Abstract
Aims
We aimed to compare seasonal exudate quality and quantity between Sphagnum moss, Eriophorum vaginatum (graminoid) and Vaccinium myrtillus (ericoid shrub).
Methods
Exudates were collected in May, July and September 2014 using a culture-based method and characterized by total organic carbon (TOC) and nitrogen (TN) contents with exudation fluxes expressed on a root-mass basis. Organic acids, sugars and amino acids in the exudates were identified by ion exchange chromatography. C and N exudate fluxes, in situ exudation fluxes and exudate contribution to soil dissolved organic matter (DOM) were estimated. Differences in exudate biodegradability were assessed by 13C pulse labeling.
Results
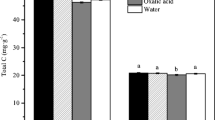
E. vaginatum had the largest exudation fluxes, Sphagnum the lowest, and V. myrtillus intermediate, being the greatest in July. All species mostly exuded organic acids except Sphagnum in September when sugars (allose, xylose) and amino acids (cystine) dominated. Sphagnum exudates were more C-rich and less degradable than the vascular species exudates, which released both organic and inorganic N forms. E. vaginatum exudates were richer in amino acids and citrate especially in July. Exudates contributed up to 20% to soil DOM.
Conclusions
Plant species composition greatly affects exudate quantity, quality and timing. Plant exudates represent considerable contributions to soil DOM.





Similar content being viewed by others
Abbreviations
- C:
-
Carbon
- DOC:
-
Dissolved organic carbon
- DOM:
-
Dissolved organic matter
- DN:
-
Dissolved nitrogen
- LMW:
-
Low molecular weight
- N:
-
Nitrogen
- TOC:
-
Total organic carbon
- TN:
-
Total nitrogen
References
Abrahao A, Lambers H, Sawaya ACHF, Mazzafera P, Oliveira RS (2014) Convergence of a specialized root trait in plants from nutrient-impoverished soils: phosphorus-acquisition strategy in a nonmycorrhizal cactus. Oecologia 176:345–355
Aerts R, Chapin FS (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Aulakh MS, Wassmann R, Bueno C, Kreuzwieser J, Rennenberg H (2001) Characterization of root exudates at different growth stages of ten rice (Oryza sativa L.) cultivars. Plant Biol 3:139–148
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32:666–681. https://doi.org/10.1111/j.1365-3040.2009.01926.x
Beauchemin NJ, Furnholm T, Lavenus J, Svistoonoff S, Doumas P, Bogusz D, Laplaze L, Tisa LS (2012) Casuarina root exudates alter the physiology, surface properties, and plant infectivity of Frankia sp. strain CcI3. Appl Environ Microbiol 78:575–580. https://doi.org/10.1128/AEM.06183-11
Bragazza L (2006) A decade of plant species changes on a mire in the Italian alps: vegetation-controlled or climate-driven mechanisms? Clim Chang 77:415–429
Bragazza L, Limpens J (2004) Dissolved organic nitrogen dominates in European bogs under increasing atmospheric N deposition. Glob Biogeochem Cycles 18:GB4018. https://doi.org/10.1029/2004GB002267
Bragazza L, Parisod J, Buttler A, Bardgett RD (2013) Biogeochemical plant-soil microbe feedback in response to climate warming in peatlands. Nat Clim Chang 3:273–277
Bragazza L, Bardgett RD, Mitchell EAD, Buttler A (2015) Linking soil microbial communities to vascular plant abundance along a climate gradient. New Phytol 205:1175–1182
Breeuwer A, Robroek BJM, Limpens J, Heijmansa MMPD, Schouten MGC, Berendse F (2009) Decreased summer water table depth affects peatland vegetation. Basic Appl Ecol 10:330–339
Cairney JWG, Meharg AA (2003) Ericoid mycorrhiza: a partnership that exploits harsh edaphic conditions. Eur J Soil Sci 54:735–740. https://doi.org/10.1046/j.1365-2389.2003.00555.x
Carvalhais LC, Dennis PG, Fedoseyenko D, Hajirezaei MR, Borriss R, von Wiren N (2011) Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J Plant Nutr Soil Sci 174:3–11
