight environment on living systems. With that, space biology and microbiology rapidly became an important subject, addressing one main question: how is life affected by the space environment? The two space environmental factors with the most impact on living systems are radiation and microgravity (or near weightlessness). The term “milligravity” would be more adequate, but the term microgravity is used for historic reasons (Boudreault and Armstrong 1988).
Exposing microorganisms to real microgravity was (and is) costly and time-consuming. Therefore, ground-based facilities and devices for simulation of microgravity (Herranz et al. 2013) were developed throughout the years. Experiments with simulated microgravity are validated through comparison with real microgravity experiments, when possible. This section provides a brief historical review on the development of (micro) biology experiments with a particular focus on (1) the knowledge gained regarding fungal adaptation to the space environment, (2) the culturing hardware used for (bio)technology experiments in space and (3) the platforms used to expose the test organisms to simulated or real microgravity.
2.1 Early 1970s–Mid-1980s: Exposing Living Systems and Searching for Effects
NASA’s Skylab programme marked the beginning of space biology in the early 1970s, testing the effects of zero gravity on human embryonic lung cells under microgravity. The equipment they used was called Woodlawn Wanderer Nine and can be considered as the first bioreactor in space, where cell cultures were grown in fully automated perfusion chambers with a medium exchange every 12 h (Montgomery et al. 1977, 1978). In the early 1980s, equipment such as the EURECA (European Retrievable Carrier) or LifeSat (a reusable reentry satellite) enabled unmanned space biotechnology experiments (Nellessen 1995; Halstead and Morey-Holton 1990). Later, the European Space Agency developed the so-called Biorack facility in a joint effort with the NASA for the Spacelab programme that carried scientific payloads on manned shuttle flights from 1983 to 1998 (Manieri et al. 1996). Biorack was used for the first time in 1985, allowing multiple users to perform experiments with various organisms, e.g. small plants, insects and yeast cells (Walther et al. 1994). It was equipped with two incubators: one for 18–30 °C and one for 30–40 °C, one freezer of −15 °C, a cooler with a temperature of 4 °C and centrifuges (Oser 1984). The first experiments with fungi in both real and simulated space conditions started also in the 1970s. Polyporus brumalis was flown in 1977 on the Salyut 6 mission, revealing that sporulation is severely affected, but mushroom formation is independent from gravity (Moore 1996). More on cultivation hardware and platforms for real and simulated microgravity are provided in Sect. 4.
2.2 Mid-1980s–Mid-1990s: Discovery and Characterization of Cellular Processes
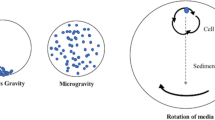
The slime mould Physarum polycephalum was tested in spaceflight mission experiments in 1985 and 1992 and in simulated microgravity experiments on Earth using a fast-rotating clinostat. The clinostat (Fig. 18.1) was developed in the 1960s to enable experiments with reduced gravity (Sobick and Sievers 1979). These approaches showed, among other things, that the contraction period of plasmodial strands decreased under space conditions (Block et al. 1994). Moreover, an experiment with the model fungus Neurospora crassa analysed the effect of microgravity on circadian rhythms, using a space shuttle flight, but no effect on appearance or duration of circadian rhythms was observed (Aplatov 1992). Another experiment showed minor deviations compared to conditions on Earth, but simulated microgravity experiments with the clinostat were not able to reproduce those (Ferraro et al. 1989). Also in 1992, A. niger, another highly pigmented spore-forming fungus, was found to be resistant to simulated space conditions (i.e. low temperature, high vacuum and proton irradiation). Spores of A. niger were able to survive low temperature and high vacuum for 24 h with a survival rate of 48%. When exposed to protons corresponding to solar cosmic rays for 250 years in near Earth orbitals, 28% of A. niger spores survived (Koike et al. 1992).
(a) Clinostat principle of operation: microgravity is simulated by continuously rotating around one axis, perpendicular to the direction of the gravity vector. The continuous rotation prevents cell sedimentation, which translates to a functional simulation of the microgravity environment. (b) Clinostat with Petri dishes, for solid media cultivation. (c) Clinostat adapted for slide-flask rotation, for liquid media cultivation. (d) BioServe’s Clinostat. Credit: BioServe Space Technologies
Until 1992, most experiments related to cell cultivations were done in small vessels or even syringes at either ambient cabin temperature or in an incubator allowing for batch analysis only. Mixing of liquids was not possible, and parameters such as dissolved oxygen, carbon dioxide or pH could not be controlled. In addition, the values for temperature, vibration or radiation could not easily be monitored (Bjursted 1992). The thin film bioreactor developed by Hughes-Fulford and Scheld in 1989 was meant to study the impact of space conditions on cellular functions and could be filled with 6 ml of medium. A fluid management system with four rotary valves allowed for removal and refilling of the medium, as well as in-flight cell fixation. However, mixing of the medium was not possible (Hughes-Fulford and Scheld 1989). The space bioreactor from NASA’s Johnson Space Center was designed to fit into a Biorack container with capacity for 500 ml culture volume. In this bioreactor, aeration was achieved by oxygenation of the medium prior to perfusion through the bioreactor (Bjursted 1992). The same laboratory developed the rotating wall vessel for microcarrier cell culture. As the name suggests, the solid body of the vessel rotates around its horizontal axis. Aeration is done by a silicone rubber membrane inside the vessel. The system was tested using hamster kidney fibroblasts, and cell densities of 107 cells/ml were reached (Schwarz et al. 1992).
Furthermore, bioreactors were also developed for the purpose of nutrient recycling, meaning the conversion of biomass with a controlled ecological life-support system (Westgate et al. 1992). One of the first dynamic cell culture systems was developed in Switzerland in 1988 and was operated with an internal osmotic pump to continuously supply the system with fresh medium (1 μl/h). The design allowed up to 7-day-long experiments with a working volume of 200 μl. The device was tested on hamster kidney cells in batch and perfusion mode (Gmünder et al. 1988). A phase-separated membrane bioreactor was developed in the late 1980s as a controlled ecological life-support system. The liquid phase was separated from air via silicone rubber tubing. Given the fact that the tubing is permeable for oxygen and carbon dioxide, oxygen could diffuse into the medium, and carbon dioxide was able to diffuse into the tubing. The initial setup was a vessel of about 9 l of total volume of which roughly 10% were occupied by silicone tubing. The bioreactor was tested with Saccharomyces cerevisiae and stirred using a marine impeller. However, oxygen became limiting quickly (Petersen et al. 1989). Therefore, changes were made in the design to enable higher oxygen transfer rates. By increasing the total volume and the percentage of tubing, oxygen transfer rates increased. Changing the gas and liquid phase (liquid now in the tubing) improved the oxygen transfer rate. According to their calculations, a 1 l bioreactor would be sufficient to recycle the carbon waste stream of ten astronauts (Villeneuve and Dunlop 1992). Two years later the group published their findings of the reactor operating in a parabolic flight with the conclusion that oxygen mass transfer and mixing are not affected by gravity (Villeneuve et al. 1994).
Continuous cultivation was published in 1994 by the group of Cogoli for the cultivation of S. cerevisiae and Candida tropicalis, and sensors to measure pH, temperature and redox potential were available (Walther et al. 1994). A sample of 1 ml could be taken, and the whole system could run for 8 days with a final volume of 3 ml. In 1995, the Second International Microgravity Laboratory mission was performed aboard a space shuttle, using Biorack (Manieri et al. 1996), studying blood cells, isolated tissue cells, bacteria, plant cells, protoplasts, yeasts, sea urchin larvae, fruit flies, toad eggs, isolated mouse bones and plant seedlings.
2.3 Mid-1990s–Early 2000s: Microgravity as a Research Topic for Biology and Biotechnology
The Shuttle-Mir programme took space biology to a new level, housing several scientific experiments in microgravity from 1995 to 1998 (NASA 2007). A 4-month-long cultivation of cartilage was done on the Russian space station Mir from September 1996 to January 1997 (Freed et al. 1999). The reactor was a slow turning lateral vessel with a volume of 125 ml. About 50–100 ml of medium was replaced by fresh medium once a day. Gas exchange was done every 6 h by recirculating medium through a silicone membrane. Cultivation was done at 37 °C. The pH was kept between 6.9 and 7.4, and dissolved oxygen could be maintained between 71 and 127 mm Hg (0.09–0.17 bar). Differences were seen in the morphology and wet weight: the cartilage cultivated in space was spherical, unlike the discoid structure observed on Earth; roughly half of the collagen wet weight was found for the cartilage cultivated in space compared to Earth. Nevertheless, these differences could also be attributed to environmental conditions during launch, landing or, for instance, cosmic radiation (Freed et al. 1999).
Experiments on mushroom-forming fungi like Polyporus brumalis, Lentinus tigrinus, and Coprinus cinereus were also performed to study the effect of spore formation in clinostat experiments. A validation of these clinostat experiments in space was published for P. brumalis, revealing absence of spore-bearing tissue in the fruiting body of P. brumalis under space conditions (Moore 1996).
S. cerevisiae was also cultivated in a miniaturized bioreactor for continuous cultivation. Cultivation was possible for 8 days, and pH, temperature, flow rate and pressure were measured using microsensors. Glucose consumption, alcohol production and cell morphology were similar between samples taken in space and for the control on ground (Walther et al. 2000).
2.4 Early 2000s–Onwards: The International Space Station as a Research Laboratory
Along the years, more platforms for simulation of microgravity have been made available, e.g. sounding rockets, parabolic flights and drop towers (Herranz et al. 2013). These, however, can only provide microgravity for a short period of time. Orbital spaceflight was still the preferred platform for long-duration real microgravity experiments, turning the ISS into a laboratory. Inhabited continuously since November 2000, the ISS has been the home of hundreds of biological experiments and other scientific experiments. The Cell Culture Unit flight hardware was developed for the ISS by Payload Systems Inc. in collaboration with MIT and NASA’s Ames Research Center. The unit can operate in microgravity and artificial gravity. Volumes of 3, 10 and 30 ml can be used, and the system provides a recirculation loop, a gas exchanger silicone membrane, reservoirs and waste disposal as well as an automatic sampling system and storage of samples. Temperatures can be controlled between 4 and 45 °C, and light can be used at a variety of wavelengths and intensities. In addition, the system is equipped with optical biosensors measuring oxygen and pH. Different human cells like myoblasts or osteoblasts were tested in that system and also S. cerevisiae and Euglena gracilis. S. cerevisiae was, for instance, cultivated at 23 °C in a gas-permeable 30 ml reservoir bag for 4 days. A longer cultivation was not possible due to gas bubbles blocking perfusion of the medium (Freed and Vunjak-Novakovic 2002).
In 2004, studies on microbial antibiotic production by Streptomyces plicatus, a spore-forming, filamentous bacterium, were performed (Benoit et al. 2006) and coupled with the development and optimization of a spaceflight reactor called Multiple Orbital Bioreactor with Instrumentation and Automated Sampling (short: MOBIAS). The reactor was a permeable silicone culture bag placed in an alumina mesh case. The bag allowed for 40 ml of medium, waste could be removed, and samples could be taken periodically and either fixed or preserved. Actinomycin D production was followed for 72 days in fed-batch mode. In the first 12 days, actinomycin D levels exceeded ground control (by 28.5%); however, all samples taken from 12 to 72 days revealed that actinomycin D concentration was higher on ground than in flight cultures (Benoit et al. 2006).
