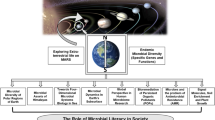
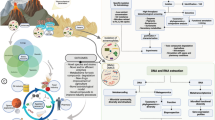
Abstract
In the era of deep space exploration, extremophile research represents a key area of research w.r.t space survival. This review thus delves into the intriguing realm of ‘Space and Astro Microbiology’, providing insights into microbial survival, resilience, and behavioral adaptations in space-like environments. This discussion encompasses the modified behavior of extremophilic microorganisms, influencing virulence, stress resistance, and gene expression. It then shifts to recent studies on the International Space Station and simulated microgravity, revealing microbial responses that impact drug susceptibility, antibiotic resistance, and its commercial implications. The review then transitions into Astro microbiology, exploring the possibilities of interplanetary transit, lithopanspermia, and terraforming. Debates on life's origin and recent Martian meteorite discoveries are noted. We also discuss Proactive Inoculation Protocols for selecting adaptable microorganisms as terraforming pioneers. The discussion concludes with a note on microbes’ role as bioengineers in bioregenerative life support systems, in recycling organic waste for sustainable space travel; and in promoting optimal plant growth to prepare Martian and lunar basalt. This piece emphasizes the transformative impact of microbes on the future of space exploration.



Similar content being viewed by others
References
Lammer H, Güdel M, Kulikov Y, Ribas I et al (2012) Variability of solar/stellar activity and magnetic field and its influence on planetary atmosphere evolution. Earth Planet Sp 64:179–199. https://doi.org/10.5047/eps.2011.04.002
Cottin H, Kotler JM, Billi D, Cockell C et al (2017) Space as a tool for astrobiology: review and recommendations for experimentations in earth orbit and beyond. Space Sci Rev 209:83–181. https://doi.org/10.1007/s11214-017-0365-5
Horneck G, Bücker H, Reitz G (1994) Long-term survival of bacterial spores in space. Adv Space Res 14:41–45. https://doi.org/10.1016/0273-1177(94)90448-0
Milojevic T, Weckwerth W (2020) Molecular mechanisms of microbial survivability in outer space: a systems biology approach. Front Microbiol 11:923. https://doi.org/10.3389/fmicb.2020.00923
Lopez JV, Peixoto RS, Rosado AS (2019) Inevitable future: space colonization beyond Earth with microbes first. FEMS Microbiol Ecol 95:fiz127. https://doi.org/10.1093/femsec/fiz127
Antunes A, Meyer-Dombard DR (2023) Editorial: rising stars in space microbiology: 2022. Front Microbiol 14:1322924. https://doi.org/10.3389/fmicb.2023.1322924
Lal R (2010) Microbiology in space. Indian J Microbiol 50:1. https://doi.org/10.1007/s12088-010-0047-4
Kanapskyte A, Hawkins EM, Liddell LC, Bhardwaj SR, Gentry D, Santa Maria SR (2021) Space biology research and biosensor technologies: past, present, and future. Biosensors 11:38. https://doi.org/10.3390/bios11020038
Padma TV (2023) India’s moon mission: four things Chandrayaan-3 has taught scientists. Nature 621:456–456. https://doi.org/10.1038/d41586-023-02852-7
Horneck G, Klaus DM, Mancinelli RL (2010) Space microbiology. Microbiol Mol Biol Rev 74:121–156. https://doi.org/10.1128/MMBR.00016-09
Raeva NV, Klemparskaya NN (1964) On the mechanism of increased radio-resistance in lactoprophylaxis of acute radiation sickness. Bull Exp Biol Med 58:800–803
Baker PW, Leff L (2004) The effect of simulated microgravity on bacteria from the mir space station. Microgravity Sci Technol 15:35–41. https://doi.org/10.1007/BF02870950