Chaparro JM, Badri DV, Bakker MG, Sugiyama A, Manter DK, Vivanco JM (2013) Root exudation of phytochemicals in Arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PLoS One 8:e55731. https://doi.org/10.1371/journal.pone.0055731
Chapin FS, Moilanen L, Kielland K (1993) Preferential use of organic nitrogen for growth by a non-mycorrhizal arctic sedge. Nature 361:150–153
Cholewa E, Griffith M (2004) The unusual vascular structure of the corm of Eriophorum vaginatum: implications for efficient retranslocation of nutrients. J Exp Bot 55:731–741
Clymo RS, Hayward PM (1982) The ecology of Sphagnum. pp. 229–289. In: Bryophyte ecology, Smith AJE (ed.), Chapman and Hall, London
Comont L, Laggoun-Défarge F, Disnar JR (2006) Evolution of organic matter indicators in response to major environmental changes: the case of a formerly cut-over peatbog (le Russey, Jura Mountains, France). Org Geochem 37:1736–1751
Crow SE, Wieder RK (2005) Sources of CO2 emission from a northern peatland: root respiration, exudation, and decomposition. Ecology 86:1825–1834
Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245:35–47
DeLarue F, Laggoun-Défarge F, Disnar JR, Lottier N, Gogo S (2011) Organic matter sources and decay assessment in a sphagnum-dominated peatland (le Forbonnet, Jura Mountains, France): impact of moisture conditions. Biogeochemistry 106:39–52
Dessureault-Rompre J, Nowack B, Schulin R, Luster J (2007) Spatial and temporal variation in organic acid anion exudation and nutrient anion uptake in the rhizosphere of Lupinus albus L. Plant Soil 301:123–134
Deubel A, Gransee A, Merbach W (2000) Transformation of organic rhizodepositions by rhizosphere bacteria and its influencen on the availability of tertiary calcium phosphate. J Plant Nutr Soil Sci 163:387–392. https://doi.org/10.1002/1522-2624(200008)
Dieleman CM, Branfireun BA, McLaughlin JW, Lindo Z (2015) Climate change drives a shift in peatland ecosystem plant community: implications for ecosystem function and stability. Glob Chang Biol 21:388–395
Drake JE, Darby BA, Giasson MA, Kramer MA, Phillips RP, Finzi AC (2013) Stoichiometry constrains microbial response to root exudation – insights from a model and a field experiment in a temperate forest. Biogeosciences 10:821–838
Dunn C, Jones TG, Roberts S, Freeman C (2016) Plant species effects on the carbon storage capabilities of a blanket bog complex. Wetlands 36:47–58
Fenner N, Ostle N, Freeman C, Sleep D, Reynolds B (2004) Peatland carbon efflux partitioning reveals that Sphagnum photosynthate contributes to the DOC pool. Plant Soil 259:345–354
Fenner N, Ostle NJ, McNamara N, Sparks T, Harmens H, Reynolds B, Freeman C (2007) Elevated CO2 effects on peatland plant community carbon dynamics and DOC production. Ecosystems 10:635–647. https://doi.org/10.1007/s10021-007-9051-x
Finlay R (2005) Action and interaction in the mycorrhizal hyphosphere – a re-evaluation of the role of mycorrhizas in nutrient acquisition and plant ecology. In: BassiriRad H (ed) Nutrient acquisition by plants: an ecological perspective. Ecological Studies 181. Springer-Verlag, Berlin, pp 221–276
Finlay RD (2008) Ecological aspects of mycorrhizal symbiosis: with special emphasis on the functional diversity of interactions involving the extraradical mycelium. J Exp Bot 59:1115–1126. https://doi.org/10.1093/jxb/ern059
Freeman C, Fenner N, Ostle NJ, Kang H, Dowrick DJ, Reynolds B, Lock MA, Sleep D, Hughes S, Hudson J (2004) Export of dissolved organic carbon from peatlands under elevated carbon dioxide levels. Nature 430:195–198. https://doi.org/10.1038/nature02707