The project EXPOSE (EXPOSE-E, EXPOSE-R and EXPOSE-R2) from 2008 to 2016 studied the effect of radiation on different fungi, such as A. sydowii, A. versicolor, Penicillium aurantiogriseum and P. expansum (Horneck and Zell 2012; Horneck et al. 2014; Rabbow et al. 2017). In EXPOSE-R all fungal spores survived an exposure for 22 months, with P. aurantiogriseum displaying the lowest survival rate. The morphology was analysed for A. versicolor and A. sydowii, and both did not show morphological changes (Novikova et al. 2015). In 2010, an ESA experiment examined growth and survival of potential fungal spacecraft contaminants on the ISS (Hasegan et al. 2011). Spores of Ulocladium chartarum, A.niger, Basipetospora halophile and Cladosporium herbarum were left at microgravity for 5 months, and germination of spores, colony-forming units, growth rates as well as sporulation were analysed after the flight. Experiments examining spreading and adhesion of fungi on wafers made from iron, silica or polycarbonate were also performed, revealing that spores of A. niger and U. chartarum were ~90% viable on all wafer materials. Additional experiments were done with U. chartarum, showing the fungus’ ability to sporulate in space, albeit in a decreased number when compared to ground experiments. Furthermore, a reduced proportion of aerial mycelium was seen in a 14-day spaceflight (Gomoiu et al. 2013). In 2011, A. terreus spores were exposed to simulated solar vacuum determining differences in survivability, morphology and phenotype (Sarantopoulou et al. 2011). In 2015, the survivability of two antarctic cryptoendolithic fungi, Cryomyces antarcticus and C. minteri, to simulated Martian conditions on board the ISS was also tested. Both fungi were exposed to these conditions for 1.5 years, and colony-forming units were determined afterwards. Both strains showed with less than 2% a very low survival (Onofri et al. 2015).
Further studies with S. cerevisiae consisted of a liquid bioreactor cultivation for 3 days in a low-shear microgravity environment in the BioLab of the Columbus laboratory of the ISS. Transcriptomic analyses revealed that cell—cell adhesion (flocculation) supports yeast survival under adverse conditions by enhancing the mating efficiency of cells (Goossens et al. 2015). In summary, experiments with fungi have been done, and experimental equipment for use in space has been developed. However, most experiments are related to cultivation of fungi on solid medium, and compounds of interest, as described in Sect. 3, are produced mostly in liquid culture. In the next section, the applications of fungal biotechnology will be described detailing A. niger as the pioneer of modern fungal biotechnology. Recent developments on fungal research in space as well as future challenges of fungal space biotechnology will be addressed in Sect. 4.
3 Fungal Biotechnology on Earth
3.1 Application Potentials of Fungal Biotechnology: A Brief Survey
For millennia, fungi have been companions of mankind. Mushroom-forming fungi are still collected from forests and used in our eating culture because of their unique taste or even due to their curative properties for the disease treatment. Yeasts were unwittingly domesticated through uncontrolled and spontaneous fermentations to make food products such as bread, wine, beer and chocolate. Modern biotechnology of fungi, i.e. their controlled cultivation in bioreactors, was born in 1919, when Pfizer started to exploit the ability of the filamentous fungus A. niger to overproduce and secrete citric acid. Since then, this organic acid has been produced and applied as preservative, acidulant, flavour enhancer and antioxidant in food, pharmaceutical and cosmetics industries; A. niger has since become a multipurpose production platform not only for citric acid but also for proteins, enzymes and natural products (Cairns et al. 2018; Show et al. 2015; Meyer et al. 2015).
With the advent of molecular cloning techniques in the late 1970s and the scientific progress in genetic engineering of fungal cell factories during the last two decades, it became possible to rationally design yeast and filamentous fungi to produce more advanced products (Nielsen et al. 2013; Meyer et al. 2016; Jin and Cate 2017; Baker 2018; Cairns et al. 2018). For example, bioethanol, organic acids, biofuels, platform chemicals, pharmaceuticals, antibiotics, proteins, enzymes and vitamins are all fungal-based products to name a few (Table 18.1). Commercial and medicinal mushrooms nowadays are not collected from forests anymore but are produced on lignocellulosic agricultural waste products. Notably, not only are they of interest for food and pharmaceutical applications but they are currently also studied as novel sustainable alternatives to produce packaging, construction material and even textiles from their biomass (Nai and Meyer 2016). Finally, mycoremediation is another important biotechnological application of fungi. Here, mainly mushroom-forming fungi are used to degrade or sequester toxic pollutants including metals from the environment (Chatterjee et al. 2017; Kapahi and Sachdeva 2017).
Fungi are thus producers, decomposers and recyclers and will likely become important drivers for the closed loop-based circular economy (Stahel 2016). Its business models “reuse” and “recycle” should, of course, also apply to the fungal biotechnology of the future. One exemplary scenario would be that spent mushroom substrate leftover from lignocellulosic plant-based biomass used to cultivate commercial mushrooms could be reused as substrate for other mushroom-forming fungi to produce textiles and construction materials (Grimm and Wosten 2018). If these textiles and materials are not of use anymore, they can be reused as substrate for various fermentation processes utilizing filamentous fungal cell factories such as A. niger to produce platform chemicals, enzymes or antibiotics. Hence, filamentous fungi are well suited per se for sustainable production of goods on Earth and also in space. Realizing the holistic concept of a circular economy is not only of crucial importance for a sustainable future of mankind on Earth [for details see (Stahel 2016)], but also it should be a paradigm for any space biotechnological endeavours of humanity.
In the following section, it is proposed that A. niger could become an essential companion of astronauts for the autonomous production of food, enzymes and antibiotics and an important player for use in terraforming efforts. This fungus is one of the most robust and efficient production systems used in biotechnology. As a multipurpose cell factory producing a diverse range of organic acids, proteins, enzymes and natural products on Earth (Table 18.1 and see below), it holds great promise to become exploited in the near future for space biotechnological purposes as well.
3.2 Aspergillus niger, the Pioneer of Modern Fungal Biotechnology
“Living with limits. Getting more from less. Producing commodities and high-value products from renewable resources including waste” (Meyer et al. 2015). This is not only the driving force and quintessence of the bioeconomy and the circular economy; it also outlines the lifestyle and product portfolio of the saprophyte A. niger. Because it first digests and then ingests nutrients from the environment, A. niger secretes high amounts of enzymes hydrolysing biopolymers such as starch, (ligno-) cellulose, pectin, xylan, proteins and lipids. This feature is exploited by many biotech companies to manufacture enzymes for use in different industries such as paper and pulp, food and feed, laundry and textile and biofuel (Table 18.1); for more details, see references in Meyer et al. (2015). Further, to outcompete cohabitant bacteria and fungi in its natural environment, it is a naturally good secretor of organic acids including citric acid, oxalate and gluconate with applications in food, beverage, pharmaceutical and cosmetics industries (Andersen et al. 2009). Also, it has been successfully redesigned to overproduce the building block itaconate used for the production of several synthetic polymers (Hossain et al. 2016) and galactaric acid, the building block for nylon (Kuivanen et al. 2016).
A. niger’s metabolic diversity has been expanded to overproduce the secondary metabolites enniatin, bassianolide and beauvericin with high pharmaceutical application potential due to their antibacterial, antifungal and anticancer activities (Boecker et al. 2018). Hence, A. niger is a powerful multipurpose cell factory producing a wide range of products, which—in such a diversity and efficiency—are not matched by any other fungal cell factory. Most importantly, highly sophisticated genetic engineering, systems biology and synthetic biology tools have been established during the last decade to understand and reprogramme the genetic and metabolic landscape of A. niger. These tools include targeted genetic transformation systems ranging from ku70 to CRISPR/Cas9 technologies (Meyer et al. 2007; Carvalho et al. 2010; Kuivanen et al. 2016; Sarkari et al. 2017; Zheng et al. 2018b, a; Song et al. 2018); highly controlled mono- and polycistronic Tet-on-based expression systems ensuring high product titres (Geib and Brock 2017; Schuetze and Meyer 2017); a well-annotated genome sequence (Pel et al. 2007); a high-quality transcriptomic database, most recently updated (Schäpe et al. 2018); and optimized cultivation protocols for stirred-tank and wave-mixed bioreactor cultivations ensuring reproducible growth, morphology and physiology of A. niger (Kurt et al. 2018). Notably, the medium composition and environmental conditions to which A. niger can be subjected and cultivated span a broad spectrum too. It can be cultivated over a wide range of temperatures (10–50 °C), pH (2–11), salinity (0–34%) and water activity (0.6–1), under nutrient-poor or nutrient-rich conditions (Meyer et al. 2011).
Survival under low gravity and cosmic irradiation coupled with flexible culture conditions, as well as a versatile substrate and product spectrum of A. niger suggests that this fungus would indeed be of high interest to be studied further for any potential space biotechnological applications. In the next section, we will thus discuss the tools and technologies, which have to be developed for A. niger to take a new step for mankind: fungal space biotechnology. We further propose that A. niger serves as an excellent model system to examine the influence of cosmic radiation and low gravity on genetic, physiological and morphogenetic processes on eukaryotic systems.
4 Future Challenges in Fungal Space Biotechnology
Despite the efforts in studying the effect of the space environment in fungi and in establishing fungal cultures in the spaceflight context efforts (Sect. 2), there is still lack of information regarding fungi and fungal cultures in the space environment. Challenges to overcome span four interdependent areas: (1) knowledge on fungal biology in the space environment; (2) design of spaceflight experiments; (3) adequate fungal culturing methodology, including the hardware for simulated and real microgravity; and (4) high-throughput research and self-sustained life-support systems in space.
4.1 Fungi in the Space Environment
Filamentous fungi such as A. niger are naturally and commonly found as part of the microbiota of spacecraft-associated environments: aboard Mir and the ISS (Makimura et al. 2001; Checinska et al. 2015; Venkateswaran et al. 2014; Be et al. 2017) as well as in spacecraft assembly facilities, also known as clean rooms (Checinska et al. 2015). Galactic cosmic ray exposure is higher on the ISS (registered 286 μGy/day in 2016) than on Earth (Sato et al. 2018; Berger et al. 2017) and even higher when travelling to the Moon or Mars. Thus, exposure to radiation needs to be considered particularly when thinking of long-duration missions into deep space (out of Earth’s magnetic field), as it is the case for a mission to Mars. This is because radiation-induced mutations can lead to functional changes, particularly in microbial species on board, due to their short generation lifetime (Romsdahl et al. 2018a; Meyer et al. 2007). For instance, a study aboard Mir showed that a bacterial gene (repsL) cloned in the yeast S. cerevisiae had a mutation rate 2–3 times higher in spaceflight than on ground (Fududa et al. 2000). On the EXPOSE-E experiment PROTECT, Bacillus spp. spores were shown to have mutation rates four times higher after exposure to outer space conditions for 1.5 years (Moeller et al. 2012). A. niger was shown to be considerably resistant to UV radiation even when defective in DNA repair (Carvalho et al. 2010).
Assuming that proper radiation shielding is provided, the predominant environmental factor of spaceflight is microgravity—also known as weightlessness. Once settled on a new planetary body such as the Moon or Mars, these will have a gravitational regime different from the one on Earth (1 g). All living systems will thus be exposed to partial gravity (1/6 g on the Moon and 1/3 g on Mars). As gravity affects fungal growth rate, sporulation, gene expression and proteome, it can lead to different quality and quantity of compounds produced (Taylor 2015; Najrana and Sanchez-Esteban 2016). In filamentous fungi, the tip of a growing hypha is thought to be the most active region of protein secretion, and a strong correlation between sustained polarized growth and protein secretion at the hyphal tip is generally accepted (Taheri-Talesh et al. 2008; Takeshita et al. 2014; Wosten et al. 1991; Robertson 1965).
Spores of A. niger were shown to maintain viability aboard the ISS regarding ability for dispersal, adhesion to spacecraft material and growth (and subsequent biodeterioration) on the different surfaces (Klintworth and Reher 1999; Alekhova et al. 2005). Characterization of different Aspergillus strains isolated from the ISS, e.g. A. fumigatus (Knox et al. 2016) and A. niger (Romsdahl et al. 2018b), revealed significant spaceflight-induced changes. For instance, more pigmentation, increased growth rate and differences in proteome profiles (mainly stress resistance and nutrient acquisition) were observed, when compared with the Earth-type strains. Characterization of A. niger showed an overall increased secondary metabolite production (Romsdahl et al. 2018b). Draft genome sequences of fungal strains exposed to microgravity at the ISS, among them A. niger, were made available and have been published (Singh et al. 2017). Moreover, characterization of A. nidulans grown on board the ISS was recently reported. Whole genome sequencing revealed that ISS conditions altered the A. nidulans genome in specific regions, and differential expression of genes involved in stress response, carbohydrate metabolic processes and secondary metabolite biosynthesis was observed (Romsdahl et al. 2018a). Moreover, a study growing Penicillium rubens in low-shear modelled microgravity showed increased expression of the gene coding for the acyl-coenzyme A: isopenicillin N acyltransferase—an enzyme involved in penicillin biosynthesis (Sathishkumar et al. 2015, 2016).