Saffary R, Nandakumar R, Spencer D, Robb FT et al (2002) Microbial survival of space vacuum and extreme ultraviolet irradiation: strain isolation and analysis during a rocket flight. FEMS Microbiol Lett 215:163–168. https://doi.org/10.1111/j.1574-6968.2002.tb11386.x
Aunins TR, Erickson KE, Prasad N, Levy SE, Jones A, Shrestha S, Mastracchio R, Stodieck L, Klaus D, Zea L, Chatterjee A (2018) Spaceflight modifies Escherichia coli gene expression in response to antibiotic exposure and reveals role of oxidative stress response. Front Microbiol 9:310. https://doi.org/10.3389/fmicb.2018.00310
Nickerson CA, Ott CM, Mister SJ, Morrow BJ, Burns-Keliher L, Pierson DL (2000) Microgravity as a novel environmental signal affecting Salmonella enterica serovar Typhimurium virulence. Infect Immun 68:3147–3152. https://doi.org/10.1128/IAI.68.6.3147-3152.2000
Wilson JW, Ott CM, Ramamurthy R, Porwollik S, McClelland M, Pierson DL, Nickerson CA (2002) Low-shear modelled microgravity alters the Salmonella enterica serovar typhimurium stress response in an RpoS-independent manner. Appl Environ Microbiol 68:5408–5416. https://doi.org/10.1128/AEM.68.11.5408-5416.2002
Wilson JW, Ramamurthy R, Porwollik S, McClelland M, Hammond T, Allen P, Ott CM, Pierson DL, Nickerson CA (2002) Microarray analysis identifies Salmonella genes belonging to the low-shear modeled microgravity regulon. Proc Natl Acad Sci USA 99:13807–13812. https://doi.org/10.1073/pnas.212387899
Wilson JW, Ott CM, Honer zu Bentrup K, Ramamurthy R, Quick L, Porwollik S et al (2007) Space flight alters bacterial gene expression and virulence and reveals a role for global regulator Hfq. Proc Natl Acad Sci USA 104:16299–16304. https://doi.org/10.1073/pnas.0707155104
Lynch SV, Mukundakrishnan K, Benoit MR, Ayyaswamy PS, Matin A (2006) Escherichia coli biofilms formed under low-shear modeled microgravity in a ground-based system. Appl Environ Microbiol 72:7701–7710. https://doi.org/10.1128/AEM.01294-06
Crabbe A, Boever PD, Houdt RV, Moors H, Mergeay M, Cornelis P (2008) Use of the rotating wall vessel technology to study the effect of shear stress on growth behavior of Pseudomonas aeruginosa PA01. Environ Microbiol 10:2098–2110. https://doi.org/10.1111/j.1462-2920.2008.01631.x
Crabbe A, Boever PD, Houdt RV, Monsieurs P, Nickerson C, Leys N, Cornelis P (2010) Response of Pseudomonas aeruginosa PAO1 to low shear modelled microgravity involves AlgU regulation. Environ Microbiol 12:1545–1564. https://doi.org/10.1111/j.1462-2920.2010.02184.x
Alan HBW (2006) Tietz clinical guide to laboratory tests, 4th edn. Saunders Elsevier, St. Louis
Gupta R, Shah P, Swiatlo E (2009) Differential gene expression in Streptococcus pneumoniae in response to various iron sources. Microb Pathog 47:101–109. https://doi.org/10.1016/j.micpath.2009.05.003
Castro SL, Nelman-Gonzalez M, Nickerson CA, Ott CM (2011) Induction of attachment-independent biofilm formation and repression of Hfq expression by low-fluid-shear culture of Staphylococcus aureus. Appl Environ Microbiol 77:6368–6378. https://doi.org/10.1128/AEM.00175-11
Lawal A, Jejelowo OA, Rosenzweig JA (2010) The effects of low-shear mechanical stress on Yersinia pestis virulence. Astrobiology 10:881–888. https://doi.org/10.1089/ast.2010.0493
Dornmayr-Pfaffenhuemer M, Legat A, Schwimbersky K, Fendrihan S, Stan-Lotter H (2011) Responses of haloarchaea to simulated microgravity. Astrobiology 11:199–205. https://doi.org/10.1089/ast.2010.0536