Fukumoto T, Kano A, Ohtani K, Yamasaki-Kokudo Y, Kim BG, Hosotani K, Saito M, Shirakawa C, Tajima S, Izumori K, Ohara T, Shigematsu Y, Tanaka K, Ishida Y, Nishizawa Y, Tada Y, Ichimura K, Gomi K, Akimitsu K (2011) Rare sugar d-allose suppresses gibberellin signaling through hexokinase-dependent pathway in Oryza sativa L. Planta 234:1083–1095
Fukumoto T, Kano A, Ohtani K, Inoue M, Yoshihara A, Izumori K, Tajima S, Shigematsu Y, Tanaka K, Ohkouchi T, Ishida Y, Nishizawa Y, Tada Y, Ichimura K, Gomi K, Yoo SD, Sheen J, Akimitsu K (2013) Phosphorylation of D-allose by hexokinase involved in regulation of OsABF1 expression for growth inhibition in Oryza sativa L. Planta 237:1379–1391
Fustec J, Lesuffleur F, Mahieu S, Cliquet J-B (2010) Nitrogen rhizodeposition of legumes. A review. Agron Sustain Dev 30:57–66. https://doi.org/10.1051/agro/2009003
Gavazov K, Hagedorn F, Buttler A, Siegwolf R, Bragazza L (2016) Environmental drivers of carbon and nitrogen isotopic signatures in peatland vascular plants along an altitude gradient. Oecologia 180:257–264
Gerke J (1994) Kinetics of soil phosphate desorption as affected by citric acid. Z Pflanzenernähr Bodenkd 157:17–22
Giesler R, Lundstrom U (1993) Soil solution chemistry—effects of bulking soil samples. Soil Sci Soc Am J 57:1283–1288
Gransee A, Wittenmayer L (2000) Qualitative and quantitative analysis of water-soluble root exudates in relation to plant species and development. J Plant Nutr Soil Sci 163:381–385
Grayston SJ, Vaughan D, Jones D (1996) Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Appl Soil Ecol 5:29–56
Hájek T, Ballance S, Limpens J, Zijlstra M, Verhoeven JTA (2011) Cell-wall polysaccharides play an important role in decay resistance of sphagnum and actively depressed decomposition in vitro. Biogeochemistry 103:45–57
Hayward PM, Clymo RS (1982) Profiles of water content and pore size in sphagnum and peat, and their relation to peat bog ecology. Proc Royal Soc Series B-Biol Sci 215:299–325
Heijmans MMPD, van der Knaap YAM, Holmgren M, Limpens J (2013) Persistent versus transient tree encroachment of temperate peat bogs: effects of climate warming and drought events. Glob Chang Biol 19:2240–2250
Hill BH, Elonen CM, Jicha TM, Kolka RK, Lehto LLP, Sebestyen SD, Seifert-Monson LR (2014) Ecoenzymatic stoichiometry and microbial processing of organic matter in northern bogs and fens reveals a common P-limitation between peatland types. Biogeochemistry 120:203–224
Hinsinger P, Bengough AG, Vetterlein D, Young IM (2009) Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil 321:117–152
Höffland E, van den Boogaard R, Nelemans J, Findenegg G (1992) Biosynthesis and root exudation of citric and malic acids in phosphate-starved rape plants. New Phytol 122:675–680
Hu T, Liu S-Q, Amombo E, Fu J-M (2015) Stress memory induced rearrangements of HSP transcription, photosystem II photochemistry and metabolism of tall fescue (Festuca arundinacea Schreb.) in response to high-temperature stress. Front Plant Sci 6:403
Jones DL (1998) Organic acids in the rhizosphere – a critical review. Plant Soil 205:25–44
Jones DL, Darrah PR (1994) Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant Soil 166:247–257
Jones DL, Hodge A, Kuzyakov Y (2004) Plant and mycorrhizal regulation of rhizodeposition. New Phytol 163:459–480
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil-root interface. Plant Soil 321:5–33
Kano A, Gomi K, Yamasaki-Kokudo Y, Satoh M, Fukumoto T, Ohtani K, Tajima S, Izumori K, Tanaka K, Ishida Y, Tada Y, Nishizawa Y, Akimitsu K (2010) A rare sugar, d-Allose, confers resistance to Rice bacterial blight with upregulation of defense-related genes in Oryza sativa. Phytopathology 100:85–90
Kano A, Fukumoto T, Ohtani K, Yoshihara A, Ohara T, Tajima S, Izumori K, Tanaka K, Ohkouchi T, Ishida Y, Nishizawa Y, Ichimura K, Tada Y, Gomi K, Akimitsu K (2013) The rare sugar D-allose acts as a triggering molecule of rice defence via ROS generation. J Exp Bot 64:4939–4951