Furthermore, research on fungi in indoor-closed space habitats is being done with respect to microbial contamination and diversity. For example, the human presence was also shown to impact fungal diversity of inflated lunar/Martian analogue habitats (Blachowicz et al. 2017). A spaceflight experiment has also been developed to test the growth of the filamentous fungus P. rubens on different spacecraft-relevant surface materials (Zea et al. 2018).
Transcriptomics, proteomics, metabolomics and compound production studies are crucial to provide valuable insights into the adaptation mechanism of filamentous fungi to spacecraft environments and their potential role in space biotechnology. It is, however, highly dependent on the ability to grow and cultivate fungi under real microgravity. The lack of opportunities for spaceflight experiments, as well as its high cost, is one of the main challenges to overcome. This will enable new studies as well as validation of previous studies performed in simulated microgravity.
4.2 Designing Microbial Spaceflight Experiments
Experiments aboard the ISS are dependent on different factors when compared to ground laboratory work, and many space agencies prepare user guides for low gravity platforms (ESA 2014; NASA 2010; Inokuchi et al. 2007). Currently most of the microbial samples set on board a spacecraft need to be brought back to Earth to be analysed. This means that samples need to be prepared for three different time points: pre-, during-, and postflight. Due to cost, security and safety, time or environmental constraints, space experiments are usually kept as simple and as flexible as possible.
Most spaceflight experiments are highly dependent on the help of the astronauts, such that increasing experiment complexity (e.g. number of samples or steps) means more astronaut time allocated. There is also an increasing number of proposals for ISS experiments, not only by researchers but also by students from different universities worldwide (Nadir and Sato 2017; Boeing and miniPCR; ESA). Safety measures also need to be taken into account when designing a spaceflight experiment, to prevent contamination and other possible hazards and to guarantee the biosafety of the astronauts and the spacecraft itself. This is done through the use of standard operation procedures (SOPs) and expert revision and evaluation of the experiment biosafety (Pierson and Ott 2011; NASA 2015a). In a microbial cultivation experiment, it implies safety of the culturing hardware, safety of the chemical solutions used (e.g. culturing media, fixative, etc.) and safety of the test organism (Basu et al. 2017). Thus, an experimental payload necessitates different levels of containment according to the safety requirements (Coil et al. 2016). It is also important to note that “uploading” and “downloading” any resource from the ISS, including scientific experiments, is highly dependent on commercial resupply missions, such as the ones now provided by SpaceX aboard the Dragon cargo spacecraft (SpaceX 2017), by Energia aboard Progress-MS cargo spacecraft (Zak 2018) or by JAXA aboard HTV cargo spacecraft (NASA 2018b). Therefore, an experimental timeline will likely need to adjust to the resupply timeline, kept in “standby” in the cold (4 °C or −20 °C) or by being chemically fixed (Zea et al. 2018). Postflight analysis is also limited, as changes in gene expression can happen in seconds, and RNA or proteins to be analysed may degrade, even in fixed/conserved samples. If growth of the test organism is not stopped during spaceflight, return to Earth may imply a quick readaptation to terrestrial conditions and shadow any spaceflight-related effect. Besides, the forces exerted on samples during reentry and return to Earth may affect them. Moreover, follow-up experiments must wait for a new flight opportunity to be tested.
4.3 Cultivation Hardware for Simulated and Real Microgravity
Scientists have long raised the awareness for the need of appropriate hardware to be developed in collaboration, and that specific biological objects should be selected as potential candidates for bioprocessing in space (Cogoli and Tschopp 1982). There is still a great amount of valuable information to be gained from experiments aboard a spacecraft in low Earth orbit, such as the ISS. But the future of space exploration envisions new and more challenging space environments. For instance, the lunar gateway (also known as Lunar Orbital Platform-Gateway or Deep Space Gateway) being developed to orbit the Moon, will be one step further in Earth-independent resource management (NASA 2017a). In any of these cases, the demands of culturing hardware and conditions that enable space biotechnology research are high. Section 2 discussed several simulated and real space hardware options for cell cultivations developed over the years. However, most of them have not been developed (or tested) specifically for filamentous fungal cultures, and only a few were tested in experiments addressing compound production. Thus, to establish (fungal) biotechnology in space, one of the challenges to address is to obtain the appropriate cultivation hardware. This can be achieved by either (1) adapting existing and available hardware to address future space biotechnology scenarios or (2) developing new hardware specifically for new purposes. There are two main types of hardware to be considered: hardware for simulated microgravity experiments on Earth and hardware for real microgravity experiments aboard spacecraft/satellites.
4.3.1 Simulated Microgravity
Many devices have been developed to simulate microgravity, such as the random positioning machine (or 3D clinostat) (van Loon 2007), the rotating wall vessel bioreactor (RWV) and the clinostat. Both the RWV and the clinostat were already being used in the early 1990s as the first RWV bioreactor flown to space took place in 1991 with mammalian cells (Walther et al. 2000) and continue to be the two main devices used today. The RWV bioreactor was initially invented by the NASA for 3D-cell cultures but was later applied for microgravity simulations to what is now known as high aspect ratio vessel (HARV), which allows for cultivations of liquid cultures up to 50 ml. In turn, the clinostat can harbour several different culturing flasks (liquid media) or solid media Petri dishes. A detailed review by Klaus (2001) on the use of clinostats and RWV to simulate microgravity stresses that these devices do not actually remove the force of gravity (g) but rather average gravity to near zero by the constant rotation of the samples. Both devices provide “functional weightlessness” for suspension cell cultures through the ability to simulate spaceflight parameters that affect particle sedimentation and buoyancy. Here, the RWV bioreactor was considered optimal for culturing cells or tissues, as it enables gas and medium mixing, but simulates weightlessness to a lesser degree, whereas a clinostat is able to reproduce an environment similar to that which would be experienced within an unstirred, undisturbed container in actual weightlessness (Klaus 2001)—Fig. 18.1. For simulated microgravity, the company Synthecon, in the United States, has readily available, off-the-shelf, autoclavable or disposable HARVs (Synthecon 2019).
Recently, the use of a fast-rotating clinostat using slide flasks was reported for simulated microgravity experiments with adherent cells (Herranz et al. 2013). This is particularly interesting because A. niger spores can be considered adherent cells when inoculated into a fully filled flask, allowed to settle and then incubated under simulated microgravity. The HARV device has also been used to study how low-shear modelled microgravity affects phenotype, gene expression and chitin metabolism in P. rubens. Microgravity-induced changes were noted in cell wall transporters, but penicillin production was not hampered (Sathishkumar et al. 2014, 2015, 2016). Also the fungistatic activity of a chitosan derivative against A. niger was tested under HARV simulated microgravity, to address potential disinfectants in space stations for removal of fungal contaminants (Devarayan et al. 2015).
4.3.2 Real Microgravity
Most of the devices for cultivation in real microgravity are developed by research centres, space agencies or individual labs in the field of space microbiology. This is because spaceflight experiments are usually funded by a space agency (the ESA, NASA, JAXA, ASI, etc.) and often include the development of specific hardware. This results in a wide range of similar devices that can be used to culture microorganisms in real microgravity (i.e. aboard the ISS). Earth-gravity simulating controls, in space, have also been developed. For instance, the KUBIK centrifuge is used for 1 g control experiments aboard the ISS. The different culturing hardware is usually placed in platforms for biological research on the ISS, for instance, national/agency experiment facilities such as ESA’s BioLab in the Columbus module. The BioLab (Biological Experiment Laboratory) facility is divided into two sections: the automated section or core unit and the manual section, designed for crew interaction with the experiments. The core unit consists of a large incubator, two centrifuges, a microscope, a spectrophotometer, a sample-handling mechanism and automatic temperature-controlled stowage, to keep small amounts of sample (ESA). JAXA’s module on the ISS—Kibo—provides a protein crystallization research facility as well as a cell biology experiment facility housing up to six standard canisters. It allows for temperature, humidity and CO2 settings (Ishioka et al. 2004). The Center for Advancement of Science in Space (CASIS) of the United States has a list of hardware available for biological and biotechnological experiments in spaceflight conditions. NASA’s Ames Research Center has developed a series of incubation cassettes, which share only the gas supply, allowing cell cultures to be maintained in a fluid flow path that provides medium circulation, gassing, introduction of new medium and removal of used medium and automated sampling and solution injection. Another hardware that has been used in multiple space biology experiments is the Biological Research in Canisters (BRIC). The BRIC typically consists of housed Petri dishes or culture chambers, contained within aluminium cylinders or boxes. It was used to study the effects of the microgravity environment on Bacillus subtilis, Staphylococcus epidermidis (Fajardo-Cavazos and Nicholson 2016) and Arabidopsis thaliana (Basu et al. 2017) and was part of a recent experiment with eight different fungal species (Venkateswaran 2018). Notably, there is considerably increasing involvement of the private sector in the area of cell culture in space, as companies are hired by the space agencies to develop culturing hardware specific for the spaceflight context. A few examples are given in Table 18.2.
BioServe Space Technologies has also developed culturing hardware for spaceflight, including the Group Activation Pack (GAP) and Fluid Processing Apparatus (FPA), of which over 5000 and 600 have been used in space to date, respectively. The FPA is similar to a small test tube consisting of a glass barrel with silicone septa; it may have gas-permeable membranes, and it allows sequential mixing of up to four different fluids within a sterile environment. For example, the FPA can harbour growth medium, inoculum, antibiotic and fixative in four separate compartments that can be mixed during an experiment (Zea et al. 2017; Crabbe et al. 2013; Coleman et al. 2008). More recently, the BioCell culturing hardware was developed, which can be modified for different culturing volumes (either single- or multiwell) and can be used both in simulated and real microgravity environments—for simulated microgravity experiments, it can be placed in the clinostat (Fig. 18.1). Experiments with BioServe’s BioCell were already performed studying spaceflight-induced changes in gene expression in human fibroblast cells (Lu et al. 2017). The OptiCell™ cell culture system was also used to culture yeast under spaceflight conditions (Hammond et al. 2012; Nislow et al. 2015).
Kayser Italia has also developed hardware for spaceflight biological experiments, in cooperation with different institutions (Vukich et al. 2013). For example, the BIOBOX is a programmable space-qualified incubator that allows fully automated execution of biological experiments and has participated in different space missions between 1992 and 2011. Different types of cultivation hardware have been developed since 2003 and were applied to yeast batch or solid culture and bacteria aerobic and anaerobic culture, among others. Kayser Italia is also developing a biomining reactor prototype as part of ESA’s BioRock project, planned to study microbe-mineral interactions in space (Loudon et al. 2017).
KIWI-microgravity has also developed various types of culturing hardware. The type IV hardware, for instance, was developed by Airbus and the German Aerospace Center (DLR) and was used on the ISS SpaceX-3 mission within the project CellBox (KIWI-microgravity 2017). For more information, the Center for Advancement of Science in Space (CASIS), in the United States, has a list of facilities available for biology and biotechnology experiments (NASA 2019b). There are also several companies providing space application services, such as NanoRacks (2019) or ESA’s ICECUBES (ICECUBES), which have platforms and services available for scientific experiments aboard the ISS.
Earth-based hardware is also undergoing development to test different culturing conditions that could be applicable for spaceflight. For example, the low-shear single-use bioreactor called Cell-tainer was used to cultivate A. niger under wave-mixed conditions (Kurt et al. 2018). These might be an interesting alternative for use at the ISS, as bag systems are preferred in the spaceflight context. In the future, miniaturized cultivation in microtiter (or smaller) scale should be achieved for high-throughput screenings or others (Karouia et al. 2017; Cairns et al. 2018). However, for the production of compounds of interest, the main challenge will be to cultivate at a large scale. Most culturing hardware available today is below the 100 ml cultivation volume. This is because the higher the volume, the more chances of bubble formation. However, for compound production and sustainable resource provision in long-term missions, large-scale cultivation is necessary.