Purevdorj-Gage B, Sheehan KB, Hyman LE (2006) Effects of low-shear modelled microgravity on cell function, gene expression, and phenotype in Saccharomyces cerevisiae. Appl Environ Microbiol 72:4569–4575. https://doi.org/10.1128/AEM.03050-05
Altenburg SD, Nielsen-Preiss SM, Hyman LE (2008) Increased filamentous growth of Candida albicans in simulated microgravity. Genom Proteom Bioinf 6:42–50. https://doi.org/10.1016/S1672-0229(08)60019-4
Searles SC, Woolley CM, Petersen RA, Hyman LE, Nielsen-Preiss SM (2011) Modelled microgravity increases filamentation, biofilm formation, phenotypic switching, and antimicrobial resistance in Candida albicans. Astrobiology 11:825–836. https://doi.org/10.1089/ast.2011.0664
Kawaguchi Y, Shibuya M, Kinoshita I, Yatabe J et al (2020) DNA damage and survival time course of Deinococcal cell pellets during 3 years of exposure to outer space. Front Microbiol 11:2050. https://doi.org/10.3389/fmicb.2020.02050
Nicholson WL (2009) Ancient micronauts: Interplanetary transport of microbes by cosmic impacts. Trends Microbiol 17:243–250. https://doi.org/10.1016/j.tim.2009.03.004
Wassmann M, Moeller R, Rabbow E, Panitz C et al (2012) Survival of spores of the UV-Resistant Bacillus subtilis Strain MW01 after exposure to low-earth orbit and simulated Martian conditions: data from the space experiment ADAPT on EXPOSE-E. Astrobiology 12:498–507. https://doi.org/10.1089/ast.2011.0772
Billi D, Viaggiu E, Cockell CS, Rabbow E et al (2011) Damage escape and repair in dried Chroococcidiopsis spp. from hot and cold deserts exposed to simulated space and martian conditions. Astrobiology 11:65–73. https://doi.org/10.1089/ast.2009.0430
Baqué M, de Vera JP, Rettberg P, Billi D (2013) The BOSS and BIOMEX space experiments on the EXPOSE-R2 mission: endurance of the desert cyanobacterium Chroococcidiopsis under simulated space vacuum, Martian atmosphere, UVC radiation and temperature extremes. Acta Astronaut 91:180–186. https://doi.org/10.1016/j.actaastro.2013.05.015
Santomartino R, Waajen AC, de Wit W, Nicholson N et al (2020) No effect of microgravity and simulated mars gravity on final bacterial cell concentrations on the international space station: applications to space bioproduction. Front Microbiol 11:579156. https://doi.org/10.3389/fmicb.2020.579156
Leuko S, Rettberg P, Pontifex A, Burns B (2014) On the response of halophilic Archaea to space conditions. Life 4:66–76. https://doi.org/10.3390/life4010066
Mancinelli RL, Klovstad M (2000) Martian soil and UV radiation: microbial viability assessment on spacecraft surfaces. Planet Space Sci 48:1093–1097. https://doi.org/10.1016/S0032-0633(00)00083-0
Cockell CS, Raven JA (2004) Zones of photosynthetic potential on Mars and the early Earth. Icarus 169:300–310. https://doi.org/10.1016/j.icarus.2003.12.024
Hassler DM, Zeitlin C, Wimmer-Schweingruber RF, Ehresmann B et al (2014) Mars’ surface radiation environment measured with the mars science laboratory’s curiosity rover. Science 343:1244797. https://doi.org/10.1126/science.1244797
Cottin H, Rettberg P (2019) EXPOSE-R2 on the international space station (2014–2016): results from the PSS and BOSS astrobiology experiments. Astrobiology 19:975–978. https://doi.org/10.1089/ast.2019.0625
Tesei D, Chiang AJ, Kalkum M, Stajich JE et al (2021) Effects of simulated microgravity on the proteome and secretome of the polyextremotolerant black fungus Knufia chersonesos. Front Genet 12:638708. https://doi.org/10.3389/fgene.2021.638708