Kaštovská E, Straková P, Edwards K, Urbanová Z, Bárta J, Mastný J, Šantrůčková H, Picek T (2017) Cotton-grass and blueberry have opposite effect on peat characteristics and nutrient transformation in peatland. Ecosystems. https://doi.org/10.1007/s10021-017-0159-3
Kazda J, Muller HJ, Stackebrandt E, Daffe M, Muller K, Pitulle C (1992) Mycobacterium madagascariense sp. nov. Int J Syst Bacteriol 42:524–528
King JS, Albaugh TJ, Allen HL, Buford M, Strain BR, Dougherty p (2002) Below-ground carbon input to soil is controlled by nutrient availability and fine root dynamics in loblolly pine. New Phytol 154: 389–398
Kuijken RCP, Snel JFH, Heddes MM, Bouwmeester HJ, Marcelis LFM (2015) The importance of sterile rhizosphere when phenotyping for root exudation. Plant Soil 387:131–142
Kuiper JJ, Mooij WM, Bragazza L, Robroek BJ (2014) Plant functional types define magnitude of drought response in peatland CO2 exchange. Ecology 95:123–131
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371. https://doi.org/10.1016/j.soilbio.2010.04.003
Kuzyakov Y, Xu X (2013) Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol 198:656–669. https://doi.org/10.1111/nph.12235
Laine AM, Bubier J, Riutta T, Nilsson MB, Moore TR, Vasander H, Tuittila ES (2012) Abundance and composition of plant biomass as potential controls for mire net ecosytem CO2 exchange. Botany 90:63–74
Leadley PW, Reynolds JF, Chapin FS (1997) A model of nitrogen uptake by Eriophorum vaginatum roots in the field: ecological implications. Ecol Monogr 67:1–22
Leppala M, Kukko-Oja K, Laine J, Tuittila E-S (2008) Seasonal dynamics of CO2 exchange during primary succession of boreal mires as controlled by phenology of plants. Ecoscience 15:460–471
Lesuffleur F, Cliquet JB (2010) Characterisation of root amino acid exudation in white clover (Trifolium repens L.) Plant Soil 333:191–201. https://doi.org/10.1007/s11104-010-0334-1
Lesuffleur F, Paynel F, Bataillé MP, Le Deunff E, Cliquet JB (2007) Root amino acid exudation: measurement of high efflux rates of glycine and serine from six different plant species. Plant Soil 294:235–246. https://doi.org/10.1007/s11104-007-9249-x
Lesuffleur F, Salon C, Jeudy C, Cliquet JB (2013) Use of a 15N2 labelling technique to estimate exudation by white clover and transfer to companion ryegrass of symbiotically fixed N. Plant Soil 369:187–197. https://doi.org/10.1007/s11104-012-1562-3
Lynch JP, Ho MD (2005) Rhizoeconomics: carbon costs of phosphorus acquisition. Plant Soil 269:45–56
Macko SA, Engel MH, Hartley G, Hatcher P, Helleur R, Jackman P, Silfer JA (1991) Isotopic compositions of individual carbohydrates as indicators of early diagenesis of organic matter in peat. Chem Geol 93:147–161
Marschner P, Crowley D, Rangel Z (2011) Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis – model and research methods. Soil Biol Biochem 43:883–894
Mitsch WJ, Gosselink JG (2000) Wetlands, 3rd edn. John Wiley and Sons, New York
Neumann G, Romheld V (1999) Root excretion of carboxylic acids and proton in phosphorus-deficient plants. Plant Soil 211:121–130
Neumann G, Romheld V (2012) Rhizosphere chemistry in relation to plant nutrition. In: Marschner P (ed) Marschner’s mineral nutrition of higher plants, 3rd edn. Elsevier, Amsterdam, pp 347–368
Paterson E (2003) Importance of rhizodeposition in the coupling of plant and microbial productivity. Eur J Soil Sci 54:741–750. https://doi.org/10.1046/j.1365-2389.2003.00557
Phillips RP, Erlitz Y, Bier R, Bernhardt ES (2008) New approach for capturing soluble root exudates in forest soils. Funct Ecol 22:990–999
Raynaud X, Lata JC, Leadley PW (2006) Soil microbial loop and nutrient uptake by plants: a test using a coupled C: N model of plant-microbial interactions. Plant Soil 287:95–116