4.4 Life-Support Systems and High-Throughput Research
Recent research on how to sustain human life in an isolated environment show that it is highly dependent on the conversion of waste material to food and on nitrogen cycling (Clauwaert et al. 2017). Providing that knowledge on how to design safe and accurate spaceflight microbiology experiments in LEO and beyond increases, research in space should be sustainable, and a natural part of the spacecraft habitat. Two main kinds of life-support systems have been discussed: the bioregenerative life-support systems and the closed ecological life-support systems. Establishment of the latter by a miniaturized bioreactor with microalgae was tested during spaceflight, showing that algae can adapt to the space environment easily, which may be valuable for future designs of more complex bioreactor and controlled ecological life-support systems (Wang et al. 2006). In every case, plants have been considered as a fundamental part (Arena et al. 2012).
Up until recently, the ISS had an environmental control and life-support system (ECLSS). Since September 2018, the Advanced Closed Loop System (ACLS) (ESA 2018b) was established. The ACLS recycles carbon dioxide on the station into oxygen. For years oxygen on the ISS was extracted from water that is brought from Earth, a costly and limiting drawback. The new system recycles half of the carbon dioxide thereby saving about 400 l of water that needs to be launched to the International Space Station each year. The ACLS was built by Airbus as International Standard Payload Rack which is about 2 m high, 1 m wide and 85.9 cm deep. Installed in the US Destiny module, it can generate about 50% of the water needed for oxygen production on the space station (ESA 2018a).
Long-duration deep space missions, such as to Mars, are expected to use matured and upgraded versions of ISS life support (Jones et al. 2014), called deep space life support. Testing the establishment of bioregenerative life-support systems for long-term crewed missions to the Moon or Mars was done, achieving 20.5% nitrogen recovery from urine, oxygen and water recycling, 55% of food regeneration, 41% solid waste degradation and insect in situ production during the 105-day long experiment (Fu et al. 2016). In this study, microorganisms were used to degrade plant waste material; however, no details were provided on the extent of the microbial contribution to the overall system.
To prepare for deep space, the MELiSSA project was designed by the European Space Agency to test the complete recycling of gas, liquid and solid wastes during long-distance space exploration. MELiSSA stands for Micro-Ecological Life-Support System Alternative, but although it includes microorganisms to a significant extent, its five major compartments count with no fungal species thus far (Hendrickx et al. 2006) (Lasseur et al. 2010). Nevertheless, with the ability to efficiently recycle by decomposing waste products (Villena and Gutierrez-Correa 2007), fungi could greatly contribute to a mission-long life-support system of a space habitat. Indeed, fungi can be applied to soil biomining or to water/waste management through bioleaching (Al-Sohaibani 2011) or mycoremediation (Kapahi and Sachdeva 2017).
It is also important to note that there is still a gap in knowledge concerning the health consequences of exposure to microgravity and radiation gradually over long periods of time (Cucinotta 2014; Gueguinou et al. 2009). Could spaceflight-associated immune system weakening preclude the expansion of human presence beyond Earth’s orbit? Astronauts on the ISS are known to have a weakened immune system; thus, maintaining health on deep space missions will be a critical issue (Fernandez-Gonzalo et al. 2017; Boice 2017). Moreover, medications are expected to degrade at a higher rate in space compared to the ground (Putcha et al. 2011; Taylor 2015). However, neither how pharmacokinetics are altered in the space environment nor the potential impact of spaceflight on drug stability, efficacy and safety is well understood (Kast et al. 2017). The challenge is then to develop new, space-hardy medication, for long-duration missions. Genetic engineering and culture setup of fungi such as A. niger can allow “on-demand” production of case-specific antibiotics and other compounds but only if self-sustained science is enabled in the spaceflight environment. This includes the need for a science-resource support system on board enabling (1) in situ high-throughput miniaturized technology, (2) large-scale microbial cultivation and testing of compound production, and (3) inclusion of science/biotechnology-related resources, directly or indirectly in the spacecraft’s closed loop life-support system.
An extensive review on technologies in space for biological research is provided in Karouia et al. (2017). In brief, high-throughput technologies such as MinION, miniPCR and WetLab-2 that enable DNA amplification (Boguraev et al. 2017), RNA isolation and PCR analysis (Parra et al. 2017) are available on board the ISS. WetLab-2 is a research platform for conducting real-time quantitative gene expression analysis aboard the ISS, allowing investigators to obtain real-time gene expression data from samples processed and analysed aboard the space station. Knowledge of these mechanisms can be applied towards developing countermeasures for protecting human health during long-term space missions and also for treating diseases on Earth. Further, it can be used to validate terrestrial analyses of samples returned from the space station by providing quantitative gene expression benchmarking prior to sample return to Earth. Moreover, GeneLab, an -omics database for spaceflight, has been assembled (Ray et al. 2018). A recent development on tools for quantitative intracellular metabolomics, with easy sampling of A. niger chemostat cultures, was reported and could potentially be applied to spaceflight (Lameiras et al. 2015). More details can be found in the review by Karouia et al. (2017).
The success of life-support systems and spaceflight research will depend on the ability to manage and sustain the available resources. Fungal-based biotechnology could play an important role in resource provision, from food or antibiotic production to waste management. The challenges for fungal biotechnology in space are summarized in Table 18.3.
5 Sustainability in Spaceflight: Lessons Learned from Aviation
From emulating birds to perfecting the manufacturing process to up to two airplanes from a model a day (Boeing 2017), the development of aviation was driven mostly by competition. Humankind achieved global connection both physically and virtually, with the first flight 115 years ago (NASA 2018a). Today, the fuel consumption per revenue passenger kilometre has nearly halved, and ticket prices have decreased to beat train and bus providers (Kharina and Rutherford 2015). A similar development can be seen in spaceflight endeavours. Space is at its tipping point of technology readiness, like aviation was before manufacturing became lean and operations streamlined for big scale projects. Soon, the cost to fly to the Moon could go from $75 million to as little as $100,000, according to Space Adventures (Becker 2017). However, there is still a long way to go in terms of sustainable development.
Sustainability is an evolving and emergent topic across sectors and the focus of negotiations for development in the aviation and space industry (Nakamura et al. 2010) from the urge to respond to the modern world’s needs. The understanding of sustainability in aviation and space activities is commonly set as the need to minimize the impact of the environmental repercussions through, e.g. changes in propulsion systems, aerodynamic optimization or advancements in flight operation procedures to minimize carbon dioxide emission (Braun-Unkhoff and Riedel 2014).
Currently aviation combines many solutions such as flight procedures, aircraft design and material and process optimization to overcome new fossil fuel alternatives. The current focus of IASA initiatives is the “Power-to-Liquid” (PtL) method. PtL is an approach to reduce circulatory-economic greenhouse gas and is based on the Fischer-Tropsch synthesis which has already been fully implemented and tested in reactors (INERATEC 2017). The German Environment Agency sees great potential in renewable aviation fuel supply through PtL in comparison to other renewable sources of fuel supply (Schmidt et al. 2016). Liquid hydrogen as propellant was a major accomplishment by the NASA, powering the space shuttle programme for decades (NASA 2015b). Future research is looking into possibilities such as quantized inertia converting Unruh radiation into thrust. Conventional chemical rockets are highly cost-intensive, and with quantized inertia, prices for launches would decrease rapidly, and rockets would be more efficient and overall sustainable (McCulloch 2015).
Even so, there is more to sustainability than striving for renewable energy concepts. Instead sustainability comprises three parts: economy, environment and society. With the ability to reach farther into space and deploy technology on higher scale and faster pace, it is crucial to implement sustainable efforts within every phase of the process and to understand that sustainability is not slowing development down. Rather sustainability is catalysing and optimizing the way humanity interacts with available resources for future generations. As vital research is taken into space, it is critical that the expansion of knowledge is properly manifested to ensure the ongoing development.
Private and governmental space agencies are currently striving for sustainable efforts. For instance, SpaceX has developed a recyclable launch system (SpaceX 2015), Virgin Galactic has new materials and launches from high altitude (Branson 2016), and since 2015, several countries have established combined efforts to meet sustainable development goals (ESA 2017). Despite the effort’s history, there is a need for common ground and regulations across nations. This vision was achieved in aviation with the International Civil Aviation Agency, by providing standards and recommended practices which are transferred into corresponding laws (ICAO).
As long-duration, far-reaching missions become the norm, the utmost priority is to define where space starts and what area of responsibility every nation is granted. The United Nations recognized the need for long-term sustainability of outer space activities in the report of the Committee on the Peaceful Uses of Outer Space (UN 2018). Additional efforts need to be considered in (1) cross-sector engagement, (2) evaluation of expert knowledge and resources available vs. the knowledge and resources required, (3) certification of processes, (4) evaluation of sustainable approaches and proposals, with determination of impact, and (5) identification of status quo in the different areas of research and development both by space agencies and by private companies.
The urge to sustain an isolated habitat, be it on Earth, on a spacecraft or another planet, demands the establishment of a bioeconomy (European Commission 2018). We are convinced that fungal biotechnology can help to achieve this sustainable economic vision.
6 Conclusion
On a spacecraft or on a Moon or Mars colony, astronauts and accompanying microorganisms are expected to remain in indoor-closed and controlled habitats for a long period of time, depending on their life-support systems. However, there is still a gap in knowledge concerning the health consequences of exposure to microgravity and radiation received gradually over long periods of time. Similarly to hospitals or submarines on Earth, these will have an artificial atmosphere, air filtration, controlled temperature, moisture, etc. which usually results in high microbial diversity. The high radiation exposure and microgravity environment will cause (un)expected changes in all living systems. Thus, while the (unplanned) presence of filamentous fungi in indoor-closed environments can put health and material safety at risk, a well-designed and planned use of fungal cultures can greatly contribute to the sustainability of long-term space missions. Studying filamentous fungi isolated from the ISS provides valuable insights on the physiological and genetic modifications provoked by the spaceflight environment.
Filamentous fungi can play an important role in three main space biotechnology areas: (1) production of compounds of interest such as antibiotics, vitamins, enzymes, conservatives, etc., (2) in situ resource utilization techniques such as biomining (e.g. the soils of the Moon or Mars), and (3) life-support systems in water/waste management through decomposing of (ligno-)cellulose products, bioleaching or mycoremediation. Thus, filamentous fungi, in particular A. niger, could become essential companions of astronauts for the autonomous production of relevant compounds and important players for use in terraforming efforts. The resilience coupled with a versatile substrate and product spectrum suggests that A. niger would indeed be of high interest to be studied further for any potential space biotechnological applications in the context of long-term space exploration. To face these challenges, it is critical that long-term, self-sustained science is performed in space. Realizing the holistic concept of a circular economy is not only of crucial importance for a sustainable future of mankind on Earth; it should be a paradigm for any space biotechnological endeavours of humanity.