Blachowicz A, Chiang AJ et al (2019) Proteomic characterization of Aspergillus fumigatus isolated from air and surfaces of the international space station. Fungal Genet Biol 124:39–46. https://doi.org/10.1016/j.fgb.2019.01.001
Ott E, Kawaguchi Y, Kölbl D et al (2020) Molecular repertoire of Deinococcus radiodurans after 1 year of exposure outside the international space station within the Tanpopo mission. Microbiome 8:150. https://doi.org/10.1186/s40168-020-00927-5
Singh NK, Bezdan D, Checinska SA, Wheeler K et al (2018) Multi-drug resistant Enterobacter bugandensis species isolated from the International space station and comparative genomic analyses with human pathogenic strains. BMC Microbiol 18:175. https://doi.org/10.1186/s12866-018-1325-2
Pickerson CA, Ott CM, Wilson JW, Ramamurthy R, Pierson DL (2004) Microbial responses to microgravity and other low-shear environments. Microbiol Mol Biol Rev 68:345–361. https://doi.org/10.1128/MMBR.68.2.345-361.2004
Wilson JW, Ott CM et al (2007) Space flight alters bacterial gene expression and virulence and reveals a role for global regulator Hfq. PNAS 104:16299–16304. https://doi.org/10.1073/pnas.0707155104
Zea L (2015) Drug Discovery and Development in Space, IAC-15-A1.8x27627, 66th International Astronautical Congress, Jerusalem, Israel, October 12–16
Benoit MR, Li W, Stodieck LS, Lam KS et al (2006) Microbial antibiotic production aboard the International Space Station. Appl Microbiol Biotechnol 70:403–411. https://doi.org/10.1007/s00253-005-0098-3
Liu C (2017) The theory and application of space microbiology: China’s experiences in space experiments and beyond: the theory and application of space microbiology. Environ Microbiol 19:426–433. https://doi.org/10.1111/1462-2920.13472
Bijlani S, Stephens E, Singh NK, Venkateswaran K, Wang CC (2021) Advances in space microbiology. Iscience 24:102395. https://doi.org/10.1016/j.isci.2021.102395
Corydon TJ, Schulz H, Richter P, Strauch SM, Böhmer M, Ricciardi DA, Wehland M, Krüger M, Erzinger GS, Lebert M et al (2023) Current knowledge about the Impact of microgravity on gene regulation. Cells 12:1043. https://doi.org/10.3390/cells12071043
Sharma G, Curtis PD (2022) The impacts of microgravity on bacterial metabolism. Life 12:774. https://doi.org/10.3390/life12060774
Huang B, Li DG, Huang Y et al (2018) Effects of spaceflight and simulated microgravity on microbial growth and secondary metabolism. Mil Med Res 5:18. https://doi.org/10.1186/s40779-018-0162-9
Ulrich N, Nagler K, Laue M, Cockell CS, Setlow P, Moeller R (2018) Experimental studies addressing the longevity of Bacillus subtilis spores—the first data from a 500-year experiment. PLoS ONE 13:e0208425. https://doi.org/10.1371/journal.pone.0208425
Facius R, Bücker H, Horneck G, Reitz G, Schäfer M et al (1979) Dosimetric and biological results from the Bacillus subtilis biostack experiment with the Aapollo-Soyuz test project. Life Sci Space Res. https://doi.org/10.1016/B978-0-08-023416-8.50020-9
Ciferri O, Tiboni O, Di G, Orlandoni AM, Marchesi ML (1986) Effects of microgravity on genetic recombination in Escherichia coli. Naturwissenschaften 73:418–421. https://doi.org/10.1186/s40168-019-0666-x
Zea L, Larsen M, Estante F, Qvortrup K, Moeller R, Dias de Oliveira S, Stodieck L, Klaus D (2017) Phenotypic changes exhibited by E. coli cultured in Space. Front Microbiol 8:1598. https://doi.org/10.3389/fmicb.2017.01598
Wilson JW, Ott CM, Quick L, Davis R et al (2008) Media ion composition controls regulatory and virulence response of Salmonella in spaceflight. PLoS ONE 23:e3923. https://doi.org/10.1371/journal.pone.0003923