Riutta T, Laine J, Tuittila ES (2007) Sensitivity of CO2 exchange of fen ecosystem components to water level variation. Ecosystems 10:718–733
Robroek BJM, Albrecht RJH, Hamard S, Pulgarin A, Bragazza L, Buttler A, Jassey VE (2015a) Peatland vascular plant functional types affect dissolved organic matter chemistry. Plant Soil 407:135–143
Robroek BJM, Jassey VEJ, Kox MAR, Berendsen RL, Mills RTE, Cécillon L, Puissant J, Meima-Franke M, Bakker PAHM, Bodelier PLE (2015b) Peatland vascular plant functional types affect methane dynamics by altering microbial community structure. J Ecol 103:925–934
Ryan MH, Tibbett M, Edmonds-Tibbett T, Suriyagoda LDB, Lambers H, Cawthray GR, Pang J (2012) Carbon trading for phosphorus gain: the balance between rhizosphere carboxylates and arbuscular mycorrhizal symbiosis in plant phosphorus acquisition. Plant Cell Environ 35:2170–2180
Saarnio S, Wittenmayer L, Merbach W (2004) Rhizospheric exudation of Eriophorum vaginatum L. – potential link to methanogenesis. Plant Soil 267:343–355
Sakoguchi H, Yoshihara A, Shintani T, Okuma K, Izumori K, Sato M (2016) Growth inhibitory effect of D-arabinose against the nematode Caenorhabditis elegans: discovery of a novel bioactive monosaccharide. Bioorg Med Chem Lett 26:726–729
Shaver GR, Fetcher N, Chapin FS (1986) Growth and flowering in Eriophorum vaginatum—annual and latitudinal variation. Ecology 67:1524–1535
Ström L, Ekberg A, Mastepanov M, Christensen TR (2003) The effect of vascular plants on carbon turnover and methane emissions from a tundra wetland. Glob Chang Biol 9:1185–1192
Tam JP, Wang S, Wong KH, Tan WL (2015) Antimicrobial peptides from plants. Pharmaceuticals 8:711–757. https://doi.org/10.3390/ph8040711
van der Krift TAJ, Kuikman PJ, Möller F, Berendse F (2001) Plant species and nutritional-mediated control over rhizodeposition and root decomposition. Plant Soil 228:191–200
Verhoeven JTA, Liefveld WM (1997) The ecological significance of organochemical compounds in Sphagnum. Acta Bot Neerl 46:117–130
Warren CR (2015) Wheat roots efflux a diverse array of organic N compounds and are highly proficient at their recapture. Plant Soil 397:147–162
Warren CR (2016) Simultaneous efflux and uptake of metabolites by roots of wheat. Plant Soil 406:359–374
White MD, Klecker M, Hopkinson RJ, Weits DA, Mueller C, Naumann C, O’Neill R, Wickens J, Yang J, Brooks-Bartlett JC, Garman EF, Grossmann TN, Dissmeyer N, Flashman E (2017) Plant cysteine oxidases are dioxygenases that directly enable arginyl transferase-catalysed arginylation of N-end rule targets. Nat Commun 8:14690. https://doi.org/10.1038/ncomms14690
Yong Wu F, Chuen Chung AK, Yee Tam NF, Hung Wong M (2012) Root exudates of wetland plants influenced by nutrient status and types of plant cultivation. Intern J Phytoremed 14:543–553
Yu ZC (2012) Northern peatland carbon stocks and dynamics: a review. Biogeosciences 9:4071–4085
Acknowledgements
Many people helped in collecting and processing the root exudates, especially Iveta Chomová, Iveta Fleischmannová, Jiří Mastný, Daniel Vaněk and Tomáš Hubáček. Their assistance was and is greatly appreciated. Many thanks also to the administration of the Šumava National Park, especially Ivana Bufková, for access to the study site. The study was supported through a grant from the Czech Science Foundation (GAČR 13-17398S) awarded to TP.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Eric Paterson
Rights and permissions
About this article
Cite this article
Edwards, K.R., Kaštovská, E., Borovec, J. et al. Species effects and seasonal trends on plant efflux quantity and quality in a spruce swamp forest. Plant Soil 426, 179–196 (2018). https://doi.org/10.1007/s11104-018-3610-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3610-0