References
Aguiar TQ, Silva R, Domingues L (2017) New biotechnological applications for Ashbya gossypii: challenges and perspectives. Bioengineered 8(4):309–315. https://doi.org/10.1080/21655979.2016.1234543
Ahmad M, Hirz M, Pichler H, Schwab H (2014) Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl Microbiol Biotechnol 98(12):5301–5317. https://doi.org/10.1007/s00253-014-5732-5
Alekhova TA, Aleksandrova AA, Novozhilova TY, Lysak LV, Zagustina NA, Bezborodov AM (2005) Monitoring of microbial degraders. Appl Biochem Microbiol 41(4):382–389
Al-Sohaibani SA (2011) Heavy metal tolerant filamentous fungi from municipal sewage for bioleaching. Asian J Biotechnol 3(3):226–236
Andersen MR, Lehmann L, Nielsen J (2009) Systemic analysis of the response of Aspergillus niger to ambient pH. Genome Biol 10(5):R47. https://doi.org/10.1186/gb-2009-10-5-r47
Aplatov AM (1992) Circadian rhythms in a long term duration space flight. Adv Space Res 12(1):249–252
Appels FVW, Dijksterhuis J, Lukasiewicz CE, Jansen KMB, Wosten HAB, Krijgsheld P (2018) Hydrophobin gene deletion and environmental growth conditions impact mechanical properties of mycelium by affecting the density of the material. Sci Rep 8(1):4703. https://doi.org/10.1038/s41598-018-23171-2
Arena C, De Micco V, De Santo AV (2012) Bioregenerative life support systems in space. Ann Kinesiol 3(1):87–98
Baker SE (2018) Protein hyperproduction in fungi by design. Appl Microbiol Biotechnol 102(20):8621–8628. https://doi.org/10.1007/s00253-018-9265-1
Barcelos MCS, Lupki FB, Campolina GA, Nelson DL, Molina G (2018) The colors of biotechnology: general overview and developments of white, green and blue areas. FEMS Microbiol Lett 365(21). https://doi.org/10.1093/femsle/fny239
Basu P, Kruse CPS, Luesse DR, Wyatt SE (2017) Growth in spaceflight hardware results in alterations to the transcriptome and proteome. Life Sci Space Res (Amst) 15:88–96. https://doi.org/10.1016/j.lssr.2017.09.001
Be NA, Avila-Herrera A, Allen JE, Singh N, Checinska Sielaff A, Jaing C, Venkateswaran K (2017) Whole metagenome profiles of particulates collected from the International Space Station. Microbiome 5(1):81. https://doi.org/10.1186/s40168-017-0292-4
Becker R (2017) How much are SpaceX tourists actually paying to fly around the Moon? https://www.theverge.com/2017/2/28/14763632/spacex-private-moon-flight-price-cost-estimate-nasa-space-adventures. 2019
Benoit MR, Li W, Stodieck LS, Lam KS, Winther CL, Roane TM, Klaus DM (2006) Microbial antibiotic production aboard the International Space Station. Appl Microbiol Biotechnol 70(4):403–411. https://doi.org/10.1007/s00253-005-0098-3
Berger T, Burmeister S, Matthiä D, Przybyla B, Reitz G, Bilski P, Hajek M, Sihver L, Szabo J, Ambrozova I, Vanhavere F, Gaza R, Semones E, Yukihara EG, Benton ER, Uchihori Y, Kodaira S, Kitamura H, Boehme M (2017) DOSIS & DOSIS 3D: radiation measurements with the DOSTEL instruments onboard the Columbus Laboratory of the ISS in the years 2009–2016. J Space Weather Space Clim 7:A8
Betzel C, Martirosyan A, Ruyters G (2017) Protein crystallization on the International Space Station ISS. In: Ruyters G, Betzel C, Grimm D (eds) Biotechnology in space. Springer, Cham, pp 27–39. https://doi.org/10.1007/978-3-319-64054-9_3
Bjursted H (1992) Proceedings of the 13th annual meeting of the IUPS commission on gravitational physiology. Paper presented at the 13th annual meeting of the IUPS commission on gravitational physiology, San Antonio, TX
Blachowicz A, Mayer T, Bashir M, Pieber TR, De Leon P, Venkateswaran K (2017) Human presence impacts fungal diversity of inflated lunar/Mars analog habitat. Microbiome 5(1):62. https://doi.org/10.1186/s40168-017-0280-8
Block I, Wolke A, Briegleb W (1994) Gravitational response of the slime mold Physarum. Adv Space Res 14(8):21–34
Boecker S, Gratz S, Kerwat D, Adam L, Schirmer D, Richter L, Schutze T, Petras D, Sussmuth RD, Meyer V (2018) Aspergillus niger is a superior expression host for the production of bioactive fungal cyclodepsipeptides. Fungal Biol Biotechnol 5:4. https://doi.org/10.1186/s40694-018-0048-3
Boeing (2017) Commercial airplanes fact sheet. http://investors.boeing.com/investors/fact-sheets/default.aspx. 2019
Boeing miniPCR genes in space. https://www.genesinspace.org/. 2019
Boguraev AS, Christensen HC, Bonneau AR, Pezza JA, Nichols NM, Giraldez AJ, Gray MM, Wagner BM, Aken JT, Foley KD, Copeland DS, Kraves S, Alvarez Saavedra E (2017) Successful amplification of DNA aboard the International Space Station. NPJ Microgravity 3:26. https://doi.org/10.1038/s41526-017-0033-9
Boice JD Jr (2017) Space The Final Frontier-Research Relevant to Mars. Health Phys 112(4):392–397. https://doi.org/10.1097/HP.0000000000000656
Boudreault R, Armstrong DW (1988) Space biotechnology current and future perspectives. Trends Biotechnol 6:91–95
Branson R (2016) Creating a sustainable business. https://www.virgin.com/richard-branson/creating-sustainable-business. 2019
Braun-Unkhoff M, Riedel U (2014) Alternative fuels in aviation. CEAS Aeronaut J 6:83–93. https://doi.org/10.1007/s13272-014-0131-2
Brungs S, Hauslage J, Hilbig R, Hemmersbach R, Anken R (2011) Effects of simulated weightlessness on fish otolith growth: clinostat versus rotating-wall vessel. Adv Space Res 48(5):792–798. https://doi.org/10.1016/j.asr.2011.04.014
Cairns TC, Nai C, Meyer V (2018) How a fungus shapes biotechnology: 100 years of Aspergillus niger research. Fungal Biol Biotechnol 5:13. https://doi.org/10.1186/s40694-018-0054-5
Cardwell G, Bornman JF, James AP, Black LJ (2018) A review of mushrooms as a potential source of dietary vitamin D. Nutrients 10(10):E1498. https://doi.org/10.3390/nu10101498
Carvalho ND, Arentshorst M, Jin Kwon M, Meyer V, Ram AF (2010) Expanding the ku70 toolbox for filamentous fungi: establishment of complementation vectors and recipient strains for advanced gene analyses. Appl Microbiol Biotechnol 87(4):1463–1473. https://doi.org/10.1007/s00253-010-2588-1
Chatterjee S, Sarma MK, Deb U, Steinhauser G, Walther C, Gupta DK (2017) Mushrooms: from nutrition to mycoremediation. Environ Sci Pollut Res Int 24(24):19480–19493. https://doi.org/10.1007/s11356-017-9826-3
Checinska A, Probst AJ, Vaishampayan P, White JR, Kumar D, Stepanov VG, Fox GE, Nilsson HR, Pierson DL, Perry J, Venkateswaran K (2015) Microbiomes of the dust particles collected from the International Space Station and Spacecraft Assembly Facilities. Microbiome 3:50. https://doi.org/10.1186/s40168-015-0116-3
Clauwaert P, Muys M, Alloul A, De Paepe J, Luther A, Sun X, Ilgrande C, Christiaens MER, Hu X, Zhang D, Lindeboom REF, Sas B, Rabaey K, Boon N, Ronsse F, Geelen D, Vlaeminck SE (2017) Nitrogen cycling in bioregenerative life support systems: challenges for waste refinery and food production processes. Prog Aerosp Sci 91:87–98. https://doi.org/10.1016/j.paerosci.2017.04.002
Cogoli A, Tschopp A (1982) Biotechnology in space laboratories. Adv Biochem Eng 22:1–50
Cohen R, Persky L, Hadar Y (2002) Biotechnological applications and potential of wood-degrading mushrooms of the genus Pleurotus. Appl Microbiol Biotechnol 58(5):582–594. https://doi.org/10.1007/s00253-002-0930-y
Coil DA, Neches RY, Lang JM, Brown WE, Severance M, Cavalier D, Eisen JA (2016) Growth of 48 built environment bacterial isolates on board the International Space Station (ISS). PeerJ 4:e1842. https://doi.org/10.7717/peerj.1842
Coleman CB, Allen PL, Rupert M, Goulart C, Hoehn A, Stodieck LS, Hammond TG (2008) Novel Sfp1 transcriptional regulation of Saccharomyces cerevisiae gene expression changes during spaceflight. Astrobiology 8(6):1071–1078. https://doi.org/10.1089/ast.2007.0211
Crabbe A, Nielsen-Preiss SM, Woolley CM, Barrila J, Buchanan K, McCracken J, Inglis DO, Searles SC, Nelman-Gonzalez MA, Ott CM, Wilson JW, Pierson DL, Stefanyshyn-Piper HM, Hyman LE, Nickerson CA (2013) Spaceflight enhances cell aggregation and random budding in Candida albicans. PLoS One 8(12):e80677. https://doi.org/10.1371/journal.pone.0080677
Cucinotta FA (2014) Space radiation risks for astronauts on multiple international space station missions. PLoS One 9(4):e96099. https://doi.org/10.1371/journal
Dedolph RR, Oemick DA, Wilson BR, Smith GR (1967) Causal basis of gravity stimulus nullification by clinostat rotation. Plant Physiol 42(10):1373–1383
Devarayan K, Sathishkumar Y, Lee YS, Kim BS (2015) Effect of microgravity on fungistatic activity of an alpha-aminophosphonate chitosan derivative against Aspergillus niger. PLoS One 10(10):e0139303. https://doi.org/10.1371/journal.pone.0139303
ESA (2014) ESA user guide to low gravity platforms. https://www.esa.int/Our_Activities/Human_and_Robotic_Exploration/Research/European_user_guide_to_low_gravity_platforms
ESA (2017) ESA and the sustainable development goals. http://m.esa.int/Our_Activities/Preparing_for_the_Future/Space_for_Earth/ESA_and_the_Sustainable_Development_Goals. 2019
ESA (2018a) Advanced closed loop system. http://www.esa.int/Our_Activities/Human_and_Robotic_Exploration/Research/Advanced_Closed_Loop_System. 2019
ESA (2018b) Next-generation life-support system heading to space station. https://www.esa.int/Our_Activities/Human_and_Robotic_Exploration/International_Space_Station/Next-generation_life-support_system_heading_to_Space_Station. 2019
ESA. BIOLAB: Biological Laboratory in Columbus. http://wsn.spaceflight.esa.int/docs/Factsheets/8%20Biolab%20LR.pdf. 2019
ESA. New space station opportunity for university students. https://www.esa.int/Education/Orbit_Your_Thesis/New_space_station_opportunity_for_university_students. 2019
European Commission (2018) A sustainable bioeconomy for Europe: strengthening the connection between economy, society and the environment. COM:673
Fajardo-Cavazos P, Nicholson WL (2016) Cultivation of Staphylococcus epidermidis in the human spaceflight environment leads to alterations in the frequency and spectrum of spontaneous rifampicin-resistance mutations in the rpoB gene. Front Microbiol 7:999. https://doi.org/10.3389/fmicb.2016.00999
Fernandez-Gonzalo R, Baatout S, Moreels M (2017) Impact of particle irradiation on the immune system: from the clinic to mars. Front Immunol 8:177. https://doi.org/10.3389/fimmu.2017.00177
Ferraro JS, Fuller CA, Sulzman FM (1989) The biological clock of Neurospora in a microgravity environment. Adv Space Res 9(11):251–260