Checinska SA, Urbaniak C, Mohan GBM, Stepanov VG et al (2019) Characterization of the total and viable bacterial and fungal communities associated with the international space station surfaces. Microbiome 7:50. https://doi.org/10.1186/s40168-019-0666-x
Lam KS, Gustavson DR, Pirnik DL, Pack E et al (2002) The effect of space flight on the production of Actinomycin D by Streptomyces plicatus. J Ind Microbiol Biotechnol 29:299–302. https://doi.org/10.1038/sj.jim.7000312
Liu M, Gao H, Shang P, Zhou X, Ashforth E, Zhuo Y et al (2011) Magnetic field is the dominant factor to induce the response of Streptomyces avermitilis in altered gravity simulated by diamagnetic levitation. PLoS ONE 6:e24697. https://doi.org/10.1371/journal.pone.0024697
Xiao Y, Liu Y, Wang G, Hao Z, An Y (2010) Simulated microgravity alters growth and microcystin production in Microcystis aeruginosa (cyanophyta). Toxicon 56:1–7. https://doi.org/10.1016/j.toxicon.2010.01.026
Lam KS, Mamber SW, Pack EJ, Forenza S et al (1998) The effects of space flight on the production of Monorden by Humicola fuscoatra WC5157 in solid-state fermentation. Appl Microbiol Biotechnol 4:579–583. https://doi.org/10.1007/s002530051216
Fang X, Zhao Z, Gu H (2005) A study on space mutation of Streptomyces fradiae. Space Med Med Eng (Beijing) 18:121–125
Yang W, Han L, Mandlaa M, Zhang H et al (2017) A plate method for rapid screening of Ketogulonicigenium vulgare mutants for enhanced 2-keto-l-gulonic acid production. Braz J Microbiol 48:397–402. https://doi.org/10.1016/j.bjm.2017.02.002
Fang A, Pierson D, Mishra S et al (2000) Growth of Streptomyces hygroscopicus in rotating-wall bioreactor under simulated microgravity inhibits Rapamycin production. Appl Microbiol Biotechnol 54:33–36. https://doi.org/10.1007/s002539900303
Huang B, Liu N, Rong X, Ruan J, Huang Y (2015) Effects of simulated microgravity and spaceflight on morphological differentiation and secondary metabolism of Streptomyces coelicolor A3(2). Appl Microbiol Biotechnol 99:4409–4422. https://doi.org/10.1007/s00253-015-6386-7
Fang A, Pierson D, Mishra S et al (1997) Production by Bacillus brevis in simulated microgravity. Curr Microbiol 34:199–204. https://doi.org/10.1007/s002849900168
Tixador R, Richoilley G, Gasset G, Templier J et al (1985) Study of minimal inhibitory concentration of antibiotics on bacteria cultivated in vitro in space (Cytos 2 experiment). Aviat Space Environ Med 56:748–751
Lapchine L, Moatti N, Gasset G, Richoilley G, Templier J, Tixador R (1986) Antibiotic activity in space. Drugs Exp Clin Res 12:933–938
Knox BP, Blachowicz A, Palmer JM, Romsdahl J, Huttenlocher A, Wang CC et al (2016) Characterization of Aspergillus fumigatus isolates from air and surfaces of the International Space Station. mSphere 1:e00227-16. https://doi.org/10.1128/mSphere.00227-16
Valtonen M, Nurmi P, Zheng JQ, Cucinotta FA et al (2009) Natural transfer of viable microbes in space from planets in extra-solar systems to a planet in our solar system and vice versa. Astrophy J 690:210–215. https://doi.org/10.1088/0004-637X/690/1/210
Lingam M, Loeb A (2017) Enhanced interplanetary panspermia in the TRAPPIST-1 system. Proc Natl Acad Sci USA 114:6689–6693. https://doi.org/10.1073/pnas.1703517114
Cockell CS (2008) The interplanetary exchange of photosynthesis. Orig Life Evol Biosph 38:87–104. https://doi.org/10.1007/s11084-007-9112-3