Freed LE, Vunjak-Novakovic G (2002) Spaceflight bioreactor studies of cells and tissues. Adv Space Biol Med 8:177–95
Freed LE, Pellis N, Searby N, de Luis J, Preda C, Bordonaro J, Vunjak-Novakovic G (1999) Microgravity cultivation of cells and tissues. Gravit Space Biol Bull 12(2):57–66
Fu Y, Li L, Xie B, Dong C, Wang M, Jia B, Shao L, Dong Y, Deng S, Liu H, Liu G, Liu B, Hu D, Liu H (2016) How to establish a Bioregenerative Life Support System for long-term crewed missions to the Moon or Mars. Astrobiology 16(12):925–936. https://doi.org/10.1089/ast.2016.1477
Fududa T, Fukuda K, Takahashi A et al (2000) Analysis of deletion mutations of the rpsL gene in the yeast Saccharomyces cerevisiae detected after long-term flight on the Russian space station Mir. Mutat Res 470:125–132
Fujita J, Yamane Y-I, Fukuda H, Kizaki Y, Wakabayashi S, Shigeta S, Suzuki O, Ono K (2003) Production and properties of phytase and acid phosphatase from a sake koji mold, Aspergillus oryzae. J Biosci Bioeng 95(4):348–353
Geib E, Brock M (2017) ATNT: an enhanced system for expression of polycistronic secondary metabolite gene clusters in Aspergillus niger. Fungal Biol Biotechnol 4:13. https://doi.org/10.1186/s40694-017-0042-1
Ghosh B, Ray RR (2011) Current commercial perspective of Rhizopus oryzae: a review. J Appl Sci 11(14):2470–2486. https://doi.org/10.3923/jas.2011.2470.2486
Gmünder FK, Nordau C-G, Tschopp A, Huber B, Cogoli A (1988) Dynamic cell culture system a new cell cultivation instrument for biological experiments in space. J Biotechnol 7:217–228
Gomoiu I, Chatzitheodoridis E, Vadrucci S, Walther I (2013) The effect of spaceflight on growth of Ulocladium chartarum colonies on the International Space Station. PLoS One 8(4):e62130. https://doi.org/10.1371/journal.pone.0062130
Gomoiu I, Chatzitheodoridis E, Vadrucci S, Walther I, Cojoc R (2016) Fungal spores viability on the international space station. Orig Life Evol Biosph 46(4):403–418. https://doi.org/10.1007/s11084-016-9502-5
Goncalves FA, Colen G, Takahashi JA (2014) Yarrowia lipolytica and its multiple applications in the biotechnological industry. Scientific World Journal 2014:476207. https://doi.org/10.1155/2014/476207
Goossens KV, Ielasi FS, Nookaew I, Stals I, Alonso-Sarduy L, Daenen L, Van Mulders SE, Stassen C, van Eijsden RG, Siewers V, Delvaux FR, Kasas S, Nielsen J, Devreese B, Willaert RG (2015) Molecular mechanism of flocculation self-recognition in yeast and its role in mating and survival. MBio 6(2):e00427-15. https://doi.org/10.1128/mBio.00427-15
Grimm D (2017) Cell biology in Space. In: Ruyters G, Betzel C, Grimm D (eds) Biotechnology in space. Springer, Cham, pp 59–72. https://doi.org/10.1007/978-3-319-64054-9_5
Grimm D, Wosten HAB (2018) Mushroom cultivation in the circular economy. Appl Microbiol Biotechnol 102(18):7795–7803. https://doi.org/10.1007/s00253-018-9226-8
Gueguinou N, Huin-Schohn C, Bascove M, Bueb JL, Tschirhart E, Legrand-Frossi C, Frippiat JP (2009) Could spaceflight-associated immune system weakening preclude the expansion of human presence beyond Earth’s orbit? J Leukoc Biol 86(5):1027–1038. https://doi.org/10.1189/jlb.0309167
Halstead TW, Morey-Holton ER (1990) LifeSat: a satellite for space biological research. Paper presented at the 20th Intersociety Conference on Environmental Systems Williamsburg, VA
Hammond TG, Allen PL, Zea L, Fanchiang C, Nislow C, Stodieck LS (2012) Specialized hardware in support of yeast genomics studies on board the Space Shuttle and ISS. Paper presented at the 1st international space station research and development conference, Denver, CO, June 26–28
Harper LD, Neal CR, Poynter J, Schalkwyk JD, Wingo DR (2016) Life support for a low-cost lunar settlement: no showstoppers. New Space 4(1):40–49. https://doi.org/10.1089/space.2015.0029
Hasegan D, Gomoiu I, Chatzitheodoridis E (2011) CFS-A—growth and survival of coloured fungi in space. ESA. http://eea.spaceflight.esa.int/portal/exp/?id=9214. Accessed 4 Feb 2018
Hemmersbach R, Strauch SM, Seibt D, Schuber M (2006) Comparative studies on gravisensitive protists on ground (2D and 3D clinostats) and in microgravity. Microgravity Sci Technol 18(3):257–259. https://doi.org/10.1007/BF02870423
Hendrickx L, De Wever H, Hermans V, Mastroleo F, Morin N, Wilmotte A, Janssen P, Mergeay M (2006) Microbial ecology of the closed artificial ecosystem MELiSSA (Micro-Ecological Life Support System Alternative): reinventing and compartmentalizing the Earth’s food and oxygen regeneration system for long-haul space exploration missions. Res Microbiol 157(1):77–86. https://doi.org/10.1016/j.resmic.2005.06.014
Herranz R, Anken R, Boonstra J, Braun M, Christianen PC, de Geest M, Hauslage J, Hilbig R, Hill RJ, Lebert M, Medina FJ, Vagt N, Ullrich O, van Loon JJ, Hemmersbach R (2013) Ground-based facilities for simulation of microgravity: organism-specific recommendations for their use, and recommended terminology. Astrobiology 13(1):1–17. https://doi.org/10.1089/ast.2012.0876
Horn A, Ullrich O, Huber K, Hemmersbach R (2011) PMT (Photomultiplier) clinostat. Microgravity Sci Technol 23(1):67–71. https://doi.org/10.1007/s12217-010-9234-5
Horneck G, Zell M (2012) Introduction to the EXPOSE-E mission. Astrobiology 12(5):373. https://doi.org/10.1089/ast.2012.0831
Horneck G, Panitz C, Zell M (2014) Introduction to the EXPOSE-R Mission. Int J Astrobiol 14(01):1–2. https://doi.org/10.1017/s147355041400069x
Hossain AH, Li A, Brickwedde A, Wilms L, Caspers M, Overkamp K, Punt PJ (2016) Rewiring a secondary metabolite pathway towards itaconic acid production in Aspergillus niger. Microb Cell Fact 15(1):130. https://doi.org/10.1186/s12934-016-0527-2
Hu Y, Zhu B (2016) Study on genetic engineering of Acremonium chrysogenum, the cephalosporin C producer. Synth Syst Biotechnol 1(3):143–149. https://doi.org/10.1016/j.synbio.2016.09.002
Hughes-Fulford M, Scheld HW (1989) Thin film bioreactors in space. Adv Space Res 9(11):111–117
ICAO. SARPs—Standards and recommended practices. https://www.icao.int/safety/SafetyManagement/Pages/SARPs.aspx. 2019
ICECUBES. Space Applications Services NV. 2019
INERATEC (2017) Processes: Power-to-lliquid. https://ineratec.de/en/processes/. 2019
Inokuchi H, Fukui K, Kogure K, Takaoki M, Kinoshita K, Izumi R, Fujimori Y (2007) Planning guide for space experiment research. JAXA. http://iss.jaxa.jp/en/kuoa/pdf/PlanningGuide.pdf
Ishioka N, Suzuki H, Asashima M, Kamisaka S, Mogami Y, Ochiai T, Aizawa-Yano S, Higashibata A, Ando N, Nagase M, Ogawa S, Shimazu T, Fukui K, Fujimoto N (2004) Development and verification of hardware for life science experiments in the Japanese Experiment Module “Kibo” on the International Space Station. J Gravit Physiol 11(1):81–91
Jin YS, Cate JH (2017) Metabolic engineering of yeast for lignocellulosic biofuel production. Curr Opin Chem Biol 41:99–106. https://doi.org/10.1016/j.cbpa.2017.10.025
Jones HW, Hodgson EW, Kliss MH (2014) Life support for deep space and Mars. Paper presented at the 44th international conference on environmental systems
Kapahi M, Sachdeva S (2017) Mycoremediation potential of Pleurotus species for heavy metals: a review. Bioresour Bioprocess 4(1):32. https://doi.org/10.1186/s40643-017-0162-8
Karnaouri A, Topakas E, Antonopoulou I, Christakopoulos P (2014) Genomic insights into the fungal lignocellulolytic system of Myceliophthora thermophila. Front Microbiol 5:281. https://doi.org/10.3389/fmicb.2014.00281
Karouia F, Peyvan K, Pohorille A (2017) Toward biotechnology in space: high-throughput instruments for in situ biological research beyond Earth. Biotechnol Adv 35(7):905–932. https://doi.org/10.1016/j.biotechadv.2017.04.003
Kast J, Yu Y, Seubert CN, Wotring VE, Derendorf H (2017) Drugs in space: pharmacokinetics and pharmacodynamics in astronauts. Eur J Pharm Sci 109S:S2–S8. https://doi.org/10.1016/j.ejps.2017.05.025
Kharina A, Rutherford D (2015) Fuel efficiency trends for new commercial jet aircraft: 1960 to 2014. The International Council on Clean Transportation
KIWI-microgravity (2017) my_biorack. http://www.kiwi-microgravity.com/wp-content/uploads/2018/03/my_biorack-2017-web.pdf. 2019
Klaus D (2001) Clinostats and bioreactors. Gravit Space Biol Bull 14(2):55–64
Klintworth R, Reher HJ (1999) Biological induced corrosion of materials II: new test methods and experiences from mir station. Acta Astronaut 44(7–12):569–578
Knox BP, Blachowicz A, Palmer JM, Romsdahl J, Huttenlocher A, Wang CC, Keller NP, Venkateswaran K (2016) Characterization of Aspergillus fumigatus isolates from air and surfaces of the International Space Station. mSphere 1(5):e00227-16. https://doi.org/10.1128/mSphere.00227-16
Koike J, Oshima T, Koike KA, Taguchi H, Tanaka R, Nishimura K, Miyaj M (1992) Survival rates of some terrestrial microorganisms under simulated space conditions. Adv Space Res 12(4):271–274
Kuivanen J, Wang YJ, Richard P (2016) Engineering Aspergillus niger for galactaric acid production: elimination of galactaric acid catabolism by using RNA sequencing and CRISPR/Cas9. Microb Cell Fact 15(1):210. https://doi.org/10.1186/s12934-016-0613-5
Kurt T, Marba-Ardebol AM, Turan Z, Neubauer P, Junne S, Meyer V (2018) Rocking Aspergillus: morphology-controlled cultivation of Aspergillus niger in a wave-mixed bioreactor for the production of secondary metabolites. Microb Cell Fact 17(1):128. https://doi.org/10.1186/s12934-018-0975-y
Lameiras F, Heijnen JJ, van Gulik WM (2015) Development of tools for quantitative intracellular metabolomics of Aspergillus niger chemostat cultures. Metabolomics 11(5):1253–1264. https://doi.org/10.1007/s11306-015-0781-z
Lasseur C, Brunet J, de Weever H, Dixon M, Dussap G, Godia F, Leys N, Mergeay M, Straeten DVD (2010) MELiSSa: the European project of a closed life support system. Gravit Space Biol 23(2):3–12
Li A, Punt P (2013) Industrial production of organic acids by fungi. Applications of microbial engineering. CRC, Boca Raton
Loudon C-M, Nicholson N, Finster K, Leys N, Byloos B, Van Houdt R, Rettberg P, Moeller R, Fuchs FM, Demets R, Krause J, Vukich M, Mariani A, Cockell C (2017) BioRock: new experiments and hardware to investigate microbe–mineral interactions in space. Int J Astrobiol 17(4):303–313. https://doi.org/10.1017/s1473550417000234
Lu T, Zhang Y, Kidane Y, Feiveson A, Stodieck L, Karouia F, Ramesh G, Rohde L, Wu H (2017) Cellular responses and gene expression profile changes due to bleomycin-induced DNA damage in human fibroblasts in space. PLoS One 12(3):e0170358. https://doi.org/10.1371/journal.pone.0170358