Zagorski ZP (2007) Question 2: relation of panspermia-hypothesis to astrobiology. Orig Life Evol Biosph 37:351–355. https://doi.org/10.1007/s11084-007-9074-5
Mileikowsky C (2000) Natural transfer of viable microbes in pace 1. From mars to earth and earth to mars. Icarus 145:391–427. https://doi.org/10.1006/icar.1999.6317
Thomas-Keprta KL, Clemett SJ, Bazylinski DA, Kirschvink JL, McKay DS, Wentworth SJ et al (2002) Magnetofossils from ancient Mars: a robust biosignature in the Martian meteorite ALH84001. Appl Environ Microbiol 68:3663–3672. https://doi.org/10.1128/AEM.68.8.3663-3672.2002
Meyer C, Fritz J, Misgaiski M, Stöffler D et al (2011) Shock experiments in support of the lithopanspermia theory: The influence of host rock composition, temperature, and shock pressure on the survival rate of endolithic and epilithic microorganisms: shock experiments in support of lithopanspermia. Meteorit Planet Sci 46:701–718. https://doi.org/10.1111/j.1945-5100.2011.01184.x
Stöffler D, Horneck G, Ott S, Hornemann U et al (2007) Experimental evidence for the potential impact ejection of viable microorganisms from mars and mars-like planets. Icarus 186:585–588. https://doi.org/10.1016/j.icarus.2006.11.007
Benardini JN, Sawyer J, Venkateswaran K, Nicholson WL (2003) Spore UV and acceleration resistance of endolithic Bacillus pumilus and Bacillus subtilis isolates obtained from Sonoran desert Basalt: implications for lithopanspermia. Astrobiology 3:709–717. https://doi.org/10.1089/153110703322736033
Link L, Sawyer J, Venkateswaran K et al (2003) Extreme spore UV resistance of Bacillus pumilus isolates obtained from an ultraclean spacecraft assembly facility. Microb Ecol 47:159–163. https://doi.org/10.1007/s00248-003-1029-4
Fajardo-Cavazos P, Langenhorst F, Melosh HJ, Nicholson WL (2009) Bacterial spores in granite survive hypervelocity launch by spallation: implications for lithopanspermia. Astrobiology 9:647–657. https://doi.org/10.1089/ast.2008.0326
Panitz C, Horneck G, Rabbow E, Rettberg P, Moeller R, Cadet J, Douki T, Reitz G (2015) The SPORES experiment of the EXPOSE-R mission: Bacillus subtilis spores in artificial meteorites. Int J Astrobiology 14:105–114. https://doi.org/10.1017/S1473550414000251
Cortesão M, Schütze T, Marx R, Moeller R, Meyer V (2020) Fungal biotechnology in space: why and how? In: Nevalainen Helena (ed) Grand challenges in fungal biotechnology. Springer International Publishing, Cham, pp 501–535. https://doi.org/10.1007/978-3-030-29541-7_18
Paulino-Lima IG, Janot-Pacheco E, Galante D, Cockell C et al (2011) Survival of Deinococcus radiodurans against laboratory-simulated solar wind charged particles. Astrobiology 11:875–882. https://doi.org/10.1089/ast.2011.0649
Schultz J, Modolon F, Peixoto RS, Rosado AS (2023) Shedding light on the composition of extreme microbial dark matter: alternative approaches for culturing extremophiles. Front Microbiol 14:1167718. https://doi.org/10.3389/fmicb.2023.1167718
Billi D, Gallego Fernandez B, Fagliarone C, Chiavarini S, Rothschild LJ (2021) Exploiting a perchlorate-tolerant desert cyanobacterium to support bacterial growth for in situ resource utilization on mars. Int J Astrobiol 20:29–35. https://doi.org/10.1017/S1473550420000300
Napoli A, Micheletti D, Pindo M, Larger S, Cestaro A, de Vera JP et al (2022) Absence of increased genomic variants in the cyanobacterium Chroococcidiopsis exposed to mars-like conditions outside the space station. Sci Rep 12:8437. https://doi.org/10.1038/s41598-022-12631-5