Machida M, Yamada O, Gomi K (2008) Genomics of Aspergillus oryzae: learning from the history of Koji mold and exploration of its future. DNA Res 15(4):173–183. https://doi.org/10.1093/dnares/dsn020
Makimura K, Hanazawa R, Takatorl K, Tamura Y, Fujisaki R, Nishiyama Y, Abe S, uchlda K, Kawamura Y, Ezaki T, Yamaguchi H (2001) Fungal flora on board the Mir-Space Station, identification by morphological features and ribosomal DNA sequences. Microbiol Immunol 45(5):357–363
Manieri P, Brinckmann E, Brillouet C (1996) The BIORACK facility and its performance during IML2 Spacelab mission. J Biotechnol 47:71–82
McCulloch ME (2015) Testing quantised inertia on the emdrive. Europhys Lett 111(6):60005
Meyer V, Arentshorst M, El-Ghezal A, Drews AC, Kooistra R, van den Hondel CA, Ram AF (2007) Highly efficient gene targeting in the Aspergillus niger kusA mutant. J Biotechnol 128(4):770–775. https://doi.org/10.1016/j.jbiotec.2006.12.021
Meyer V, Wu B, Ram AF (2011) Aspergillus as a multi-purpose cell factory: current status and perspectives. Biotechnol Lett 33(3):469–476. https://doi.org/10.1007/s10529-010-0473-8
Meyer V, Fiedler M, Nitsche B, King R (2015) The cell factory Aspergillus enters the big data era: opportunities and challenges for optimising product formation. In: Krull R, Bley T (eds) Filaments in bioprocesses. Springer, Cham, pp 91–132. https://doi.org/10.1007/10_2014_297
Meyer V, Andersen MR, Brakhage AA, Braus GH, Caddick MX, Cairns TC, de Vries RP, Haarmann T, Hansen K, Hertz-Fowler C, Krappmann S, Mortensen UH, Penalva MA, Ram AFJ, Head RM (2016) Current challenges of research on filamentous fungi in relation to human welfare and a sustainable bio-economy: a white paper. Fungal Biol Biotechnol 3:6. https://doi.org/10.1186/s40694-016-0024-8
Moeller R, Reitz G, Nicholson The Protect Team WL, Horneck G (2012) Mutagenesis in bacterial spores exposed to space and simulated Martian conditions: data from the EXPOSE-E spaceflight experiment PROTECT. Astrobiology 12(5):457–468. https://doi.org/10.1089/ast.2011.0739
Montgomery P, Cox J, Reynolds R, Paul J, Hayflick L, Stock D, Shuiz W, Kimzey S (1977) Biomedical results from Skylab. Technical paper N77-33780:221-234, US Government printing office, Washington, DC
Montgomery POJ, Cook JE, Reynolds RC, Paul S, Hayflick L, Stock D, Schulz WW, Kimsey S, Thirolf RG, Rogers T, Campbell D (1978) The response of single human cells to zero gravity. In Vitro 14(2):165–173
Moore D (1996) Graviresponses in fungi. Adv Space Res 17(617):73–82
Nadir AJ, Sato K (2017) A hitchhiker’s guide to an ISS experiment in under 9 months. NPJ Microgravity 3:6. https://doi.org/10.1038/s41526-016-0003-7
Nai C, Meyer V (2016) The beauty and the morbid: fungi as source of inspiration in contemporary art. Fungal Biol Biotechnol 3:10. https://doi.org/10.1186/s40694-016-0028-4
Najrana T, Sanchez-Esteban J (2016) Mechanotransduction as an adaptation to gravity. Front Pediatr 4:140. https://doi.org/10.3389/fped.2016.00140
Nakamura H, Kajikawa Y, Suzuki S (2010) Academic landscape of aviation and environment. Paper presented at the 27th international congress of the aeronautical sciences, Nice, 19–24 Sept
NanoRacks (2019). http://nanoracks.com/products/nanolabs/. 2019
NASA (2007) Shuttle-Mir. https://www.nasa.gov/mission_pages/shuttle-mir/. 2019
NASA (2010) Reference guide to the International Space Station. https://www.nasa.gov/pdf/508318main_ISS_ref_guide_nov2010.pdf. 2019
NASA (2015a) Biosafety Review Board (BRB). https://www.nasa.gov/feature/biosafety-review-board-brb. 2019
NASA (2015b) Liquid hydrogen—the fuel of choice for space exploration. https://www.nasa.gov/content/liquid-hydrogen-the-fuel-of-choice-for-space-exploration. 2019
NASA (2017a) Deep Space Gateway to open opportunities for distant destinations. https://www.nasa.gov/feature/deep-space-gateway-to-open-opportunities-for-distant-destinations. 2019
NASA (2017b) Group Activation Pack-Fluid Processing Apparatus (GAP-FPA). https://www.nasa.gov/mission_pages/station/research/experiments/375.html. 2019
NASA (2018a) 115 years ago: wright brothers make history at Kitty Hawk. https://www.nasa.gov/feature/115-years-ago-wright-brothers-make-history-at-kitty-hawk. 2019
NASA (2018b) About the H-II Transfer Vehicle (HTV). https://www.nasa.gov/mission_pages/station/structure/elements/htv_about.html. 2019
NASA (2018c) Biological Research in Canisters (BRIC). https://www.nasa.gov/mission_pages/station/research/experiments/708.html. 2019
NASA (2019a) Bioculture System (Bioculture System Facility). https://www.nasa.gov/mission_pages/station/research/experiments/1125.html. 2019
NASA (2019b) International Space Station—facility by category. https://www.nasa.gov/mission_pages/station/research/experiments/facilities_hardware.html#Biology-and-Biotechnology. 2019
NASA Space Station Research Explorer on NASA.gov. https://www.nasa.gov/mission_pages/station/research/experiments/explorer/search.html?#q=&i=&p=&c=Biology. 2019
Nellessen W (1995) The development of the European retrievable carrier “EURECA”. Adv Space Res 16(8):5–16
Newbert R, Barton B, Greaves P, Harper J, Turner G (1997) Analysis of a commercially improved Penicillium chrysogenum strain series: involvement of recombinogenic regions in amplification and deletion of the penicillin biosynthesis gene cluster. J Ind Microbiol Biotechnol 19:18–27
Nielsen J, Larsson C, van Maris A, Pronk J (2013) Metabolic engineering of yeast for production of fuels and chemicals. Curr Opin Biotechnol 24(3):398–404. https://doi.org/10.1016/j.copbio.2013.03.023
Nislow C, Lee AY, Allen PL, Giaever G, Smith A, Gebbia M, Stodieck LS, Hammond JS, Birdsall HH, Hammond TG (2015) Genes required for survival in microgravity revealed by genome-wide yeast deletion collections cultured during spaceflight. Biomed Res Int. https://doi.org/10.1155/2015/976458
Novikova N, Deshevaya E, Levinskikh M, Polikarpov N, Poddubko S, Gusev O, Sychev V (2015) Study of the effects of the outer space environment on dormant forms of microorganisms fungi and plants in the exposer experiment. Int J Astrobiol 14(1):137–142. https://doi.org/10.1017/S1473550414000731
Okabe M, Lies D, Kanamasa S, Park EY (2009) Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Appl Microbiol Biotechnol 84(4):597–606. https://doi.org/10.1007/s00253-009-2132-3
Onofri S, de Vera JP, Zucconi L, Selbmann L, Scalzi G, Venkateswaran KJ, Rabbow E, de la Torre R, Horneck G (2015) Survival of Antarctic cryptoendolithic fungi in simulated Martian conditions on board the International Space Station. Astrobiology 15(12):1052–1059. https://doi.org/10.1089/ast.2015.1324
Oser H (1984) The life sciences programme of the ESA and opportunities for radiation biology experiments. Adv Space Res 4(10):265–275
Parra M, Jung J, Boone TD, Tran L, Blaber EA, Brown M, Chin M, Chinn T, Cohen J, Doebler R, Hoang D, Hyde E, Lera M, Luzod LT, Mallinson M, Marcu O, Mohamedaly Y, Ricco AJ, Rubins K, Sgarlato GD, Talavera RO, Tong P, Uribe E, Williams J, Wu D, Yousuf R, Richey CS, Schonfeld J, Almeida EAC (2017) Microgravity validation of a novel system for RNA isolation and multiplex quantitative real time PCR analysis of gene expression on the International Space Station. PLoS One 12(9):e0183480. https://doi.org/10.1371/journal.pone.0183480
Pel HJ, de Winde JH, Archer DB, Dyer PS, Hofmann G, Schaap PJ, Turner G, de Vries RP, Albang R, Albermann K, Andersen MR, Bendtsen JD, Benen JA, van den Berg M, Breestraat S, Caddick MX, Contreras R, Cornell M, Coutinho PM, Danchin EG, Debets AJ, Dekker P, van Dijck PW, van Dijk A, Dijkhuizen L, Driessen AJ, d’Enfert C, Geysens S, Goosen C, Groot GS, de Groot PW, Guillemette T, Henrissat B, Herweijer M, van den Hombergh JP, van den Hondel CA, van der Heijden RT, van der Kaaij RM, Klis FM, Kools HJ, Kubicek CP, van Kuyk PA, Lauber J, Lu X, van der Maarel MJ, Meulenberg R, Menke H, Mortimer MA, Nielsen J, Oliver SG, Olsthoorn M, Pal K, van Peij NN, Ram AF, Rinas U, Roubos JA, Sagt CM, Schmoll M, Sun J, Ussery D, Varga J, Vervecken W, van de Vondervoort PJ, Wedler H, Wosten HA, Zeng AP, van Ooyen AJ, Visser J, Stam H (2007) Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat Biotechnol 25(2):221–231. https://doi.org/10.1038/nbt1282
Petersen GR, Seshafl PK, Dunlop EH (1989) Phase separated membrane bioreactor: results from model system studies. Adv Space Res 9(8):185–193
Pierson DL, Ott CM (2011) Microbiological lessons learned from the space shuttle. American Institute of Aeronautics and Astronautics
Putcha L, Taylor PW, Boyd JL (2011) Biopharmaceutical challenges of therapeutics in space: formulation and packaging considerations. Ther Deliv 2(11):1373–1376. https://doi.org/10.4155/TDE.11.115
Rabbow E, Rettberg P, Parpart A, Panitz C, Schulte W, Molter F, Jaramillo E, Demets R, Weiss P, Willnecker R (2017) EXPOSE-R2: the astrobiological ESA mission on board of the International Space Station. Front Microbiol 8:1533. https://doi.org/10.3389/fmicb.2017.01533
Ray S, Gebre S, Fogle H, Berrios DC, Tran PB, Galazka JM, Costes SV (2018) GeneLab: omics database for spaceflight experiments. Bioinformatics 35(10):1753–1759. https://doi.org/10.1093/bioinformatics/bty884
Research SS Select BioServe Space Technologies space flight experiment hardware. http://www.spacestationresearch.com/wp-content/uploads/SelectBioserveHardware.pdf. 2019
Richter L, Wanka F, Boecker S, Storm D, Kurt T, Vural Ö, Süßmuth R, Meyer V (2014) Engineering of Aspergillus niger for the production of secondary metabolites. Fungal Biol Biotechnol 1:4
Ritala A, Hakkinen ST, Toivari M, Wiebe MG (2017) Single cell protein-state-of-the-art, industrial landscape and patents 2001-2016. Front Microbiol 8:2009. https://doi.org/10.3389/fmicb.2017.02009
Robertson NF (1965) Presidential address: the fungal hypha. Trans Br Mycol Soc 48:1–8
Rodriguez Perez S, Garcia Oduardo N, Bermudez Savon RC, Fernandez Boizan M, Augur C (2008) Decolourisation of mushroom farm wastewater by Pleurotus ostreatus. Biodegradation 19(4):519–526. https://doi.org/10.1007/s10532-007-9157-z
Romsdahl J, Blachowicz A, Chiang AJ, Chiang YM, Masonjones S, Yaegashi J, Countryman S, Karouia F, Kalkum M, Stajich JE, Venkateswaran K, Wang CCC (2018a) International Space Station conditions alter genomics, proteomics, and metabolomics in Aspergillus nidulans. Appl Microbiol Biotechnol 103(3):1363–1377. https://doi.org/10.1007/s00253-018-9525-0