Cosciotti B, Balbi A, Ceccarelli A, Fagliarone C, Mattei E, Lauro SE et al (2019) Survivability of anhydrobiotic cyanobacteria in salty ice: implications for the habitability of icy worlds. Life 9:86. https://doi.org/10.3390/life9040086
Blachowicz A, Chiang AJ, Elsaesser A, Kalkum M, Ehrenfreund P, Stajich JE et al (2019) Proteomic and metabolomic characteristics of extremophilic fungi under simulated mars conditions. Front Microbiol 10:1013. https://doi.org/10.3389/fmicb.2019.01013
Olsson-Francis K, Doran PT, Ilyin V, Raulin F, Rettberg P, Kminek G et al (2023) The COSPAR planetary protection policy for robotic missions to mars: a review of current scientific knowledge and future perspectives. Life Sci Space Res (Amst) 36:27–35. https://doi.org/10.1016/j.lssr.2022.12.001
Garrett-Bakelman FE, Darshi M, Green SJ, Gur RC et al (2019) The NASA twins study: a multidimensional analysis of a year-long human spaceflight. Science 364:eaau8650. https://doi.org/10.1126/science.aau8650
Hendrickx L, De WH, Hermans V, Mastroleo F et al (2006) Microbial ecology of the closed artificial ecosystem MELiSSA (micro-ecological life support system alternative): reinventing and compartmentalizing the Earth’s food and oxygen regeneration system for long-haul space exploration missions. Res Microbiol 157:77–86. https://doi.org/10.1016/j.resmic.2005.06.014
De Micco V, Amitrano C, Mastroleo F et al (2023) Plant and microbial science and technology as cornerstones to bioregenerative life support systems in space. NPJ Microgravity 9:69. https://doi.org/10.1038/s41526-023-00317-9
Koehle AP, Brumwell SL, Seto EP et al (2023) Microbial applications for sustainable space exploration beyond low Earth orbit. NPJ Microgravity 9:47. https://doi.org/10.1038/s41526-023-00285-0
Volpin F, Badeti U, Wang C, Jiang J et al (2020) Urine treatment on the international space station: current practice and novel approaches. Membranes (Basel) 10:327. https://doi.org/10.3390/membranes10110327
Grossi CEM, Fantino E, Serral F, Zawoznik MS et al (2020) Methylobacterium sp. 2A is a plant growth-promoting Rhizobacteria that has the potential to improve potato crop yield under adverse conditions. Front Plant Sci 11:71. https://doi.org/10.3389/fpls.2020.00071
Bijlani S, Singh NK, Eedara VVR, Podile AR et al (2021) Methylobacterium ajmalii sp. Nov., isolated from the International Space Station. Front Microbiol 12:639396. https://doi.org/10.3389/fmicb.2021.639396
Handy D, Hummerick ME, Dixit AR, Ruby AM, Massa G, Palmer A (2021) Identification of plant growth promoting bacteria within space crop production systems. Front Astron Space Sci 8:735834. https://doi.org/10.3389/fspas.2021.735834
Verbeelen T, Leys N, Ganigué R, Mastroleo F (2021) Development of nitrogen recycling strategies for bioregenerative life support systems in space. Front Microbiol 12:700810. https://doi.org/10.3389/fmicb.2021.700810
Acknowledgements
JK, JK and AN extend their sincere gratitude to Gargi, Maitreyi and Shivaji colleges for providing the academic environment and resources essential for the completion of this review work.
Author information
Authors and Affiliations
Contributions
JKb conceived the idea for the article, JK and JK performed the literature search, data analysis, and drafted the work, AN performed critical review of the draft.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, J., Kaur, J. & Nigam, A. Extremophiles in Space Exploration. Indian J Microbiol (2024). https://doi.org/10.1007/s12088-024-01297-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12088-024-01297-4