Romsdahl J, Blachowicz A, Chiang AJ, Singh N, Stajich JE, Kalkum M, Venkateswaran K, Wang CCC (2018b) Characterization of Aspergillus niger isolated from the International Space Station. mSystems 3(5):e00112-18. https://doi.org/10.1128/mSystems.00112-18
Ronnie GW (2013) The growth behavior of the model eukaryotic yeast Saccharomyces cerevisiae in microgravity. Curr Biotechnol 2(3):226–234. https://doi.org/10.2174/22115501113029990023
Sanchez C (2010) Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl Microbiol Biotechnol 85(5):1321–1337. https://doi.org/10.1007/s00253-009-2343-7
Sarantopoulou E, Gomoiu I, Kollia Z, Cefalas AC (2011) Interplanetary survival probability of Aspergillus terreus spores under simulated solar vacuum ultraviolet irradiation. Planet Space Sci 59(1):63–78. https://doi.org/10.1016/j.pss.2010.11.002
Sarkari P, Marx H, Blumhoff ML, Mattanovich D, Sauer M, Steiger MG (2017) An efficient tool for metabolic pathway construction and gene integration for Aspergillus niger. Bioresour Technol 245(Pt B):1327–1333. https://doi.org/10.1016/j.biortech.2017.05.004
Sathishkumar Y, Velmurugan N, Lee HM, Rajagopal K, Im CK, Lee YS (2014) Effect of low shear modeled microgravity on phenotypic and central chitin metabolism in the filamentous fungi Aspergillus niger and Penicillium chrysogenum. Antonie Van Leeuwenhoek 106(2):197–209. https://doi.org/10.1007/s10482-014-0181-9
Sathishkumar Y, Unnithan AR, Sen D, Kim CS, Lee YS (2015) Microgravity biosynthesized penicillin loaded electrospun polyurethane–dextran nanofibrous mats for biomedical applications. Colloids Surf A Physicochem Eng Asp 477:77–83. https://doi.org/10.1016/j.colsurfa.2015.01.065
Sathishkumar Y, Krishnaraj C, Rajagopal K, Sen D, Lee YS (2016) High throughput de novo RNA sequencing elucidates novel responses in Penicillium chrysogenum under microgravity. Bioprocess Biosyst Eng 39(2):223–231. https://doi.org/10.1007/s00449-015-1506-4
Sato T, Nagamatsu A, Ueno H, Kataoka R, Miyake S, Takeda K, Niita K (2018) Comparison of cosmic-ray environments on Earth, Moon, Mars and in spacecraft using phits. Radiat Prot Dosim 180(1–4):146–149. https://doi.org/10.1093/rpd/ncx192
Schäpe P, Kwon MJ, Baumann B, Gutschmann B, Jung S, Lenz S, Nitsche B, Paege N, Schütze T, Cairns TC, Meyer V (2018) Updating genome annotation for the microbial cell factory Aspergillus niger using gene co-expression networks. Nucleic Acids Res. https://doi.org/10.1093/nar/gky1183
Schmidt P, Weindorf W, Roth A, Batteiger V, Riegel F (2016) Power-to-liquids–potentials and perspectives for the future supply of renewable aviation fuel. German Environment Agency, Dessau-Roßlau
Schuetze T, Meyer V (2017) Polycistronic gene expression in Aspergillus niger. Microb Cell Factories 16(1):162–162. https://doi.org/10.1186/s12934-017-0780-z
Schuster A, Schmoll M (2010) Biology and biotechnology of Trichoderma. Appl Microbiol Biotechnol 87(3):787–799. https://doi.org/10.1007/s00253-010-2632-1
Schwarz RP, Goodwin TJ, Wolf DA (1992) Cell culture for three-dimensional modeling in rotating-wall. J Tiss Cult Meth 14:51–58
Show PL, Oladele KO, Siew QY, Zakry FAA, Lan JC-W, Ling TC (2015) Overview of citric acid production from Aspergillus niger. Front Life Sci 8(3):271–283. https://doi.org/10.1080/21553769.2015.1033653
Singh NK, Blachowicz A, Romsdahl J, Wang C, Torok T, Venkateswaran K (2017) Draft genome sequences of several fungal strains selected for exposure to microgravity at the International Space Station. Genome Announcement ASM
Sobick V, Sievers A (1979) Responses of roots to simulated weightlessness on the fast-rotating clinostat. Life Sci Space Res 17:285–290
Song L, Ouedraogo JP, Kolbusz M, Nguyen TTM, Tsang A (2018) Efficient genome editing using tRNA promoter-driven CRISPR/Cas9 gRNA in Aspergillus niger. PLoS One 13(8):e0202868. https://doi.org/10.1371/journal.pone.0202868
Sonnenberg AS, Baars JJ, Gao W, Visser RG (2017) Developments in breeding of Agaricus bisporus var. bisporus: progress made and technical and legal hurdles to take. Appl Microbiol Biotechnol 101(5):1819–1829. https://doi.org/10.1007/s00253-017-8102-2
SpaceX (2015) Reusability: the key to making human life multi-planetary. https://www.spacex.com/news/2013/03/31/reusability-key-making-human-life-multi-planetary. 2019
SpaceX (2017) Dragon. https://www.spacex.com/dragon. 2019
Stahel WR (2016) Circular economy. Nature 531(7595):435–438
Steensels J, Snoek T, Meersman E, Picca Nicolino M, Voordeckers K, Verstrepen KJ (2014) Improving industrial yeast strains: exploiting natural and artificial diversity. FEMS Microbiol Rev 38(5):947–995. https://doi.org/10.1111/1574-6976.12073
Synthecon (2019) Autoclavable rotating wall vessels—3D cell culture. http://synthecon.com/pages/autoclavable_rotating_wall_vessel_synthecon_26.asp. 2019
Tabuchi T, Nakahara T (1980) Preparation of itaconic acid. JP- Patent 55 034 017 (to Mitsubishi)
Taheri-Talesh N, Horio T, Araujo-Bazan L, Dou X, Espeso EA, Penalva MA, Osmani SA, Oakley BR (2008) The tip growth apparatus of Aspergillus nidulans. Mol Biol Cell 19(4):1439–1449. https://doi.org/10.1091/mbc.E07-05-0464
Takeshita N, Manck R, Grun N, de Vega SH, Fischer R (2014) Interdependence of the actin and the microtubule cytoskeleton during fungal growth. Curr Opin Microbiol 20:34–41. https://doi.org/10.1016/j.mib.2014.04.005
Taylor PW (2015) Impact of space flight on bacterial virulence and antibiotic susceptibility. Infect Drug Resist 8:249–262. https://doi.org/10.2147/IDR.S67275
Tsukahara M, Shinzato N, Tamaki Y, Namihira T, Matsui T (2009) Red yeast rice fermentation by selected Monascus sp. with deep-red color, lovastatin production but no citrinin, and effect of temperature-shift cultivation on lovastatin production. Appl Biochem Biotechnol 158(2):476–482. https://doi.org/10.1007/s12010-009-8553-8
UN (2018) United Nations report of the Committee on the Peaceful Uses of Outer Space. United Nations, New York
van Loon JJWA (2007) Some history and use of the random positioning machine, RPM, in gravity related research. Adv Space Res 39(7):1161–1165. https://doi.org/10.1016/j.asr.2007.02.016
Van Mulders SE, Stassen C, Daenen L, Devreese B, Siewers V, van Eijsden RG, Nielsen J, Delvaux FR, Willaert R (2011) The influence of microgravity on invasive growth in Saccharomyces cerevisiae. Astrobiology 11(1):45–55. https://doi.org/10.1089/ast.2010.0518
Venkateswaran K (2018) BRIC—Natural Product under Microgravity (BRIC-NP). https://www.nasa.gov/mission_pages/station/research/experiments/1780.html. 2019
Venkateswaran K, Vaishampayan P, Cisneros J, Pierson DL, Rogers SO, Perry J (2014) International Space Station environmental microbiome—microbial inventories of ISS filter debris. Appl Microbiol Biotechnol 98(14):6453–6466. https://doi.org/10.1007/s00253-014-5650-6
Villena GK, Gutierrez-Correa M (2007) Morphological patterns of Aspergillus niger biofilms and pellets related to lignocellulolytic enzyme productivities. Lett Appl Microbiol 45(3):231–237. https://doi.org/10.1111/j.1472-765X.2007.02183.x
Villeneuve PE, Dunlop EH (1992) Evolution of a phase separated gravity independent bioreactor. Adv Space Res 12(5):237–245
Villeneuve PE, Wenger KS, Thompson BG, Kedar T, Dunlop EH (1994) Development of a CELSS bioreactor oxygen transfer and micromixing in parabolic flight. Adv Space Res 14(11):75–78
Vukich M, Donati A, Zolesi V (2013) Kayser Italia hardware for radiation and microgravity experiments in space. Rendiconti Lincei 25(S1):7–11. https://doi.org/10.1007/s12210-013-0261-1
Walther I, van der Schoot BH, Jeanneret S, Arquint P, de Rooij NF, Gass V, Bechlera B, Lorenzi G, Cogoli A (1994) Development of a miniature bioreactor for continuous culture in a space laboratory. J Biotechnol 38:21–32
Walther I, van der Schoot BH, Boillat M, Cogoli A (2000) Performance of a miniaturized bioreactor in space flight microtechnology at the service of space biology. Enzym Microb Technol 27:778–783
Wang G, Chen H, Li G, Chen L, Li D, Hu C, Chen K, Liu Y (2006) Population growth and physiological characteristics of microalgae in a miniaturized bioreactor during space flight. Acta Astronaut 58(5):264–269. https://doi.org/10.1016/j.actaastro.2005.11.001
Westgate P, Kohlmann K, Hendrickson R, Ladisch MR (1992) Bioprocessing in space. Enzym Microb Technol 14:76–79
Wosten HAB, Moukha SM, Sietsma JH, Wessel JGH (1991) Localization of growth and secretion of proteins in Aspergillus niger. J Gen Microbiol 137:2017–2023
Yu GJ, Wang M, Huang J, Yin YL, Chen YJ, Jiang S, Jin YX, Lan XQ, Wong BH, Liang Y, Sun H (2012) Deep insight into the Ganoderma lucidum by comprehensive analysis of its transcriptome. PLoS One 7(8):e44031. https://doi.org/10.1371/journal.pone.0044031
Zak A (2018) Progress-MS series. http://www.russianspaceweb.com/progress-ms.html. 2019
Zea L, Larsen M, Estante F, Qvortrup K, Moeller R, Dias de Oliveira S, Stodieck L, Klaus D (2017) Phenotypic changes exhibited by E. coli cultured in space. Front Microbiol 8:1598. https://doi.org/10.3389/fmicb.2017.01598
Zea L, Nisar Z, Rubin P, Cortesão M, Luo J, McBride SA, Moeller R, Klaus D, Müller D, Varanasi KK, Muecklich F, Stodieck L (2018) Design of a spaceflight biofilm experiment. Acta Astronaut 148:294–300. https://doi.org/10.1016/j.actaastro.2018.04.039
Zheng X, Zheng P, Sun J, Kun Z, Ma Y (2018a) Heterologous and endogenous U6 snRNA promoters enable CRISPR/Cas9 mediated genome editing in Aspergillus niger. Fungal Biol Biotechnol 5:2. https://doi.org/10.1186/s40694-018-0047-4
Zheng X, Zheng P, Zhang K, Cairns TC, Meyer V, Sun J, Ma Y (2018b) 5S rRNA promoter for guide RNA expression enabled highly efficient CRISPR/Cas9 genome editing in Aspergillus niger. ACS Synth Biol 8(7):1568–1574. https://doi.org/10.1021/acssynbio.7b00456
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this chapter
Cite this chapter
Cortesão, M., Schütze, T., Marx, R., Moeller, R., Meyer, V. (2020). Fungal Biotechnology in Space: Why and How?. In: Nevalainen, H. (eds) Grand Challenges in Fungal Biotechnology. Grand Challenges in Biology and Biotechnology. Springer, Cham. https://doi.org/10.1007/978-3-030-29541-7_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-29541-7_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-29540-0
Online ISBN: 978-3-030-29541-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)